Abstract
Several scientific investigations have focused on providing new strategies for supporting the development of low saturated and zero trans lipid materials, as healthier fat alternatives for food application. This work evaluated the consistency, crystallization behavior, microstructure and polymorphism of six blends composed of palm and canola oils at different concentrations (100:0, 80:20, 60:40, 40:60, 20:80 and 0:100, in w/w%) added with 5.0% of fully hydrogenated palm oil (FHPO) or with a mixture of 2.5% of FHPO and 2.5% of sorbitan monostearate (SMS). The results were compared with the non-structured blends (standard samples). Through microstructure images, the formation of a more homogeneous and denser packed crystal network was observed for samples added with both crystallization modifiers (FHPO/SMS) compared to the corresponding standard samples, after stabilization at 25 °C during 3 h. In particular, enhanced crystallization modifications were observed for the 40:60 blend, in which the crystal form β′ emerged after the addition of FHPO/SMS. Moreover, the 40:60 blend structured with FHPO/SMS showed increased consistency (from 30 to 658 gF/cm2) and induced onset crystallization in a higher temperature (from 13.1 to 23.9 °C) compared with the non-structured one, due to the specific crystallization effects provided by both added structurants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Trans fatty acids are known to be strongly associated with an increase in the incidence of cardiovascular and chronic degenerative diseases, and also by negatively influencing the intrauterine growth, obesity and inflammatory processes (Dhaka et al. 2011). Saturated fatty acids when consumed in excess are also harmful to human health. Lauric acid (C12:0), myristic acid (C14:0), palmitic acid (C16:0) and stearic acid (C18:0), each of them presenting different effects on human metabolism are commonly applied to foodstuffs. Myristic acid, for example, has a strong hypercholesterolemic and atherogenic effect. Lauric and palmitic acids exhibit potential to increase LDL (low density lipoprotein) levels, although, it has been shown that palmitic acid exerts negligible deleterious effect in relation to HDL (high density lipoprotein) levels. On the other hand, stearic acid differs from other saturated fatty acids due to its blank effect on the cholesterolemic balance (Siraj et al. 2015).
Food industries are urged to adapt formulations in order to partially or totally replace trans and saturated fatty acids.
Crystallization modifiers (also called structurants or structuring agents) can be added to zero trans and/or reduced saturated fatty acids containing systems in order to induce texture patterns similar to the one existing in unhealthy lipid-based products (Siraj et al. 2015). These agents act as constructive elements in the formation of three-dimensional networks, responsible for physically sustaining the entrapped liquid oil. The structurants molecular size and shape, as well as their interactions with the lipid material and the crystallization conditions (temperature, cooling rate, shearing), will determine the final structure of the fat blend and, consequently, the consistency, melting behavior and other sensorial properties of the final product. In the last few years, several structuring agents are being considered for improving the crystallization profile of low saturated fat blends as monoacylglycerols, diacylglycerols, fatty acids, fatty alcohols, waxes, wax esters, sorbitan esters (as sorbitan monostearate, and tristearate), and phytosterols (mainly associated with γ-oryzanol). Each of these crystallization modifiers can be evaluated separately or even as a mixture of two or more types in order to excel structuration characteristics (Pernetti et al. 2007; Ribeiro et al. 2015).
Sorbitan monostearate is a non-ionic hydrophobic surfactant, capable of gelling a number of organic solvents, including vegetable oils. The prevalent structure provided by this agent is based on tubular arrangements, which are formed during cooling. When self-assembling, they associate in tubular forms and immobilize the organic solvent and give rise to a three-dimensional network (Murdan et al. 1999; Pernetti et al. 2007).
The addition of small amounts of totally hydrogenated oils (commonly named as hardfats) can also be considered as a prominent tool for enhancing lipid crystal structuration of low saturated fat blends. Fully hydrogenated palm oil, for instance, exhibits a homogeneous composition of saturated triacylglycerols with high melting point, able to act as modifier of the crystallization process, inducing the formation of specific β’ form crystals (de Oliveira et al. 2015a).
In this context, aiming to contribute for the development of healthier structured lipid blends with lower saturated fatty acid contents and without trans fatty acids, the current research focused on the structuration and evaluation of fat blends composed by different relative amounts of palm and canola oils through the addition of 5% (w/w) of fully hydrogenated palm oil (FHPO) and, alternatively, the addition of a mixture of 2.5% (w/w) of FHPO and 2.5% (w/w) of sorbitan monostearate (SMS). The structuring ability of these crystallization modifier agents was evaluated in terms of solid fat content, crystallization behavior, crystal morphology, consistency and polymorphism.
Materials and methods
Materials
Deodorized palm oil (PO) was supplied by Agropalma S/A (Brazil). Canola oil (CO) and fully hydrogenated palm oil (FHPO) were supplied by Cargill Agrícola S/A (Brazil). Sorbitan monostearate (SMS, 55% purity, product number: S7010) was purchased at Sigma-Aldrich (United States).
Sample preparation
Palm oil (PO) and canola oil (CO) were firstly heated to 100 °C and maintained at this temperature during 15 min under constant agitation for complete homogenization and effective melting of the lipid crystals. After melting, six samples with no crystallization modifiers addition were prepared by blending the following proportions of PO and CO, respectively: 100:0, 80:20, 60:40, 40:60, 20:80, and 0:100 (in w/w %). Another set of six samples was obtained as previously described and added with 5% (w/w) of fully hydrogenated palm oil (FHPO) and named as 100:0P, 80:20P, 60:40P, 40:60P, 20:80P, and 0:100P. Similarly, 2.5% (w/w) of FHPO and 2.5% of sorbitan monostearate (SMS) were added to a third set of the same six standard blends, and designated as 100:0PS, 80:20PS, 60:40PS, 40:60PS, 20:80PS, and 0:100PS. After the addition of the crystallization modifiers, the samples were heated up to 100 °C under constant stirring, during 15 min until complete melting and homogenization. All the samples were stored at room temperature (approximately 25 °C).
Analytical methodology
Fatty acid composition
Fatty acid composition determinations were performed in duplicate in a gas chromatography Agilent 6850 Series GC System, equipped with a capillary column and flame ionization detector (FID). Prior to the determinations, the samples were converted to their fatty acid methyl esters (FAME), following the procedure described by Hartman and Lago (1973). FAME were separated as described by AOCS Official Method Ce 2-66 (AOCS 2009), in a capillary column DB23 Agilent (50% cyanopropyl-methylpolysiloxane, length of 60 m, internal diameter of 0.25 mm and coated with a 0.25 μm film), under the following oven temperature conditions: at 110 °C for 5 min, from 110 to 215 °C in a rate of 5 °C/min, and with a holding time of 24 min at 215 °C. Other operating parameters were: detector temperature of 280 °C; injection temperature of 250 °C; helium used as stripping gas; split ratio of 1:50; and 1.0 µL injection volume. The qualitative evaluation was determined by comparing peak retention times with the respective fatty acid standard peaks, and the quantitative composition was accessed by area normalization.
Triacylglycerol composition
Triacylglycerol composition was determined in duplicate using a gas chromatography Agilent 6850 Series GC System, with a DB-17 HT Agilent capillary column (50% phenyl-methylpolysiloxane, length of 15 m, internal diameter of 0.25 mm, and coated by a 0.15 μm film). The operating conditions were: column flow of 1.0 mL/min; detector temperature of 375 °C; injector temperature of 360 °C; initial oven temperature of 280 °C, heated to 340 °C at 2 °C/min, and kept at 340 °C for 40 min; helium as stripping gas, injection volume of 1.0 μL, split injection rate of 1:100; sample concentration of 10 mg/mL in tetrahydrofuran. The triacylglycerol groups were identified as described by Antoniosi Filho et al. (1995) and were quantified based on relative peak area.
Solid fat content
Solid fat content (SFC) was determined using a low resolution nuclear magnetic resonance spectrometer Bruker pc 120 Minispec, according to AOCS Direct Method Cd 16b-93 (AOCS 2009). Prior to measurements, the equipment was calibrated with three Bruker-certified calibration standards (0, 31.8 and 73.5% of solid content each). Equipment parameters were: magnet temperature of 40 °C and pulse of 20 MHz. Sample preparation in a high precision dry bath TCON 2000 was performed as follows: melted at 100 °C for 15 min, conditioned at 60 °C for 5 min, chilled to 0 °C for 90 min, and then kept at each measurement temperature (10, 15, 20, 25, 30, 35, 40, 45, and 50 °C) during 30 min prior to SFC measurements. The results were expressed as the mean of two determinations.
Microstructure images
Images were obtained by polarized light microscope (Olympus, model BX 51) coupled to a digital video camera (Media Cybernetics). Initially, samples were melted at 100 °C and one drop of each sample was placed over a pre-heated glass slide (100 °C) with the aid of a capillary tube, and then covered with a coverslip. Subsequently, the slides were kept during 3 h at 25 °C in order to stabilize the crystalline structure. The slides were then placed on the heating plate and kept at 25 °C for images acquisition. The images were captured by the software Image Pro-Plus 7.0 (Media Cybernetics), under polarized light and amplification of 40 times. Three visual areas of each slide were randomly focused and only one of them was selected to represent the observed morphology.
Consistency
Consistency was determined by the texture analyzer TA-XT Plus (Stable Micro Systems). Prior to the measurement, samples were heated to 100 °C and then maintained in 50 mL plastic beakers at 5 °C for 24 h for crystals formation and, subsequently conditioned during 24 h at the measurement temperature of 15 °C. The probe was a Plexiglas® cone with non-truncated tip angle of 60°. The experiment set up conditions were: penetration depth of 10 mm and probe velocity of 2 mm/s. The compression force obtained is given in gram force (gF). According to Haighton (1959), the compression force can be converted to yield value (YV) by the following equation:
where YV is yield value, in gF/cm2; K is a constant that is dependent on the cone angle (for a cone of 60°, K is 2815); W is the compression force, in gF; p is penetration depth, in units of 0.1 mm. Samples were analyzed in quadruplicate and the results were expressed as the means of the replicates.
Crystallization behavior
Crystallization events were assessed by exothermic heat flow determined using a differential scanning calorimetry DSC Q2000 (TA Instruments). Approximately 10 mg of each melted sample was weighted in aluminum pans and sealed with covers. An empty sealed aluminum pan was used as reference and the equipment was previously calibrated with indium. The samples were subjected to the following temperature program: initial temperature of 80 °C kept during 3 min, from 80 to − 60 °C at a cooling rate of 10 °C/min, and maintained at − 60 °C for 3 min.
Polymorphism
The preferred crystalline polymorphic form was determined by a X-ray diffractometer Philips (PW 1710), according to the AOCS Cj 2-95 method (AOCS 2009), with Bragg–Brentano geometry (θ:2θ), and Cu-kα radiation (λ = 1.54056 Å, at 40 kV and 30 mA). The measurements were performed over the range 5°– 40° (2θ scale), with 0.02° step in 2θ, with an acquisition time of 2 s. Diffraction was performed at ambient temperature (~ 20 °C) and the crystal forms were determined from their characteristic short spacing (d), calculated using the Bragg’s Law (λ = 2d sinθ).
Statistical analysis
Yield value was statistically evaluated by one-way analysis of variance (ANOVA) by the software STATISTICA V.8 (StatSoft Inc., USA). Tukey’s post hoc tests were applied for statistical comparison among the means with a significance level of 5% (p < 0.05).
Results and discussion
Fatty acid composition
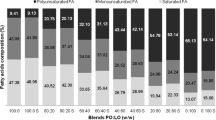
Figure 1 presents the fatty acid composition, expressed as mass percentage (%) of saturated, monounsaturated and polyunsaturated fatty acids, of the lipid raw materials—palm oil (PO), canola oil (CO), and fully hydrogenated palm oil (FHPO)—as well as blends of PO:CO without and with crystallization modifiers (5% of FHPO or combined 2.5% of FHPO with 2.5% of SMS).
Fatty acid composition (in %) of fully hydrogenated palm oil (FHPO), palm oil (100:0), canola oil (0:100), and 80:20, 60:40, 40:60, 20:80 blends of palm and canola oil, respectively, with and without crystallization modifier additions. Standard samples with no crystallization modifiers are labeled only by the proportion percentage. Blends with structurant addition received P (5% FHPO addition) or PS (2.5% FHPO and 2.5% sorbitan monostearate addition) after the respective proportion percentage
Palm oil has typical balanced composition of both saturated and unsaturated fatty acids and can usually be considered as a lipid material with no trans fatty acid content. Thus, PO fatty acid composition showed a content of 47.1 and 52.9% of saturated and unsaturated fatty acids, respectively. Among the saturated fatty acids, palmitic (Pt) and stearic (St) acids predominate, representing, respectively, 40.5 and 5.0% of the total composition. Regarding the unsaturated fatty acid profile, monounsaturated and polyunsaturated fatty acids represent, respectively, 43.5 and 9.4% of the total fatty acid content, mainly comprised of 43.2% of oleic (O) and 9.2% of linoleic (L) acids. Due to its suitable composition and the absence of trans fatty acids, palm oil is currently still a fat material largely applied in shortenings by the food industry, despite the strong trend in replacing natural sources of saturated fatty acid.
Canola oil is also used as a zero trans fatty acid raw material and is composed predominantly of unsaturated fatty acids, with approximately 91.0 and 9.0% of unsaturated and saturated fatty acids content, respectively. Oleic (O), linoleic (L) and linolenic (Ln) acids were the main fatty acids found in CO, with levels corresponding to 59.8, 21.7 and 8.3%, as also reported by Ribeiro et al. (2009a).
The fully hydrogenated palm oil (FHPO) sample displayed, predominantly, saturated fatty acids (99.4% of the total fatty acid content)—as expected for fully hydrogenated oils—and is made up mainly by stearic acid (61.1%) and palmitic acid (36.3%).
Due to its high-unsaturated fatty acid content, canola oil is used in lipid-based formulations in order to replace other lipid materials, rich in saturated fatty acids (as palm oil). Figure 1 confirms the gradual reduction of saturated fatty acids content in the blends with increasing canola oil addition, as a result of the partial or total replacement of palm oil, turning them adequate test material to evaluate the effects of adding crystallization modifiers.
As expected, a slight increment in the saturated fatty acid content can be observed with the addition of 5.0% of FHPO to the blends of palm and canola oil compared to those with no structurant addition (standard samples). However, the application of a small quantity of FHPO for promoting the crystallization of a low saturated fatty acid blends can be justified by allowing an increased load of canola oil in those blends. Alternatively, the addition of only 2.5% of FHPO together with 2.5% of sorbitan monostearate (SMS) introduced less saturated fatty acid in comparison with the blends with 5% FHPO addition.
Triacylglycerol composition
Vegetable fats and oils are frequently formed by a large number of different triacylglycerols. Even minor differences found in the fatty acid composition among lipid materials can result in major modifications in their triacylglycerol profile. Depending directly on their specific triacylglycerol compositions, fats and oils exhibit unique physicochemical properties and, consequently, particular technological performance under application. In the complementary material, Figure 1 (Online Resource 1) displays the triacylglycerol composition expressed in percentage of trisaturated (SSS), monounsaturated (SUS), diunsaturated (SUU), and triunsaturated (UUU) triacylglycerols of the lipid raw materials—PO, CO, and FHPO—and blends of PO:CO without and with crystallization modifiers (5% of FHPO or combined 2.5% of FHPO with 2.5% of SMS).
The semi-solid structure characteristic of palm oil at ambient temperature (approximately 20–25 °C) is strongly related to its triacylglycerol profile, with about 34% of SUS and 40% SUU triacylglycerols. The main triacylglycerols (TAGs) found in the palm oil samples are PtOO, PtPtO, PtOL, and OOO, at concentrations of 23.8, 21.3, 9.7, and 9.7%, respectively, similar to values reported in the literature (O’Brien 2003). Among the total content of 10% of trisaturated TAGs determined in palm oil, the major TAGs types found were PtPtPt (6.7%), and PtPtSt (2.5%). According to O’Brien (2003), the high content of palmitic acid in a lipid material—as currently determined in the PO sample—indicates preferable crystallization in the β′ form. Fats with this specific crystal polymorphism are largely applied in margarines, spreads and confectionary products, due to their desirable physical characteristics of plasticity, texture, aeration and creaming (Ghotra et al. 2002).
Canola oil sample showed predominant amounts of diunsaturated and triunsaturated TAGs, respectively, 20.0 and 80.0%, as similarly reported by O’Brien (2003). The major triacylglycerol types found in CO sample were OOO, OOL, and OOLn, reaching 23.3, 24.3, and 17.6%, respectively.
The composition of the FHPO sample was exclusively trisaturated TAGs, as expected for a vegetable oil after total hydrogenation. The main FHPO triacylglycerols found were PtStSt and PtPtSt, in amounts of 39.0 and 35.0%, respectively.
The TAG composition of the blends prepared with PO and CO at different proportions without crystallization modifiers, indicate an evident increase in the UUU content followed by a proportional decrease of the other TAG types (SSS, SSU, and SUU), with the gradual addition of canola oil, replacing palm oil content.
Blends added with 5.0 and 2.5% of FHPO showed a slight proportional increment of trisaturated triacylglycerols levels compared to the standard ones. Due to the increase in melting point provided by the selected presence of trisaturated TAGs, even small amounts of FHPO are expected to favor thermal resistance of the blends, enabling higher addition of canola oil.
Solid fat content
Solid fat content measured at different temperatures is an approach for physical characterization frequently applied in fats and oils materials in order to relate their solid/liquid lipid phase’s ratio to consistency values.
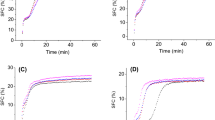
Figure 2 displays the solid fat content measured over a temperature range of the standard blends and samples added with 5.0% of FHPO (Fig. 2a) and with 2.5% of FHPO combined with 2.5% of SMS (Fig. 2b).
Solid fat content (SFC, in %) measured in different temperatures (°C) of standard blends (100:0, 80:20, 60:40, 40:60, 20:80, and 0:100 of palm and canola oil, respectively) and a samples added with 5.0% of FHPO, or b samples added with 2.5% of FHPO and 2.5% of SMS. Blends with structurant addition received P (5% FHPO addition) or PS (2.5% FHPO and 2.5% sorbitan monostearate addition) after the respective proportion percentage
In general, blends 100:0, 80:20, and 60:40 with and without structurant addition showed higher solid fat content compared to the blends with higher amounts of canola oil (40:60, 20:80, and 0:100), due to the major presence of triacylglycerols from palm oil in their composition.
The gain in solid fat contents of the samples added with 5.0% of FHPO compared to the corresponding standard samples can be visualized in Fig. 2a, and these results are due to the crystallization effect provided by the predominant saturated fatty acid composition of this structurant. Blends added with 2.5% of FHPO and 2.5% of SMS (Fig. 2b) also provided an increment in the SFC compared with the standard samples, however in a slightly less extent in comparison with samples added only with FHPO.
SFC parameter of a lipid material is one of a possible set of characteristics proper for suiting applications of fats in food products. For instance, the SFC measured at 10 °C of a lipid material compatible for margarine formulations should be less than 32%, leading to desirable spreadability in the final product stored at refrigeration temperature (from 4 to 10 °C). The SFC near 20 °C should be higher than 10% for avoiding oiling-out and keeping the product structure stable at room temperature (Ribeiro et al. 2009b). In this context and if only SFC assessment is considered, samples 60:40, 60:40P and 60:40PS are probably suitable options for application in margarine formulations.
In shortening production, fat materials can also be preliminarily selected by SFC evaluations, complemented by other procedures. Shortenings usually display semi-solid melting characteristics close to ambient temperature in order to confer desirable sensorial characteristics for the final product as texture, melting feeling in the mouth, lubrication and air incorporation. In order to be compatible with puff pastry applications, a shortening should exhibit an SFC range between 20 and 23% around 25 °C, while in bakery application, the SFC should be approximately 16% at this temperature (Ghotra et al. 2002). With these specifications, sample 100:0P will function as puff pastry shortening and sample 80:20P has the qualification for bakery shortening formulation.
Microstructure images
Fat microstructure characteristics can be appraised from the observation of the fat crystal network, formed by aggregation of fat crystal clusters in a particular large pattern (Awad and Marangoni 2006).
In lipid systems, fats with increased hardness usually present higher solid fat content. However, lipid materials with the same solid fat content can exhibit different texture and rheological properties, suggesting that compaction levels of the crystal fat network impacts their hardness/softness, spreadability and other rheological parameters (Rodrigues-Ract et al. 2010). Therefore, the results found for solid fat content, as well as crystalline morphology, microstructure and consistency evaluation techniques are often considered together to pinpoint the physical applications of fat.
The images of the samples crystal network under polarized light microscopy of the standard blends (without structurant addition) and blends added with FHPO and FHPO/SMS were obtained after isothermal crystallization at 25 °C, and are shown in Fig. 3.
Polarized light microscopy images of palm and canola oil blends in the proportions of, respectively, 100:0, 80:20, 60:40, 40:60, 20:80, and 0:100 with and without crystallization modifier addition, after 3 h of isothermal crystallization at 25 °C (the bars represent 50 μm). Standard samples with no crystallization modifiers are labeled only by the proportion percentage. Blends with structurant addition received P (5% FHPO addition) or PS (2.5% FHPO and 2.5% sorbitan monostearate addition) after the respective proportion percentage
Observing the images in Fig. 3, samples with higher content of canola oil tend to exhibit reduced crystallized area—represented by the white color, due to the presence of higher content of unsaturated fatty acids and, therefore, liquid oil. Besides, in the samples with increased amount of canola oil, the crystal distribution is sparsely dispersed along the formed network.
By adding 5.0% FHPO, blends showed increased crystallized area compared with samples without structurant, even in blends with higher canola oil content. Additionally, the images of the samples added with FHPO exhibit a more homogeneous crystallization frame with predominant presence of smaller crystals, packed in a denser network compared to the standard samples. This trend reinforces the findings related to SFC measurements, that the addition of FHPO favors the crystal formation—nucleation and growth—of the palm and canola oil blends, probably due to the higher melting point of their trisaturated triacylglycerols.
Consequently, harder lipid materials will be originated from a denser crystal-packing network together with increased crystalline area and smaller diameter crystals.
Images of blends with added FHPO and SMS display a distinguishing crystallization network compared to the ones added only with FHPO and the standard samples. The presence of both, FHPO and SMS, in the palm/canola oil blends led to the formation of smaller crystal particles diameters compared to the standard blends (no added structurant) and samples added with only FHPO. In addition, compared to the network formed by the FHPO added crystals, even denser arrangements were formed by packing the smaller crystals of blends with FHPO/SMS, without the formation of crystalline agglomerates as seen in the other blends.
Crystal network differences noticed in the structured samples are developed by the specific crystallization behavior provided by application of the structurant modifiers. Under a general perspective, crystallization modifiers are classified in two main groups: self-assembly and crystal particles systems, according to their structuring mechanism (Dassanayake et al. 2011). In this context, fully hydrogenated palm oil (FPHO) is placed in the crystal particle system group, since its composition is predominantly high-melting point triacylglycerols. Therefore, the crystal nucleation and growth evolved from the FHPO addition is based on blend structuration, entrapping the liquid phase inside the formed crystal network. On the other hand, sorbitan monostearate (SMS) is a structurant example of the self-assembly group (Dassanayake et al. 2011; Siraj et al. 2015). According to Murdan et al. (1999), SMS molecules dispersed at higher temperature in organic solvents (for instance, vegetable oils) self-assemble into rod-shaped tubular structures, after subjected to cooling conditions. Therefore, applying SMS in lipid blends lead to the immobilization of the liquid phase in a continuous three-dimensional frame, self-assembled due to reduce solubility of SMS (Murdan et al. 1999; Pernetti et al. 2007; Masuchi et al. 2014).
Consistency
Consistency is measured as the force necessary to cause a permanent deformation, and this value is usually converted to the respective Yield Value (YV) parameter, allowing comparison among fats, shortenings, and margarines (Haighton 1959). It is also correlated with other plastic fats technological and sensorial properties as spreadability, creaming, hardness, softness and overall sample texture.
Hardness of lipid materials increases with higher solid fat content (O’Brien 2003). However, with the recent application of crystallization modifiers, fats with lower solid content, can also display increased consistency values, due to the packing characteristics of the fat crystal network. Therefore, in addition to solid fat content, microstructure parameters as size, packing density, network homogeneity, and agglomeration of the fat crystals also affect the hardness behavior of the lipid material (de Oliveira et al. 2015a).
The yield values measured at 15 °C for palm and canola oil blends without and with structurants—FHPO and FHPO/SMS—are shown in Fig. 4. As expected, blends with increasing content of canola oil exhibited decreasing YV, due to raising amount of lower melting point fatty acids.
Yield value (YV, in gF/cm2) of palm and canola oil blends in the proportions of, respectively, 100:0, 80:20, 60:40, 40:60, 20:80, and 0:100 without (standard) and with crystallization modifier addition, measured at 15 °C. Standard samples with no crystallization modifiers are labeled only by the proportion percentage. Blends with structurant addition received P (5% FHPO addition) or PS (2.5% FHPO and 2.5% sorbitan monostearate addition) after the respective proportion percentage. Same letters indicate that there are no significant differences between the means evaluated by Tukey’s test (p < 0.05)
According to the classification suggested in Haighton (1959), Yield Value ranges determined for fats, margarines and shortenings are correlated with qualitative parameters of hardness and softness, starting from very soft for fats with YV lower than 50 gF/cm2 and too hard for materials with YV higher than 1500 gF/cm2. Based on these limits, samples 0:100, 0:100P, 20:80, 20:80P and 40:60 (YV < 50 gF/cm2) can be interpreted as very soft to just pourable, whilst blends 100:0, 100:0P, 100:0PS, 80:20PS and 60:40PS (YV > 1500 gF/cm2) are considered as too hard fats. In between these two extremes, the blends 80:20, 40:60PS and 20:80PS, with YV ranging from 200 to 800 gF/cm2, can be classified as satisfactory plastic and spreadable, well fitted for margarine and shortenings applications.
After the addition of FHPO or FHPO plus SMS, palm and canola oil blends displayed higher YV in comparison to the standard samples (no structurants added). As can be seen in Fig. 4, higher YV were observed in blends added with both structurants—FHPO and SMS. This result suggests that the sorbitan monostearate addition led to the formation of more homogeneous and denser crystal network packing, corroborating the observation of microscopic images (Fig. 3). It is also striking to observe that for a given blend, the higher Yield Values correspond to samples with lower solids content, as can be seen in Fig. 2.
The addition of palm hardfat (FHPO) alone as a fat structurant caused marked increment of YV in blends with higher amounts of palm oil (100:0P and 80:20P). This trend can be assigned to the chemical similarity of the predominant fatty acid chain length in both constituents.
Blends structured by combining both FHPO and SMS structurants showed the highest consistency values among the evaluated samples at 15 °C. For example, blend 100:0PS tripled and doubled the YV of the samples 100:0 and 100:0P, respectively. The most remarkable YV increase was observed in sample 40:60PS, with YV determined as 22 and 10 times higher than the YV in samples 40:60 and 40:60P, respectively. Although classified as a very soft fat, the blend 0:100PS represents the ability of FHPO plus SMS to modify the crystallization of the fat network. These results can be well correlated with the structurant ability of both FHPO and SMS together of promoting the formation of a denser and more organized fat crystal network in the palm and canola oils blends, specially by the particular self-assembly behavior of SMS molecules.
Crystallization behavior
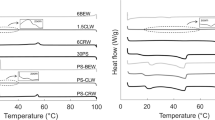
The crystallization events as measured by DSC, of the palm and canola oils blends with and without structurant addition are shown in Fig. 5.
Crystallization events of palm and canola oils blends in the proportions of, respectively, 100:0, 80:20, 60:40, 40:60, 20:80, and 0:100, a without structurant addition, b with 5.0% of fully hydrogenated palm oil (FHPO), and c with 2.5% of FHPO and 2.5% of sorbitan monostearate (SMS). Standard samples with no crystallization modifiers are labeled only by the proportion percentage. Blends with structurant addition received P (5% FHPO addition) or PS (2.5% FHPO and 2.5% sorbitan monostearate addition) after the respective proportion percentage
The DSC-crystallization curves of the standard blends are shown in Fig. 5a. Samples containing palm oil exhibited two distinct crystallization peaks, set up by the specific thermal behavior of the two main triacylglycerol groups of this lipid material. For instance, sample 100:0—pure palm oil—displayed the first crystallization peak starting at 19.8 °C, and the second peak at 6.2 °C, indicating the crystallization of predominantly monounsaturated (SUS) and diunsaturated (SUU) triacylglycerol groups, respectively. With increasing incorporation of canola oil, gradually replacing palm oil content in the blends, these two peaks were shifted to the left, decreasing their crystallization onset temperature.
A third crystallization peak emerged at approximately − 50 °C, in blends with higher content of canola oil (40:60, 20:80 and 0:100), corresponding to the crystallization of the triunsaturated (UUU) triacylglycerols, typically found in this vegetable oil (Figure 1, Online Resource 1).
Blends of palm and canola oils with structurant addition displayed higher crystallization onset temperature compared to standard ones, for the same proportion of palm and canola oils blend. This increment in the crystallization onset temperature of the first peak indicated an increase in the thermal resistance of the blend systems added with the structurants FHPO and FHPO with SMS. Temperature range of the first peak crystallization onset was between 19.8 and − 15.1 °C for the standard blends, whilst increased crystallization onset temperatures were observed after FHPO and FHPO/SMS additions with ranges of 24.2 to 21.9 °C, and 26.6 to 22.1 °C, respectively. For example, the blend with 60% of canola oil and 40% of palm oil (40:60) showed a crystallization onset temperature at 13.1 °C without structurant addition. After the addition of FHPO and FHPO/SMS, the blend 40:60 displayed increments in the onset temperature of the first crystallization peak of 8.8 and 10.8 °C, respectively, supporting the assumption that both structurants FHPO and SMS clearly affects the crystallization behavior of low saturated fatty acids blends.
After the incorporation of 5.0% of FHPO, the first crystallization peak of the blends showed increased intensity, represented by the increased peak height, as observed in Fig. 5b. This feature occurs due to the presence of higher amounts of trisaturated (SSS) triacylglycerols from the FHPO addition.
When applying non-triacylglycerol additives to lipid blends, attention should be given to confirm the effective incorporation of this molecule in the system. The appearance of abnormal peaks not originated from the triacylglycerol content of the lipid material generally indicates an imperfect incorporation of additives. As shown in Fig. 5c, SMS was efficiently incorporated in the blends, since no isolated peak appeared, specifically around 52.7 °C, the crystallization onset temperature of the pure SMS, as reported in Masuchi et al. (2014).
Therefore, the findings corroborate with the previous analyses—presented in “Microstructure images” section and “Consistency” section, confirming that the structurants FHPO and SMS added to the palm and canola oils blends were effective in modifying the crystallization behavior, consistency and microstructure of the blends, supporting the development of low saturated fatty acid blends as replacement of current unhealthy structured fat systems.
Polymorphism
In lipid materials the predominant polymorphic forms are generally termed as α, β′, and β, and determined by X-ray diffraction specific short spacings, which result from their triacylglycerols packing density.
The short spacings determined from X-ray diffraction patterns and the corresponding predominant polymorphic form of the blends with and without structurant addition are shown in Table 1.
All blends containing 80 and 100% of canola oil and the 40:60 blend without additives did not show crystallized forms at the current measurement temperature conditions by the X-ray diffraction technique. This is an expected result due to the higher content of lower melting point fatty acids in canola oil. Intense peaks with short spacings near 4.2 and 3.8 Å were observed in all crystallized blends, characterizing the predominant presence of β′ crystals, as correspondingly described in the literature for pure palm oil (Lin 2002).
After the addition of fully hydrogenated palm oil, the crystallized blends also displayed the same predominant β′ polymorphism as the standard ones, since FHPO and PO are formed by similar chemical structures, with the same triacylglycerols chain lengths, and therefore, both crystallized together, probably by the mechanism of similarity (Walstra 2003).
Samples 40:60P and 40:60PS showed β′ crystals form after the addition of both structurant systems, whilst the standard sample 40:60 did not present any crystal structure at the same measurement conditions. The appearance of a β′ crystal form in these blends with 60% of canola oil can be seen as the most important evidence that FHPO and FHPO/SMS act as crystallization modifiers, leading to the formation of higher thermal resistant lipid networks, even with higher content of unsaturated fatty acids.
De Oliveira et al. (2015b) evaluated the addition of 6% of a mixture of canola hardfat and SMS (50:50, in w/w) to blends of palm oil and canola oil up to a maximum of 50% canola oil. They reported that combining, these additives increased the consistency and thermal resistance of the lipid blends and lead to the formation of β crystals—not typically found in palm oil. In the current research the major presence of palmitic acid in the palm hardfat (FHPO) compared to the stearic acid rich canola hardfat composition, induced the β’ crystallization in blends with up to 60% canola oil. Therefore, the use of palm hardfat together with SMS could induce the formation of the same preferential polymorphism found for samples of pure palm oil (β’), favoring the application of the blend 40:60 (palm oil: canola oil) with these two crystallization modifiers (FHPO and SMS) as a preeminent alternative fat for palm oil when developing low saturated fatty acid formulations.
Conclusion
Zero trans lipid blends based on canola and palm oil were developed and displayed enhanced crystallization characteristics after the addition of 2.5% of fully hydrogenated palm oil and 2.5% of sorbitan monostearate. The simultaneous application of these two crystallization modifiers—FHPO and SMS—leads to the formation of a denser crystal fat network with increased thermal resistance and consistency, allowing to obtain healthier structured lipid materials with high amounts of unsaturated fatty acids, specially for the sample containing 60% of canola oil and 40% of palm oil added with both structurant.
References
Antoniosi Filho NR, Mendes OL, Lanças FM (1995) Computer prediction of triacylglycerol composition of vegetable oils by HRGC. Chromatographia 40:557–562
AOCS (2009) Official methods and recommended practices of the American Oil Chemists’ Society, vol 2009. AOCS press, Champaign
Awad TS, Marangoni AG (2006) Ingredient interactions affecting texture and miscrostructure of confectionary chocolate. In: Gaonkar AG, McPherson A (eds) Ingredient interactions: effects on food quality, vol 2. Taylor & Francis Group, Abingdon-on-Thames, pp 423–476
Dassanayake LSK, Kodali DR, Ueno S (2011) Formation of oleogels based on edible lipid materials. Curr Opin Colloid Interface Sci 16:432–439. https://doi.org/10.1016/j.cocis.2011.05.005
De Oliveira GM, Badan Ribeiro AP, dos Santos AO et al (2015a) Hard fats as additives in palm oil and its relationships to crystallization process and polymorphism. LWT Food Sci Technol 63:1163–1170. https://doi.org/10.1016/j.lwt.2015.04.036
De Oliveira GM, Stahl MA, Ribeiro APB et al (2015b) Development of zero trans/low sat fat systems structured with sorbitan monostearate and fully hydrogenated canola oil. Eur J Lipid Sci Technol 117:1762–1771. https://doi.org/10.1002/ejlt.201400559
Dhaka V, Gulia N, Ahlawat KS, Khatkar BS (2011) Trans fats-sources, health risks and alternative approach: a review. J Food Sci Technol 48:534–541. https://doi.org/10.1007/s13197-010-0225-8
Ghotra BS, Dyal SD, Narine SS (2002) Lipid shortenings: a review. Food Res Int 35:1015–1048. https://doi.org/10.1016/S0963-9969(02)00163-1
Haighton AJ (1959) The measurement of the hardness of margarine and fats with cone penetrometers. J Am Oil Chem Soc 36:345–348. https://doi.org/10.1007/BF02640051
Hartman L, Lago RC (1973) Rapid preparation of fatty acids methyl from lipids. Lab PR 22:474–476
Lin SW (2002) Palm oil. In: Gunstone FD (ed) Vegetable oils in food technology: composition, properties and uses. CRC Press, Boca Raton, pp 1–352
Masuchi MH, Grimaldi R, Kieckbusch TG (2014) Effects of sorbitan monostearate and monooleate on the crystallization and consistency behaviors of cocoa butter. J Am Oil Chem Soc 91:1111–1120. https://doi.org/10.1007/s11746-014-2469-3
Murdan S, Gregoriadis G, Florence AT (1999) Novel sorbitan monostearate organogels. J Pharm Sci 88:608–614. https://doi.org/10.1021/js980342r
O’Brien RD (2003) Fats and oils: formulating and processing for applications, 2nd edn. CRC Press LLC, New York
Pernetti M, van Malssen KF, Flöter E, Bot A (2007) Structuring of edible oils by alternatives to crystalline fat. Curr Opin Colloid Interface Sci 12:221–231. https://doi.org/10.1016/j.cocis.2007.07.002
Ribeiro APB, Basso RC, Grimaldi R et al (2009a) Effect of chemical interesterification on physicochemical properties and industrial applications of canola oil and fully hydrogenated cottonseed oil blends. J Food Lipids 16:362–381. https://doi.org/10.1111/j.1745-4522.2009.01152.x
Ribeiro APB, Grimaldi R, Gioielli LA, Gonçalves LAG (2009b) Zero trans fats from soybean oil and fully hydrogenated soybean oil: physico-chemical properties and food applications. Food Res Int 42:401–410. https://doi.org/10.1016/j.foodres.2009.01.012
Ribeiro APB, Masuchi MH, Miyasaki EK et al (2015) Crystallization modifiers in lipid systems. J Food Sci Technol 52:3925–3946. https://doi.org/10.1007/s13197-014-1587-0
Rodrigues-Ract JN, Cotting LN, Poltronieri TP et al (2010) Crystallization behavior of structured lipids by chemical interesterification of milkfat and sunflower oil. Cienc Tecnol Aliment 30:258–267. https://doi.org/10.1590/S0101-20612010000100038
Siraj N, Shabbir MA, Ahmad T et al (2015) Organogelators as a saturated fat replacer for structuring edible oils. Int J Food Prop 18:1973–1989. https://doi.org/10.1080/10942912.2014.951891
Walstra P (2003) Soft solids. In: Walstra P (ed) Physical chemistry of foods. Marcel Dekker, New York, pp 701–789
Acknowledgements
The authors are grateful for the Brazilian financial support received from São Paulo Research Foundation (FAPESP—Grant Numbers: 2009/53006-0 and 2013/00240-1).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Barbosa, K.M., Cardoso, L.P., Ribeiro, A.P.B. et al. Crystallization of low saturated lipid blends of palm and canola oils with sorbitan monostearate and fully hydrogenated palm oil. J Food Sci Technol 55, 1104–1115 (2018). https://doi.org/10.1007/s13197-017-3026-5
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-017-3026-5