Abstract
In this study, the response surface methodology was utilized to determine optimum conditions for extracting the polysaccharides from Rosa roxburghii Tratt fruit (RRTPs) using ultrasonic-assisted extraction, and the characterization and antioxidant activities of the RRTPs were discussed. RRTPs yield was 6.59 ± 1.34%, which was well consistent with the predicted value of 6.716%, under the following optimum conditions: ratio of water to raw material 40.18 mL/g, extraction temperature 78.8 °C, ultrasonic power 148 W, and extraction time 32.8 min. The monosaccharide composition analysis indicated that RRTPs were composed of mannose (Man), rhamnose (Rha), glucuronic acid (GlcA), galacturonic acid (GalA), glucose (Glc), galactose (Gal), arabinose (Ara) and xylose (Xyl). The molecular weight distribution analysis showed that RRTPs had four main components with molecular weights of 332.56, 183.96, 11.92 and 5.95 kDa, respectively. In vitro antioxidant studies revealed RRTPs exhibited significant antioxidant potential on hydroxyl, superoxide and DPPH radicals. In addition, antioxidant assays in vivo demonstrated that RRTPs can significantly increase the superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) and catalase (CAT) activities, and total antioxidant capacity (TAOC) to some extent, as well as decrease the level of malondialdehyde (MDA) in both serum and liver of d-Gal aging-induced mice. These data suggested that RRTPs could be a potential candidate of natural antioxidants for applications in functional food, pharmaceuticals or cosmetic industries. In summary, this work provided an effective method for the exploitation and utilization of value-added R. roxburghii Tratt fruit which would be useful to fully utilize this resource.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chestnut rose (Rosa roxburghii Tratt, RRT), also named as Chinquapin rose or Burr rose, is a wild fruit crop distributed in the provinces of northwest, central-south and southwest China (Xu et al. 2004). As a kind of heliophilous, deciduous shrub species, RRT mainly grows at a high altitude of 1000–1600 m in hilly mountain areas. It is proved that RRT has strong environmental adaptation which can grow on poor, compacted and sandy soil, and resist common diseases. It grows into a large shrub with small light green attractive foliage, and carnation-like roses in spring. The fruit of RRT has been used as traditional Chinese medicines for the treatment of cancer, immunity, aging, atherosclerosis, stress etc. (Chen et al. 2014; Hirose et al. 1981). Furthermore, current research has shown that its fruit has superoxide dismutase activity, and senescence-retarding and anti-tumor effects (Ma et al. 1997; Wen et al. 2007). Due to that mentioned above, it could be important to exploit and utilize this fruit as a potential biomass material.
Polysaccharides, widely existing in many fruits, have become an important type of bioactive natural products in recent years owing to their various biological activities such as antioxidation (Chen et al. 2016), anticoagulation (Cai et al. 2013), anti-hypoglycemic (Teng et al. 2013), anti-injury (Shi et al. 2005), anti-tumor (Liu et al. 2014), anti-inflammatory (Chen et al. 2012) and anti-fatigue activities (Tan et al. 2012). Recently, a variety of extraction technologies have been developed and employed to improve the extracting of polysaccharides from various sources, including supercritical fluid extraction (Wang and Weller 2006), solvent extraction (Cui et al. 2014), enzyme-assisted extraction (Liao et al. 2015), cellulose-assisted extraction (Zhu et al. 2016), infrared-assisted extraction (Qu et al. 2016), microwave-assisted extraction (Zhao et al. 2013), and ultrasonic-assisted extraction (Wang et al. 2015). However less work on extraction of polysaccharides from RRT fruit as well as its characterization and has been done. In this work, RRT fruit from hilly mountain areas of Guizhou Province has been used.
In terms of extraction technique of polysaccharides, conventional extraction techniques, including immersion, boiling, refluxing, or heating, generally require longer time, more solvent and higher temperature, with lower yield of the polysaccharides or even decrease of some of their pharmacological activities (Bagherian et al. 2011). As a kind of efficient extraction technique of polysaccharides from various biomaterials, ultrasonic-assisted extraction (UAE) can reduce solvent consumption, save time, and reduce damage to the structural and molecular properties of bioactive compound, while increases the yield of extracts (Teng and Choi 2014), which has been commonly applied to polysaccharide extractions from different materials in recent years (Chen et al. 2016). Thus, UAE was used to extract R. roxburghii Tratt fruit polysaccharides (RRTPs).
As an effective statistical and mathematical tool for optimizing experimental processes, response surface methodology (RSM) has been widely used to improve and optimize the polysaccharide extraction processes by many investigators (Wang et al. 2015). In this study, the Box–Behnken design (BBD), a popular form of RSM, was introduced to optimize the extraction conditions of RRTPs using the UAE. The physicochemical properties of the extracted RRTPs including the monosaccharide composition and the molecular weight, and its antioxidant activities in vitro and in vivo were also estimated so as to supply the useful information for possible application of RRT fruit.
Materials and chemicals
Ripe fruits were picked from wild growing R. roxburghii plants in the Longli County, Guizhou Province in September, 2014. The samples were first air-dried at 50 °C then ground to a fine powder in a domestic mill, sieved using 60 mesh sieve (60 mesh sieve was determined based on the preliminary studies), and stored at room temperature in glass desiccators until use.
Bovine serum albumin (BSA), ascorbic acid, l-rhamnose (Rha), d-mannose (Man), l-arabinose (Ara), d-galactose (Gal), d-glucose (Glc), d-xylose (Xyl), d-galacturonic acid (GalA), d-glucuronic acid (GlcA), and trifluoroacetic acid (TFA), d-1-phenyl-3-methyl-5-pyrazolone (PMP) and 1,1-diphenyl-2-picrylhydrazyl (DPPH), were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Dialysis tube (molecular weight cut-off, 3000 Da) was purchased from Spectrumlabs Co. (USA). Other chemicals were analytical grade.
Extraction of RRTPs using UAE technique
About 20.0 g of the dried RRT fruit powder was refluxed with petroleum ether (boiling point range 30–60 °C) for 4 h and immersed in 90% ethanol at room temperature twice (each for 4 h) to remove colored ingredients, lipids, monosaccharides, and other small molecule impurities. The pretreated residues were filtrated and air-dried at 40 °C to get pretreated RRT fruit powder.
Under designed extraction temperature (40–90 °C), time (10–50 min), ultrasonic power (60–220 W) and water to the raw material ratio (10–50 mL/g), a total of 10 g of pretreated dry powder was introduced into distilled water in a 500 mL conical flask then subjected to extraction using a 40 kHz ultrasonic processor (VS-65UE, Wuxi Woxin instrument Co., Ltd., China). After extraction by UAE method, the extracted slurry was centrifuged (4000 r/min, 10 min). The insoluble residues were re-extracted and re-centrifuged under designed extraction as mentioned above to recover the residual polysaccharides. Then, the collected supernatants were pooled together and concentrated to a fourth of the original volume using a rotary evaporator (R-215, Buchi, Switzerland) at 50 °C under reduced pressure. The concentrated solution was precipitated using four-fold volumes of ethanol (80% v/v final ethanol concentration) overnight at 4 °C. After centrifugation at 4000 r/min for 15 min, the precipitate was deproteinated according to the Sevage method (Sevag et al. 1938), washed with pure ethanol (99%), redissolved in distilled water and intensively dialyzed for 3 days. The extracts were centrifuged (4000 r/min, 15 min) and finally lyophilized in vacuum freeze dryer to obtain the dried RRTPs. The polysaccharide extraction yield (%) from this extraction was calculated following the equation:
Experimental design of BBD
According to the results of single factor experiment, the preliminary range of the extraction variables including \( X_{1} \) (water to raw material ratio), \( X_{2} \) (extraction temperature), \( X_{3} \) (ultrasound power), and \( X_{4} \) (extraction time) was determined, whereas the extraction yield of RRTPs was the dependent variable. Then, a four-factor, three-level BBD was applied to construct model building and optimize the extraction parameters for the yield of RRTPs. Each independent variable had the low, middle, and high levels which were coded as − 1, 0, and 1, respectively. Twenty-nine experiments carried out at random with three replicates were shown in Table 1. The experimental data were fitted to the following second-order polynomial mode:
where Y is the predicted response and \( \beta_{0} \) represents the intercept term; \( \beta_{i} \), \( \beta_{ii} \) and \( \beta_{ij} \) are the regression coefficients for linearity, square, and interaction, respectively; \( X_{i} \) and \( X_{j} \) are the coded levels of independent variables (\( i \, \ne \, j \)). The terms \( X_{i} X_{j} \) and \( X_{i}^{2} \) represent the interaction and quadratic terms, respectively.
Control experiment
The conventional hot water extraction (HWE) was employed in a HH-6 water-bath (Guohua Wiring Company, Shanghai, China) at the optimal extraction condition: extraction temperature of 90 °C, extraction time of 240 min, and water to raw material ratio of 40 mL/g based on the preliminary three-factor and three-level designed orthogonal optimal experiment.
Physicochemical properties of RRTPs
The carbohydrate content in RRTPs was determined by the phenol–sulfuric acid colorimetric method using d-glucose as standard (Dubois et al. 1956). Total uronic acid content was estimated by the reference method using galacturonic acid as the standard (Blunenkrantz and Asboe-Hansen 1973). Protein content was determined according to the Bradford assay using BSA as a standard (Bradford 1976).
Physicochemical properties of RRTPs were estimated using: Fehling’s test (Schneider 1979), phenol–sulfuric acid reaction (Dubois et al. 1956), α-naphthol reaction iodination reaction (Lee et al. 1994), FeCl3 reaction (Zhou 1978).
Monosaccharide composition of RRTPs
The RRTPs sample (10 mg) was completely hydrolyzed to monosaccharides with trifluoroacetic acid (4 M, 5 mL) in an oven at 120 °C for 4 h. The hydrolysate was evaporated to dry under a stream of N2 and then derivatized with 300 μL of 0.3 M NaOH and 300 μL of PMP solution (0.5 M, in methanol) for 60 min at 70 °C. At last, the resulting mixtures were added to 300 μL of 0.3 M HCl solution to stop the reaction, and the resulting product was centrifuged for 10 min. The supernatants containing PMP-labeled derivatives were filtered through a 0.45 μm syringe filters and 20 μL of the final product was injected into a ZORBAX SB-C18 (4.6 × 250 mm, particle size 5 μm, Agilent, USA) capillary column at 30 °C. The Shimadzu LC-2010A HPLC system equipped with a refractive index detector was used. The column mobile phase was 0.1 M KH2PO4 (pH 10) with 73% (solution A) and 27% acetonitrile (solution B) in water. The flow rate was 1.0 mL/min and the detector wavelength was 250 nm. The monosaccharide standards were PMP-labeled and determined by HPLC in the same way. Sugar identification was identified by their characteristic retention times. Man, Rha, GlcA, GalA, Glc, Gal, Ara and Xyl were used as the monosaccharide standards.
Molecular weight distribution of RRTPs
The molecular weight of RRTPs was determined by High performance size exclusion chromatography (HPSEC) on a Shimadzu LC-2010A HPLC system (Shimadzu Corporation, Japan) equipped with a Shodex SB-804 HQ column (300 × 8.0 mm, Showa Denko Corp., Japan) and an RID-10A refractive index detector. RRTPs (2.0 mg) were dissolved by 1.0 mL sodium nitrate (0.1 M) and loaded into the chromatography system (20 μL). The columns were maintained at 30 °C and eluted with 0.1 M sodium nitrate at a flow rate of 0.9 mL/min. Standard dextrans (Sigma–Aldrich, St. Louis, MO, USA) with different molecular weights (180, 4600, 10,000, 21,100, 393,000 and 500,000 Da) were used as molecular mass markers. The molecular weight (Mw) of RRTPs was calculated by comparison with the calibration curve. The standard curve of log(Mw) against elution time (T) was:
Antioxidant activity analysis in vitro of RRTPs
At present the mechanisms of the antioxidant activity mainly focus on radical scavenging, prevention of chain initiation, reductive capacity, binding of transition metal ion catalysts, and decomposition of peroxides (Liu et al. 2007). In this study, the DPPH, hydroxyl and superoxide radical scavenging assay were used for determining antioxidant activity in vitro of RRTPs.
The DPPH radical scavenging activity of RRTPs was measured according to the method described by Chen et al. (2012). Briefly, 1.0 mL of DPPH solution (0.4 mM in ethanol) was mixed with 3.0 mL of the RRTPs at various concentrations. After mixing vigorously for 10 s, the mixture was allowed to incubate at 25 °C for 30 min in the dark. The absorbance (Abs) of the samples was measured at 517 nm and the percentage of free radical scavenging activity was determined from the difference in the Abs between the control and the samples. Ascorbic acid was used as a positive control.
The Hydroxyl radical assay was performed by Fenton method (Dou et al. 2015).
The superoxide radical scavenging activity was estimated as previously described (Liu et al. 2014), with minor modifications. Firstly, 2.0 mL of 0.05 M Tris-HCl buffer (pH 8.2) and 1.0 mL of the RRTPs at various concentrations were incubated at 25 °C for 20 min. Thereafter, 0.4 mL of 1,2,3-phentriol (5 mM) was added and the reaction mixture was kept at 25 °C for 4 min, and the rate of Abs change (A/min) of solution was measured at 325 nm. Ascorbic acid was used as a positive control. The ability of scavenging superoxide radical was evaluated employing the following equation:
where Ai was the rate of Abs change of blank control group and Aj was the rate of Abs change of the sample.
Antioxidant activity analysis in vivo of RRTPs
Animal grouping and treatment
Male Kunming mice (6–8 weeks old, body weight (BW) = 20 ± 2 g) of specific-pathogen free grade were provided by the Experimental Animal Center of Chongqing Medical University (Chongqing, China). During the entire experimental period, mice were housed individually in cages at 22–24 °C temperature and 50–60% relative humidity with 12 h light per day, and allowed free access to tap water and standard diet. All animals’ treatments were strictly performed in compliance with the prevailing Chinese legislation about the care and use of laboratory animals and all of the experiments were approved by the Animal Care and Use Committee of Southwest University (Experimental Animal License SCXK (Chongqing) 200120008).
After 7 days of adaptation, the mice were randomly divided into seven groups (five mice per group): in RRTPs groups, mice were respectively treated with RRTPs at four different doses (100, 200, 400 and 800 mg/kg, BW per day) via gastric gavage and d-Gal (100 mg/kg BW per day) was hypodermically injected once daily; in negative control group (NCG), mice received 10 mL/kg BW per day physiological saline once daily by hypodermic injection and gastric gavage; in model control group (MCG), mice were hypodermically injected with 100 mg/kg BW per day d-Gal and gastric gavaged physiological saline (10 mL/kg BW per day) once daily; in positive control group (PCG), mice were gastric gavaged 100 mg/kg BW per day ascorbic acid and hypodermically injected d-Gal equally to the MCG once daily. All animals were carried out once daily for 6 consecutive weeks.
Biochemical assay
After overnight fasting, the mice were weighed and killed by cervical decapitation. The blood samples were collected from orbital sinus and immediately centrifuged at 4000×g at 4 °C for 10 min to obtain serum. The liver was excised, weighed, and immediately homogenized in phosphate buffer (4 °C, 0.2 M, pH 7.4). The suspension was centrifuged at 10,000×g for 10 min at 4 °C and the supernatant was collected for further biochemical analysis. The activities of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px), and total antioxidant capacity (TAOC) and malondialdehyde (MDA) level of both blood serum and liver supernatant were detected with commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the corresponding manuals.
Statistical analysis
The data were reported as the mean ± standard deviation (SD) of three measurements. Analysis of variance (ANOVA) was performed by Dunnett’s multiple-range tests, where p < 0.05 was regarded as statistically significant.
Results and discussion
Single factor effect on RRTPs extraction
Figure 1a shows the effect of ratio of water to raw material on the yield of RRTPs when the three factors (extraction temperature, ultrasonic power, and extraction time) were fixed at 80 °C, 100 W and 30 min, respectively. As shown in Fig. 1a, the extraction yield of RRTPs increased over the ratio of water to raw material range of 10–40 mL/g, and the highest value was obtained at 40 mL/g, beyond which it dropped. The driving force for the mass transfer of polysaccharide molecules between the interior plant cells and the exterior solvent could increase with the increment of the water to material ratio initially. But, as the ratio continued to increase, the distribution of ultrasonic energy density in the extraction solution decreased, resulting in the loss of production collection by hindering the dissolution of polysaccharides (Samavati and Manoochehrizadeh 2013). Thus, the water to raw material ratio of 40 mL/g was adapted for the following experiments.
Effect of different extraction parameters on the yield of the extracted RRTPs. a Ratio of water to raw material (extraction temperature, ultrasonic power and extraction time were fixed at 80 °C, 100 W and 30 min); b extraction temperature (raw material ratio, ultrasonic power and extraction time were fixed at 30 mL/g, 100 W and 30 min); c Ultrasonic power (raw material ratio, extraction temperature and extraction time were fixed at 30 mL/g, 80 °C and 30 min); d extraction time (raw material ratio, extraction temperature and ultrasonic power were fixed at 30 mL/g, 80 °C and 100 W). Values are the mean ± SD (n = 3). Different superscripts (a–e) indicate a significant difference (p < 0.05)
Figure 1b shows the effect of extraction temperature on extraction yield at constant water to raw material ratio (30 mL/g), ultrasonic power (100 W), and extraction time (30 min). Figure 1b indicated that the extraction yield increased rapidly with the increasing temperature until 80 °C and began to decline, and a maximum yield was observed at around 80 °C. The decrease could be attributed to the degradation of polysaccharides at high temperature. This was in agreement with other study (Belhaj et al. 2017). Accordingly, 80 °C of extraction temperature was selected in the subsequent extraction procedure.
To study the effect of ultrasonic power on the extraction yield of RRTPs, the ultrasonic power was set at 60, 100, 140, 180, and 220 W, while the other factors were as follows: ratio of water to raw material of 30 mL/g, extraction temperature of 80 °C, and extraction time of 30 min. As shown in Fig. 1c, the extraction yield increased as ultrasonic power increased from 60 to 140 W. However, when the ultrasonic power continuously increased, a significant decline in extraction yield was observed. It is well known that violent shock wave generated by ultrasound could rapidly disrupture the plant cells and release the inner contents into extraction solvent. However, high power could lead to the degradation of the structure of polysaccharides. Previous investigation has revealed the possibility of polysaccharides disassembly during the ultrasonic extraction (Tang et al. 2016). Consequently, 140 W of ultrasonic power was selected for the study.
The extraction yield under different extraction time was evaluated with a ratio of water to raw material of 30 mL/g, an extraction temperature of 80 °C, and an ultrasonic power of 100 W. The result reveal that the extraction yield increased at first and reached the highest value at 40 min, then decreased when the extraction time exceeded 40 min (Fig. 1d). Although the extraction of polysaccharides could be favored for a long extraction time, the molecule structure of polysaccharides might be destroyed during the extension in the extraction time. The same results were reported for the ultrasonic-assisted extraction of polysaccharides from other species (Chen et al. 2016). Thus, the extraction time was controlled in the range of 40 min.
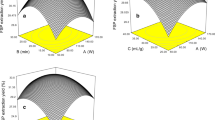
Optimization of UAE condition of RRTPs
The design matrix and corresponding BBD results were presented in Table 1. The extraction yield of RRTPs Y (%) ranged from 3.89 to 6.74%, and obtained the maximum value as the ratio of water to raw material of 40 mL/g, at 80 °C, 140 W, and a 40 min treatment time. The predicted model can be estimated through multiple regression analyses of the experimental data by the following equation:
The high model significance was confirmed by its F value (24.27) and p value (< 0.0001). In addition, high value of R2 (0.9604) and Adj-R2 (0.9208) indicated that the quadratic model was statistically significant and adequate for describing the relationship between the observed and predicted values. The coefficient of the variation (C.V.) was a relatively low value (4.58%), which suggested that the polynomial model had a high degree of reliability and the experimental data were very reliable. Meanwhile, the lack of fit F value of 3.31 and p value of 0.1301 implied that it was insignificant relative to the pure error. The smaller is the value of P, the more significant is the corresponding coefficient. The linear coefficients (X2, X3 and X4), quadratic term coefficients (X 21 , X 22 , X 23 and X 24 ) and interaction coefficients (X2X4 and X3X4) had high significance due to their relatively small p values (p < 0.05).
Using the software Design-Expert, the optimal values of independent variables and response variable for the proposed extraction were estimated. Based on the response surface plots and variance analysis, the maximum RRTPs yield and predicted yield were performed under the following conditions: extraction temperature 78.84 °C, extraction time 32.81 min, ultrasonic power 148.01 W, and ratio of water to raw material 40.18 mL/g, and the maximum predicted extraction yield of 6.716%. Taking into account practical considerations, the extraction process can be performed by using these modified conditions: extraction temperature 78.8 °C, extraction time 32.8 min, ultrasonic power 148 W, and ratio of water to raw material 40.18 mL/g. Under these actual optimum extraction conditions, the mean yield of RRTPs was 6.59 ± 1.34% (n = 3), demonstrating that the model developed in the present study was accurate and adequate for evaluating the extraction process of RRTPs.
Comparison with HWE
In the control experiment, the yield of RRTPs obtained by HWE was 4.83 ± 0.56%. Under the optimal conditions of UAE, the RRTPs yield increased by 36.44% when compared to HWE. Furthermore, compared with HWE, UAE had a higher extraction efficiency of RRTPs with a shorter extraction time and at a lower extraction temperature.
Chemical characteristics of RRTPs
The variations in total sugar, protein and uronic acid contents, as well as the monosaccharide composition and physicochemical property of RRTPs were studied. RRTPs comprised 71.59 ± 5.22% neutral sugar, 1.02 ± 0.18% protein and 19.30 ± 1.76% uronic acid. The results obtained from the phenol–sulfuric acid test, Fehling’s and α-naphthol reaction and iodination reaction indicated that RRTPs extracted by UAE consisted of non-reducibility polysaccharides but without starches.
The monosaccharide composition of RRTPs was presented in Fig. 2a, b. The HPLC analysis revealed that RRTPs extracted by UAE were composed of Man, Rha, GlcA, GalA, Glc, Gal, Ara, and Xyl at a molecular ratio of 2.64:5.13:2.71:1.20:6.69:8.01:1.00:1.55. The molecular weight distribution analysis showed that RRTPs included four main components with molecular weights of 332.563, 183.963, 11.929 and 5.953 kDa, respectively, indicating that RRTPs were heterogeneous polysaccharides.
Antioxidant activity in vitro of RRTPs
It could be seen from Fig. 3a that the extracted RRTPs showed concentration-dependent scavenging activity of DPPH and good antioxidant capacity compared to the synthetic antioxidant; ascorbic acid. The DPPH scavenging effect increased with the increasing concentration up to 0.4 mg/mL. At the concentration of 0.4 mg/mL, the DPPH radical scavenging activities of RRTPs and ascorbic acid were 83.46 and 94.68%, respectively. The half-effective concentration (EC50) value of RRTPs was no more than 0.1 mg/mL, even though the scavenging ability of RRTPs was not as strong as ascorbic acid at all experimental concentrations. This result indicated that RRTPs represent strong electron donors and could react with free radicals and terminate the radical chain reactions.
The scavenging activities of RRTPs and ascorbic acid against the hydroxyl radical, as shown in Fig. 3b, revealed the RRTPs had concentration-dependent manner and expressed strong hydroxyl radical scavenging ability. At concentrations ranging from 0.04 to 0.3 mg/mL, RRTPs had weaker activity than ascorbic acid, and then had stronger activity than that when the concentration exceeded 0.4 mg/mL. Notably, the scavenging effect of RRTPs was 79.68% at 0.6 mg/mL, while ascorbic acid showed 71.45%. This result suggested that RRTPs can be considered as a good scavenger of hydroxyl radical.
The obtained results (Fig. 3c) showed that the superoxide radical scavenging activity of RRTPs positive correlated with increasing concentrations. RRTPs exhibited strong radical scavenging activity under the experimental conditions, but at a level lower than that of ascorbic acid. The EC50 values of RRTPs and ascorbic acid were 0.27 and 0.093 mg/mL, respectively. The maximum scavenging capability (66.37%) of RRTPs was reached 83.5% that of ascorbic acid at a dose of 0.6 mg/mL, suggesting that RRTPs had a noticeable effect on scavenging superoxide radical.
Antioxidant activity in vivo of RRTPs
In the present work, d-Gal was used to establish the aging model in mice for assessment of in vivo antioxidant activity of RRTPs. As displayed in Tables 2 and 3, the aging model group (MCG) showed significant decreases in the activities of CAT, SOD, GSH-Px and TAOC in both the serum and liver as compared with the normal group (NCG), while the level of MDA in serum and liver from the MCG significantly increased compared to the NCG. These results suggested that the aging mice model was successfully established in our study.
In recent years, increasing evidence demonstrated that the development of aging was largely attributed to the cumulative damage caused by the generation of reactive oxygen species (ROS) in cells (Jing et al. 2016). CAT, SOD and GSH-Px are the most important antioxidant enzymes to inhibit free radical formation and usually used as biomarkers to indicate ROS production. Results of RRTPs and ascorbic acid of CAT, SOD and GSH-Px in serum and liver of aging mice were shown in Tables 2 and 3. All four RRTPs groups and ascorbic acid treatment group (PCG) could increase CAT, SOD and GSH-Px activity, both in serum and liver samples. Compared with MCG, RRTPs at the doses of 200, 400 or 800 mg/kg and PCG significantly enhanced the activities of these antioxidant enzyme activities. TAOC represents the ability of the non-enzymatic ROS defense system. As shown in Tables 2 and 3, all RRTPs treated groups and PCG exhibited significantly increased TAOC activity both in the serum and liver of aging mice, indicating that the non-enzymatic antioxidant defense system of aging mice had been enhanced. As MDA is a naturally occurring product of lipid peroxidation, the level of MDA is an oxidative stress marker. As shown in Tables 2 and 3, the levels of MDA in all RRTPs treated groups and PCG reduced significantly in both serum and liver, suggesting that the intake of RRTPs could resist lipid peroxidation in aging mice. These results further demonstrated that RRTPs displayed significantly antioxidant activity.
Conclusion
Maximal RRTPs yield was obtained using the optimized extraction conditions (water to raw material ratio of 40.18 mL/g, extraction temperature of 78.8 °C, ultrasonic power of 148 W, and extraction time of 32.8 min). The experimental yield (6.59 ± 1.34%, n = 3) under these conditions corresponded fairly well to the predicted. The monosaccharide composition analysis indicated that RRTPs were composed of Man, Rha, GlcA, GalA, Glc, Gal, Ara and Xyl. The molecular weight distribution analysis showed that RRTPs had four main components with molecular weights of 332.563, 183.963, 11.929 and 5.953 kDa, respectively. RRTPs antioxidant activities and in vivo results showed that RRTPs could significantly enhance the activities of antioxidant enzymes (CAT, SOD, and GSH-Px), increase TAOC values, and reduce the MDA level in both serum and liver of d-Gal aging-induced mice.
References
Bagherian H, Ashtiani FZ, Fouladitajar A, Mohtashamy M (2011) Comparisons between conventional, microwave- and ultrasound-assisted methods for extraction of pectin from grapefruit. Chem Eng Process 50(11–12):1237–1243
Belhaj D, Frikha D, Athmouni K, Bouthain B, Ahmed MB, Bouallagui Z, Kallel M, Maalej S, Zhou J, Ayadi H (2017) Box–Behnken design for extraction optimization of crude polysaccharides from Tunisian Phormidium versicolor cyanobacteria (NCC 466): partial characterization, in vitro antioxidant and antimicrobial activities. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2017.06.046
Blunenkrantz N, Asboe-Hansen G (1973) New method for quantitative determination of uronic acids. Anal Biochem 54(2):484–489
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cai WR, Xie LL, Chen Y, Zhang H (2013) Purification, characterization and anticoagulant activity of the polysaccharides from green tea. Carbohydr Polym 92(2):1086–1090
Chen RZ, Li Y, Dong H, Liu Z, Li S, Yang S, Li X (2012) Optimization of ultrasonic extraction process of polysaccharides from Ornithogalum Caudatum Ait and evaluation of its biological activities. Ultrason Sonochem 19(6):1160–1168
Chen Y, Liu ZJ, Liu J, Liu LK, Zhang ES, Li WL (2014) Inhibition of metastasis and invasion of ovarian cancer cells by crude polysaccharides from Rosa Roxburghii Tratt in vitro. Asian Pac J Cancer Prev 15(23):10351–10354
Chen GJ, Zhang SQ, Ran CX, Wang LS, Kan JQ (2016) Extraction, characterization and antioxidant activity of water-soluble polysaccharides from Tuber huidongense. Int J Biol Macromol 91:431–442
Cui GT, Zhang WX, Wang QJ, Zhang AM, Mu HB, Bai HJ, Duan JY (2014) Extraction optimization, characterization and immunity activity of polysaccharides from Fructus Jujubae. Carbohyd Polym 111:245–255
Dou J, Meng YH, Liu L, Li J, Ren DY, Guo YR (2015) Purification, characterization and antioxidant activities of polysaccharides from thinned-young apple. Int J Biol Macromol 72:31–40
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Ana Chem 28(3):350–356
Hirose M, Shibata M, Hagiwara A, Imaida K, Ito N (1981) Chronic toxicity of butylated hydroxytoluene in Wistar rats. Food Chem Toxicol 19(19):147–151
Jing YQ, Gao YZ, Wang WF, Chen YY, Lu P, Ma C, Zhang YH (2016) Optimization of the extraction of polysaccharides from tobacco waste and their biological activities. Int J Biol Macromol 91:188–189
Lee JW, Yu RY, Chen LR (1994) Principles and methods of biochemistry experiment. Beijing University Press, Beijing, pp 151–278
Liao NB, Zhong JJ, Ye XQ, Lu S, Wang WJ, Zhang RH, Xu J, Chen SJ, Liu DH (2015) Ultrasonic-assisted enzymatic extraction of polysaccharide from Corbicula fluminea: characterization and antioxidant activity. LWT-Food Sci Technol 60(2):1113–1121
Liu C, Wang C, Xu Z, Wang Y (2007) Isolation, chemical characterization and antioxidant activities of two polysaccharides from the gel and the skin of Aloe barbadensis Miller irrigated with sea water. Process Biochem 42(6):961–970
Liu X, Sun ZL, Jia AR, Shi YP, Li RH, Yang MP (2014) Extraction, preliminary characterization and evaluation of in vitro antitumor and antioxidant activities of polysaccharides from Mentha piperita. Int J Mol Sci 15(9):16302–16319
Ma YX, Zhu Y, Wang CF (1997) The aging retarding effect of ‘Long-Life CiLi’. Mech Ageing Dev 96(1–3):171–189
Qu Y, Li CX, Zhang C, Zeng R, Fu CM (2016) Optimization of infrared-assisted extraction of Bletilla striata polysaccharides based on response surface methodology and their antioxidant activities. Carbohyd Polym 148:345–353
Samavati V, Manoochehrizadeh A (2013) Polysaccharide extraction from Malva sylvestris and its anti-oxidant activity. Int J Biol Macromol 60:427–436
Schneider F (1979) Sugar analysis: official and tentative methods recommended by the international commission for uniform methods of sugar analysis. ICUMSA, Peterborough, pp 41–73
Sevag MG, Lackman DB, Smolens J (1938) The isolation of the components of streptococcal nucleoproteins in serologically active form. J Biol Chem 124:425–436
Shi YL, Yang LH, Cai DH (2005) The effect of pleurotus eryngii polysaccharide on exhausted mice’s resistance to oxidation and injury. J Phys Educ 12:56–58 (in Chinese)
Tan W, Yu KQ, Liu YY, Ouyang MZ, Yan MH, Luo R, Zhao XS (2012) Anti-fatigue activity of polysaccharides extract from Radix Rehmanniae Preparata. Int J Biol Macromol 50:59–62
Tang W, Lin L, Xie J, Wang Z, Wang H, Dong Y, Shen M, Xie M (2016) Effect of ultrasonic treatment on the physicochemical properties and antioxidant activities of polysaccharide from Cyclocarya paliurus. Carbohy Polym 151:305–312
Teng H, Choi YH (2014) Optimization of ultrasonic-assisted extraction of bioactive alkaloid compounds from rhizoma coptidis (Coptis chinensis Franch.) using response surface methodology. Food Chem 142(1):299–305
Teng Z, Qian L, Zhou Y (2013) Hypolipidemic activity of the polysaccharides from Enteromorpha prolifera. Int J Biol Macromol 62:254–256
Wang L, Weller CL (2006) Recent advances in extraction of nutraceuticals from plants. Trends Food Sci Technol 17(6):300–312
Wang YG, Wang F, Ma XQ, Sun SC, Leng FF, Zhang WJ, Wang XL (2015) Extraction, purification, characterization and antioxidant activity of polysaccharides from Piteguo fruit. Ind Crop Prod 77(10):467–475
Wen XP, Xu Q, Deng XX, Ji XB (2007) Chestnut rose (Rosa roxburghii Tratt): a promising genetic resource for fruit and ornament exploitation in China. Floric Ornam Biotechnol 1:46–50
Xu Q, Wen XP, Deng XX (2004) A simple protocol for isolating genomic DNA from Chestnut Rose (Rosa roxburghii Tratt) for RFLP and PCR analyses. Plant Mol Biol Rep 22(3):301a–301g
Zhao BT, Zhang J, Guo X, Wang JL (2013) Microwave-assisted extraction, chemical characterization of polysaccharides from Lilium davidii var. unicolor Salisb and its antioxidant activities evaluation. Food Hydrocoll 31(2):346–356
Zhou KY (1978) Organic chemistry experiment. Higher Education Press, Beijing, p 272
Zhu ZY, Dong FY, Liu XC, Lv Q, Yang Y, Liu F, Chen L, Wang TT, Wang Z, Zhang YM (2016) Effects of extraction methods on the yield, chemical structure and anti-tumor activity of polysaccharides from Cordyceps gunnii mycelia. Carbohyd Polym 140:461–471
Acknowledgements
We gratefully acknowledge the financial support by the Fundamental Research Funds for the Central Universities (XDJK2017D131).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, G., Kan, J. Ultrasound-assisted extraction, characterization, and antioxidant activity in vitro and in vivo of polysaccharides from Chestnut rose (Rosa roxburghii tratt) fruit. J Food Sci Technol 55, 1083–1092 (2018). https://doi.org/10.1007/s13197-017-3023-8
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-017-3023-8