Abstract
Nagpur mandarin orange (Citrus reticulata) peels were subjected to treatment with nanobiocatalysts in the form of cellulase and pectinase immobilized magnetic nanoparticles (MNPs). MNPs (Fe3O4) with average diameter in range of 40–90 nm were immobilized with cellulase and pectinase through APTES and glutaraldehyde. Treatment followed by extraction into organic solvents resulted in 8-9 fold increase in extraction of carotenoidic pigments compared to use of free enzymes. Optimum pH and temperature for the process were determined to be 5.0 and 50 °C, respectively. The nanobiocatalysts could be reused across three cycles with only 15 % drop in yield per cycle. Dinitrosalicylic acid assays showed that superior peel hydrolysis also led to greatest extent of pigment extraction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The orange peel represents an underutilized source of carotenoidic pigments. More than 600 natural products are collectively identified as carotenoids (Goodwin 1980; Goodwin and Britton 1988). They represent one of the most prevalent groups of naturally occurring organic pigments and are found in significant proportion in the peel of many fruits. There is increasing awareness of the correlation between the intake of carotenoids and health benefits such as prevention of lung, breast and prostate cancer and cardio-vascular disease as well as the prevention of inflammation, cataract and macular degeneration (Fraser et al. 2005; Stahl and Sies 2005; Krinsky 1994). Traditional methods for extraction of carotenoids rely heavily on the use of organic solvents (Wrolstad et al. 2005; Aravantinos-Zafiris et al. 1992). The solvents being used for such extractions are not uniform due to the diversity of carotenoid structures and their corresponding polarities. While robust procedures for solvent extraction of carotenoids have been developed, challenges remain in using such methods on a commercial scale. Promising alternative and greener modes of extraction include the use of supercritical CO2 and hydrolytic enzymes (Fernandez et al. 2015; Gnayfeed et al. 2001; Salvatore Lenucci et al. 2015). The use of enzymes for degradation of plant cell walls is not restricted only to the extraction of carotenoids but has also been used for retrieval of oils, polysaccharides and polyphenols as well as for producing sugars (Dominguez et al. 1994; Chamorro et al. 2012; Wilkins et al. 2007). Cell wall hydrolases such as cellulase and pectinase are the most commonly used enzymes owing to the similarity in constitution of plant cell walls (Nagodawithana and Reed 1993). The enzyme-assisted breakdown of the polysaccharides enveloping the cell is found to enhance the release of intracellular contents (Grohman and Baldwin 1992; Cinar 2005). The latter can subsequently be separated and purified by conventional solvent extraction methods (Aravantinos-Zafiris et al. 1992; Delgado Vargas and Pareds Lopez 1997). The main challenge in using hydrolytic enzymes for pre-treatment of plant biomass is the low reusability of biocatalysts due to problems in retrieval of enzymes from reactions as well as their inferior stability under reaction conditions (Polizzi et al. 2007; Iyer and Ananthanarayan 2008). The cost of production of carotenoid pigments from natural sources.
In this work, we explore the effect of immobilising the hydrolytic enzymes on nano particles with the overall objective of developing an efficient and reusable method of extraction of pigments from the peel of oranges. We have developed stable iron oxide magnetic nano particles (MNPs) with size in the range of 60–110 nm. The hydrolytic enzymes cellulase and pectinase are immobilised on the magnetic iron oxide nano particles via reported conjugation strategies (Jordan et al. 2011; Khoshnevisan et al. 2011). The size and morphology of the enzyme-nanoparticle constructs were characterised by Atomic Force Microscopy (AFM), X-Ray Diffraction (XRD) and Transmission Electron Microscopy (TEM). The enzyme immobilised magnetic iron oxide nano particles were found to improve the yield of extraction of pigments by nearly 8-9 fold as confirmed by UV–visible spectroscopy. In addition, the improved extraction of carotenoid pigments bears a strong correlation with the extent of peel hydrolysis. The use of cellulase and pectinase towards extraction of carotenoids from natural sources has been reported previously (Grohman and Baldwin 1992; Cinar 2005). However, there are relatively few approaches which address the issue of reusability of the biocatalyst in such processes. In this work, we demonstrate the reusability of the enzyme-MNP construct towards the extraction of pigments from separate source batches of orange peel. Our results highlight the utility of immobilised cellulolytic enzymes with respect to extraction of pigments. The reusability of the nanobiocatalyst along with their improved stability compared to free enzymes, bodes well for their active use. The elucidation of conditions for the extraction of pigments from a source like orange peel further enhances the value of the nanobiocatalyst-mediated process. Based on the increased yield of carotenoid pigments using the reusable nanobiocatalytic approach reported here, it is foreseeable that the cost of carotenoids can be significantly lowered. The current cost of carotenoid pigments ranges between INR 1000 for β-carotene to INR 4000 for lutein and zeaxanthin. The cost of enzymes and nanoparticle-constructs employed in the current work is nominal compared to these prices. It is expected that the present work will enable cost of carotenoids especially ones commonly sold as diet supplements such as lutein and zeaxanthin to be reduced by 2-3 fold. This assessment is based on the improved yield of extraction using the current work, the cost of nanobiocatalyst synthesis as well as the absence of specialized equipments/infrastructure for performing the extraction.
Experimental
Materials
Nagpur mandarin oranges (Citrus reticulata) were procured locally. Aspergillus niger Cellulase (1.3 U/mg solid), Aspergillus niger Pectinase (1.02 U/mg solid), APTES (3-Aminopropyltriethoxysilane) purity 98 %, Glutaraldehyde (25 % in H2O), Carbodiimide, Cellulose microcrystalline powder, Pectin (Polygalacturonic acid) 95 % (enzymatic) and 3,5-dinitrosalicylic acid were obtained from Sigma Aldrich (Bangalore, India). Other chemicals were obtained from Merck KGaA (Bangalore, India), and Sigma Aldrich (Bangalore, India). All glassware was soaked in piranha solution (concentrated H2SO4 and 35 % H2O2, 3:1 v/v) for 1 h and rinsed with deionised water before use.
Preparation of magnetic iron oxide nanoparticles
Iron Oxide (Fe3O4) nanoparticles were prepared by variation of reported methods of oxidative alkaline hydrolysis of ferrous ions (Mehta et al. 1997). An aqueous suspension of Fe(OH)2 was prepared by adding 1 M KOH to FeCl2 and adjusting pH to 8 while stirring. Precipitation at room temperature was followed by separation via magnetic decantation. The precipitate was washed with deionised water and ethanol, dried at room temperature, and was crushed and stored in a vacuum desiccator.
Physical adsorption of enzymes on iron oxide nanoparticles
Twenty mg of Iron Oxide nanoparticles were taken in 1 mL of 0.1 M Potassium Phosphate buffer solution (PBS) pH 7 and sonicated for 5 min at room temperature. The suspended particles were shaken gently for 6 h at room temperature using an Amerex Instruments GYROMAX 787. The particles were collected by magnetic decantation, washed 3 times with PBS and yield was calculated (98 %). 2 ml of enzyme solution (10 mg/ml) was added, the mixture was shaken for 20 h at room temperature, and was used directly thereafter.
Covalent attachment of enzymes on iron oxide nanoparticles
APTES-glutaraldehyde mediated conjugation
Functionalization of Iron Oxide NPs with APTES
One hundred fifty mg of Iron Oxide nanoparticles were dispersed in 4.5 mL of deionised water and 0.5 mL of 10 % 3-aminopropyltriethoxysilane (APTES) was added (Koh et al. 2006; Parka et al. 2011). The resulting suspension was sonicated for 5 min at room temperature followed by nitrogen purge and reflux for 3 h at 120 °C under nitrogen atmosphere. The modified particles were separated by magnetic decantation, washed with deionised water and ethanol and dried at room temperature. They were weighed, yield was determined (93 %) and stored in a vacuum desiccator.
Cross-linking with glutaraldehyde
Twenty mg of APTES modified Iron Oxide nanoparticles were taken in 8 mL of 2.5 % Glutaraldehyde and sonicated for 5 min at room temperature (Parka et al. 2011; Lei et al. 2007). The suspension was shaken gently for 6 h at room temperature. Subsequently, the particles were taken through two rounds of magnetic decantation and washing with PBS pH 7 before being weighed and yield determined (97 %). Two ml of enzyme solution (10 mg/ml) was added, the mixture was shaken for 20 h at room temperature, and was used directly thereafter. The conjugation strategy is represented in supplementary information, Scheme 1.
Carbodiimide mediated conjugation
Twenty mg of Iron Oxide nanoparticles were taken in 1 mL of 0.1 M PBS pH 7 and sonicated for 5 min at room temperature. 0.2 mL of carbodiimide solution (0.02 g/mL in 0.05 M PBS pH 7) was added and the resulting mixture was sonicated for 15 min at room temperature (Kouassi et al. 2005; Jordan et al. 2011). The particles were taken through two rounds of magnetic decantation, sonication and washing with PBS. Activated Iron Oxide nanoparticles were weighed after magnetic decantation and the yield was calculated (98 %). Two ml of enzyme solution (10 mg/ml) was added, the mixture was shaken for 20 h at room temperature, and was used directly thereafter.
Pigment extraction
(1) Mechanical Treatment: The orange peels were washed with deionised water and homogenised in a blender with 0.05 M citrate buffer pH 5 to make resulting solution of 10 g in 40 mL. (2) Enzymatic treatment: 1 g of orange peel was cut into small pieces but not homogenised. This was taken in 4 mL of 0.05 M citrate buffer pH 5 and was treated with 2 mL (10 mg/ml) of free enzyme or nanobiocatalyst suspension for 3 h at 50 °C. Samples were subsequently shaken for 21 h at room temperature. Optimum pH and temperatures were deduced from separate experiments (see “pH and temperature optimization” section). (3) Solvent Extraction: The enzyme or nanobiocatalyst treated peel was filtered. The supernatant was taken up in a separating funnel and an identical amount of organic solvent (such as methanol/ethyl acetate/DCM) was used for pigment extraction. Absorbance was measured in the wavelength range of 380–550 nm; spectra were recorded in triplicate for three different extractions.
pH and temperature optimization
Extraction of pigments using several enzyme constructs used at different pH (3, 5 and 7) of the medium of reaction was carried out. Similarly, the extraction was performed at different temperatures (40, 50, and 70 °C) with optimized solvent, chemical linker and pH. The temperatures were maintained for 3 h followed by incubation at room temperature for 21 h.
Assay of enzymes and nanobiocatalysts
The enzymes and nanobiocatalysts were assayed using a well known reported procedure (Miller 1959). 1 g of model substrate (cellulose or pectin) or ground orange peel was taken in 4 mL of 0.05 M citrate buffer pH 5.0. Two mL of enzymes (10 mg/mL) or nanobiocatalyst suspension were added to this followed by incubation at 50 °C for 1 h. Subsequently, 3 mL of dinitrosalicylic acid (DNS) reagent was added after the incubation and the samples were heated at 100 °C for 5 min. The samples were allowed to cool and were centrifuged at 5000 rpm at room temperature for 5 min. The UV–visible absorbance of the supernatant was measured at 540 nm. The DNS assays were performed in triplicate and mean values with standard deviations are reported.
Particle size and physical morphology of iron oxide nanoparticles
Iron oxide nano particles (5 mg) were taken in 1 mL of deionised water and sonicated for 5 min at room temperature. A small drop of supernatant was spread on glass cover slip that had been washed with piranha solution for 1 h. The glass cover slip with sample was dried at room temperature. Atomic Force Microscopy (AFM) was performed on NanoScope Multimode 8.0 from Bruker. The molecular structure of nano particles was confirmed by X-ray diffractometry on a Bruker AXS, Diffractometer D8 Discover where the nano particles were taken in powder form. HR-TEM was recorded on a FEI TECNAI G2 F20. The suspended bare nano particles and nanobiocatalyst were taken on carbon coated copper grid and dried before characterization.
Results and discussion
Particle size and molecular structure confirmation
AFM revealed a distinctly different morphology of bare nano particles compared to enzyme-immobilised ones. As shown in Figure S1, the cellulase and pectinase immobilised MNPs were much more homogenous compared to bare MNPs. Particle diameters in the former were in the range of 40–90 nm in contrast to 60–110 nm for the bare MNPs. This behaviour was consistent with previous reports regarding the aggregation behaviour of macromolecule-conjugated nano particles (Kuo et al. 2012). The lower dispersion of bare nano particles has been attributed to the colloidal instability of uncoated particles in water. In the present case, covalent conjugation of the enzymes cellulase and pectinase onto MNPs prevented their agglomeration thereby resulting in smaller particles and a narrower distribution of sizes (see Supplementary Information, Figure S2).
The crystalline structure of the iron oxide (2θ = 37°) nano particles was determined by powder X-ray diffraction (Figure S3). The diffraction peaks were assigned to five different lattice (atomic planes) structures and the product was confirmed as having a typical Fe2.7O4 (or Fe3O4) structure. The binding of cellulase/pectinase to Fe3O4 nanoparticles was investigated by FTIR spectroscopy. As shown in Figure S4, the characteristic IR frequencies of the enzyme at 1550 and 1410 cm−1 were also observed in the APTES-linked samples suggesting attachment of enzymes to Fe3O4 nanoparticles (Khoshnevisan et al. 2011). High Resolution Transmission Electron Microscopy (HR-TEM) was used to study the shape of bare and enzyme functionalized MNPs. As shown in Figure S5, nanoparticles functionalized with cellulase and pectinase exhibited greater dispersion and lower particle diameter compared to the bare nanoparticles.
Extraction of pigments from orange peel
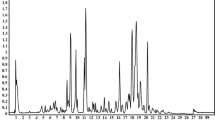
The loading efficiency of cellulase and pectinase on iron oxide MNPs was analysed by assessing the protein content through Biuret assays. The loading efficiencies of cellulase and pectinase on iron oxide MNPs were found to be 78 % for chemically attached and 52 % for physically adsorbed. These loading efficiencies are comparable to previous reports where 95 % binding efficiency of cellulase on MNPs have been reported (Khoshnevisan et al. 2011). The extraction of pigments from orange peels was followed by UV–visible spectroscopy that have been previously used for inferring the presence of specific carotenoids (Wrolstad et al. 2005). The separation and identification of individual carotenoids was not pursued since the primary aim of the study was to assess the performance of MNP-immobilized cellulase and pectinase in terms of overall extraction efficiency. The immobilization of cellulase and pectinase on iron-oxide was performed via different conjugation strategies with the linkers varying in their conjugation chemistries as well as their length. As shown in Fig. 1, the UV–visible spectrum of the extracts obtained by the treatment with different catalysts, indicates the possible presence of β- and α-carotene, cryptoxanthin, zeaxanthin and lutein. In particular, β-carotene, cryptoxanthin, zeaxanthin, α-carotene and lutein have comparable molar extinction coefficients in the range of 445–450 nm (Wrolstad et al. 2005). Different pattern of absorbance peaks was observed at 425, 445–450 and 475 nm, indicating presence of these pigments. Based on the absorbance measured at 445 nm, the nanoparticle-immobilized enzymes lead to nearly nine-fold greater extraction of pigments. The superior performance of the APTES-Glutaraldehyde conjugates may be correlated to their optimum spacing which translates to a better loading efficiency of the enzymes due to lesser steric interference.
Optimization of parameters for extraction
Factors that affect the performance of the enzyme-MNP constructs such as the conjugation linker, pH and temperature were investigated. The effect of pH on the extraction of pigments by action of cellulase and pectinase-conjugated MNPs was investigated and an optimum pH of 5.0 was identified. At the optimum pH, greater amount of pigment extraction was observed for the chemically conjugated nanobiocatalyst as compared to the physically adsorbed or bare enzyme (Figure S6). The optimum temperature for the performance of cellulase/pectinase-MNP constructs was explored by performing the treatment of orange peel with nanobiocatalysts at different temperatures. The free enzyme and the enzyme-MNP conjugates all displayed their best activity at 50 °C (Figure S7). This is in agreement with previous reports (Khoshnevisan et al. 2011) and the magnitude of pigment extraction mediated by covalently conjugated nanobiocatalyst was high than that obtained via the free enzyme. The efficacy of uptake of the pigments released from orange peel by different solvents post-treatment with cellulase/pectinase-MNPs was also investigated.
As shown in Figure S8, ethyl acetate was found to be the best to elute the pigment. Hexane showed relatively poorer uptake of the extracted pigments. A comparison of the extraction of pigments under optimum conditions emphasizes the superior performance of chemically conjugated nanobiocatalyst leading to nearly 8-9 fold greater pigment extraction (Fig. 1).
Correlation of peel hydrolysis and pigment extraction
In the present case cellulase and pectinase-mediated hydrolysis of polysaccharides present in orange peel is likely to result in the formation of reducing sugars. The DNS assay has been commonly used for estimation of reducing sugars (Ghose 1987; Jordan et al. 2011). The results of DNS assay on free enzyme and enzyme-MNP constructs between model substrates and orange peel are shown in Fig. 2.
The covalently conjugated cellulase/pectinase-MNP constructs exhibit the strongest propensity for model substrate and peel hydrolysis as well as facilitate pigment release and extraction from orange peels (vide infra). The correlation between effective peel hydrolysis and pigment extraction has not been reported previously (Cinar 2005).
Reusability of nanobiocatalysts for extraction
As shown in Fig. 3, the pigment extraction was reasonably uniform across three cycles for covalently conjugated cellulase/pectinase-MNP constructs with around 15 % drop in yield per cycle. In contrast, the performance of the physically adsorbed constructs showed a sharp drop after the first cycle itself. The significantly greater yield of extracted pigments facilitated by our nanobiocatalyst coupled with a high degree of reusability enhanced the utility of the process. The free enzyme does not have access to magnetic separation and exhibits negligible levels of activity following the first cycle.
The stability of the enzyme-MNP conjugates was verified over a period of 7 days and the covalently conjugated cellulase/pectinase-MNP exhibited consistently superior performance over this time compared to the physically adsorbed enzyme-MNPs or free enzyme.
Finally, the cost of enzymes used is in a single batch of extraction is in the range of INR 25–50. The cost of nanoparticles and other materials used in a single batch of extraction is even lower. In contrast the cost of carotenoid pigments being extracted from a single batch will be in the range of INR 100–200. Thus, considering the reusability of the current method as well as the lack of specialized infrastructure necessary for extraction, the process is expected to lower cost of production of carotenoids significantly.
Conclusions
Cellulase/pectinase linked via APTES and glutaraldehyde on iron oxide MNPs facilitated increased release of carotenoidic pigments from orange peels. Chemically conjugated nanobiocatalysts were found to outperform physically adsorbed enzymes or free enzymes by several fold, based on treatment and incubation over a 24-h span. β-carotene, cryptoxanthin and zeaxanthin were part of the carotenoids extracted with efficient uptake in ethyl acetate post-treatment with nanobiocatalysts. The peel hydrolysis and pigment extraction efficiencies dropped off under treatment conditions other than pH 5.0 and temperature 50 °C. The nanobiocatalyst mediated hydrolysis of orange peels was found to positively correlate with yield of extraction of carotenoidic pigments and was reusable across three cycles with only 15 % drop in extraction yields.
References
Aravantinos-Zafiris G, Oreopoulou V, Tzia C, Thomopoulos CD (1992) Utilisation of orange by-products orange peel carotenoids. J Sci Food Agric 59(1):77–79
Chamorro S, Viveros A, Alvarez L, Vega E, Brenes A (2012) Changes in polyphenol and polysaccharide content of grape seed extract and grape pomace after enzymatic treatment. Food Chem 133(2):308–314
Cinar I (2005) Effects of cellulase and pectinase concentrations on the colour yield of enzyme extracted plant carotenoids. Process Biochem 40:945–949
Delgado Vargas F, Pareds Lopez O (1997) Effects of enzymatic treatments on carotenoid extraction from marigold flowers (Tagetes erecta). Food Chem 58:255–258
Dominguez H, Nunez MJ, Lema JM (1994) Enzymatic pre-treatment to enhance oil extraction from fruits and oil seeds: a review. Food Chem 49(3):271–286
Fernandez K, Vega M, Aspe E (2015) An enzymatic extraction of proanthocyanidins from Pais grape seeds and skins. Food Chem 168:7–13
Fraser ML, Lee AH, Binns CW (2005) Lycopene and prostate cancer: emerging evidence. Expert Rev Anticancer Ther 5:847–854
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59(2):257–268
Gnayfeed MH, Daood HG, Illes V, Biacs PA (2001) Supercritical CO2 and subcritical propane extraction of pungent paprika and quantification of carotenoids, tocopherols and capsaicinoids. J Agric Food Chem 49:2761–2766
Goodwin TW (1980) Biochemistry of the carotenoids, vol 1: Plants, 2nd edn. Chapman and Hall, New York
Goodwin TW, Britton G (1988) Distribution and analysis of carotenoids. In: Goodwin TW (ed) Plant pigments. Academic Press, London, pp 62–132
Grohman K, Baldwin EA (1992) Hydrolysis of orange peel with pectinase and cellulase enzymes. Biotechnol Lett 14:1169–1174
Iyer PV, Ananthanarayan L (2008) Enzyme stability and stabilization-aqueous and non-aqueous environment. Process Biochem 43:1019–1032
Jordan J, Challa SSRK, Theegala C (2011) Preparation and characterization of cellulase-bound magnetite nanoparticles. J Mol Catal B Enzym 68:139–146
Khoshnevisan K, Bordbar A-K, Zare D, Davoodi D, Noruzi M, Barkhi M, Tabatabaei M (2011) Immobilization of cellulase enzyme on superparamagnetic nanoparticles and determination of its activity and stability. Chem Eng J 171:669–673
Koh I, Wang X, Varghese B, Isaacs L, Ehrman SH, English DS (2006) Magnetic iron oxide nanoparticles for biorecognition: evaluation of surface coverage and activity. J Phys Chem B 110:1553–1558
Kouassi GK, Irudayaraj J, McCarty G (2005) Examination of cholesterol oxidase attachment to magnetic nanoparticles. J Nanobiotechnol 3:1
Krinsky NI (1994) The biological properties of carotenoids. Pure Appl Chem 66:1003–1010
Kuo CH, Liu YC, Chang CM, Chen JH, Chang C, Shieh CJ (2012) Optimum conditions for lipase immobilization on chitosan-coated Fe3O4 nanoparticles. Carbohydr Polym 87:2538–2545
Lei Z, Bi S, Hu B, Yang H (2007) Combined magnetic and chemical covalent immobilization of pectinase on composite membranes improves stability and activity. Food Chem 105:889–896
Mehta RV, Upadhyay RV, Charles SW, Ramchand CN (1997) Direct binding of protein to magnetic particles. Biotechnol Tech 11(7):493–496
Miller GL (1959) Use of dinitrosalicylic acid reagent for determinations of reducing sugar. Anal Chem 31:426–428
Nagodawithana T, Reed G (1993) Enzymes in processing, 3rd edn. Academic Press, San Diego
Parka HJ, McConnella JT, Boddohib S, Kipperb MJ, Johnson PA (2011) Synthesis and characterization of enzyme-magnetic nanoparticle complexes: effect of size on activity and recovery. Colloids Surf B Biointerfaces 83:198–203
Polizzi KM, Bommarius AS, Broering JM, Chaparro-Riggers JF (2007) Stability of biocatalysts. Curr Opin Chem Biol 11:220–225
Salvatore Lenucci M, De Caroli M, Marrese PP, Iurlaro A, Recio L, Bohm V, Dalessandro G, Piro G (2015) Enzyme-aided extraction of lycopene from high-pigment tomato cultivars by supercritical carbon dioxide. Food Chem 170:193–202
Stahl W, Sies H (2005) Bioactivity and protective effects of natural carotenoids. Biochim Biophys Acta Mol Basis Dis 1740:101–107
Wilkins MR, Widmer WW, Grohmann K, Cameron RG (2007) Hydrolysis of grapefruit peel waste with cellulase and pectinase enzymes. Bioresour Technol 98:1596–1601
Wrolstad RE, Acree TE, Decker EA, Penner MH, Reid DS, Schwartz SJ, Shoemaker CF, Smith D, Sporns P (eds) (2005) Handbook of food analytical chemistry: pigments, colorants, flavor, texture and bioactive food components. Wiley, London
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kumar, S., Sharma, P., Ratrey, P. et al. Reusable nanobiocatalysts for the efficient extraction of pigments from orange peel. J Food Sci Technol 53, 3013–3019 (2016). https://doi.org/10.1007/s13197-016-2272-2
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-016-2272-2