Abstract
Breakfast cereals was formulated from blends of acha and fermented okara (soybean residue). Acha grains were cleaned, winnowed, washed, dried (at 50 °C for 4 h) and milled into flour. Okara was processed from soybean seed through the stepwise procedure of cleaning, soaking, washing, milling and sieving. The residue was divided into five portions, fermented differently for 0, 12, 24, 36, and 48 h and then dried at 50 °C for 6 h. Acha flour (UFAC) was milled and blended at 50, 60, 70, 80, 90, 100 % ratio with milled okara flour. Functional properties (bulk density, water absorption capacity, swelling capacity, wettability, viscosity and particle size distribution, microbial load, and proximate composition were carried out on the individual flour samples. The blended flours were conditioned, partially heat treated (for 10 min), aged (4 °C for 6 h), cut, toasted (120 °C for 1 h), cooled and packaged. The different toasted breakfast cereals, TBFC, were subjected to sensory evaluation, proximate composition, micronutrient composition (vitamin A, B1, B2, B3, calcium, iron, phosphorus, and zinc) and microbial analyses (mould count and total viable count). The proximate composition of the unfermented okara (UFOK) and fermented okara (FEOK 1, 2, 3, and 4 fermented at 12, 24, 36, 48 h respectively) flour samples showed that fermentation increased moisture from 4.71 ± 0.06 to 6.11 ± 0.05, crude fibre from 36.62 ± 0.01 to 46.18 ± 0.55, and carbohydrate from 2.50 ± 0.18 to 2.71 ± 0.34 contents. There was a decrease in the fat content from 16.29 ± 0.04 to 13.27 ± 0.22, ash from 1.41 ± 0.17 to 6.36 ± 0.17 and crude protein from 30.32 ± 0.21 to 33.53 ± 0.11 contents. From the sensory evaluation, the result showed that 70:30 acha-unfermented okara TBFC, FEOK 1D 60:40 acha-12 h FEOK TBFC, 70:30 acha-24 h FEOK TBFC, 50:50 acha-36 h FEOK TBFC, and 70:30 acha-48 h FEOK TBFC had the best overall acceptability for the different fermentation times. The functional properties, proximate and sensory evaluation were the basis for selection of the best products which were UFAC (control), UFOK C (70:30 acha UFOK TBFC) and FEOK 4C (70:30 acha 48 h FEOK TBFC) which were further subjected to proximate, micronutrient, and microbial analyses. There was an increase in the protein, fat, ash, crude fibre content whereas the moisture and carbohydrate contents decreased. A considerable decrease was found in vitamin A and B1 contents of the products, whereas vitamin B2 and B3 increased. Also the mineral contents of all the products were improved as indicated in the increase in ash content.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Breakfast in simple term means “breaking the fast” of the night. It is the most important meal of the day because it is the meal which is used to break the fast of the long night usually 10–12 h. Breakfast is regarded by many nutritionists as the most important meal of the day because food consumed at breakfast seem to be more utilized than the same amount eaten at night. Most researchers who have studied the effect of eating breakfast have suggested that the benefits are due mainly to the protein that is eaten from the meal because high protein breakfast were found to be better than low protein breakfast in helping to maintain a normal blood sugar level between mid-morning and lunch (Ensminger 1994). Breakfast is basically produced from cereals which are the dry seeds of those members of the grass family grown for their grains and are by far the most important plants eaten by man. Cereal is typically a low-fat, nutrient-dense food with many essential vitamins and minerals such as zinc, phosphorus, calcium among others. In addition to delivering important nutrients and essential vitamins such as iron, B-vitamins and zinc, cereals also provide the important benefits of grains.
Breakfast cereal was defined to include ready-to-eat breakfast cereal (RTEC), oats/porridge, and muesli (Williams 2014). Exclusions were breakfast bars, muesli bars, liquid breakfast products, milk drinks, and yogurt products. Breakfast cereal was also defined as dry cereal eaten at breakfast which has been processed into different forms by soaking, swelling, roasting, grinding, rolling or flaking, shredding or puffing of any cereal and is eaten as breakfast. Breakfast cereal consisting of vegetable protein foods (bread, cereals and soy bean milk) were reported to be as effective as those consisting chiefly of animal protein foods (eggs, meat and milk) in keeping the blood at a normal level The level of protein eaten as breakfast cereal may depend partly upon the amount supplied by a cereal.
Breakfast cereal products were originally sold as milled grains of wheat and oats that required further cooking in the home prior to consumption. In this century, due to efforts to reduce the amount of in-home preparation time, breakfast cereal technology has evolved from the simple procedure of milling grains for cereal products that require cooking to the manufacturing of highly sophisticated ready-to-eat products that are convenient and quickly prepared (Tribelhorn 1991).
Cereals have been used over the years as industrial raw material because of the high carbohydrate, low fat and protein contents. Cereals such as maize and sorghum are rich in sulphur-containing essential amino acids such as methionine, cysteine and tryptophan, while the storage proteins of most oil seeds and legumes are relatively low in sulphur containing amino acids, but very high in another essential amino acid, lysine compared to cereals (Kanu et al. 2009). Meanwhile, proximate composition of a food material would not give indication of limited use because sorghum, millet, fonio (acha) among others, are under-utilized. These under-utilized cereals lack some essential nutrients thereby reducing their uses. Also, their uses had been relegated to household preparation of local dishes. Their potentials could be brought to limelight when the under-utilized cereals are processed technologically into industrial or commercial products. Though, the cereal grains provide the bulk of the energy requirement in local meals because of their low cost, they are surplus in the essential sulphur-containing amino acid, methionine and cystine but poor in lysine. Relative to lysine and sulphur amino acid contents, legume and cereal proteins are nutritionally complementary, depending on the contents of the second limiting amino acids, i.e., threonine in cereals and tryptophan in legumes (Duranti 2006).
Hence, the call for blending legumes with cereals in food product development (Okafor and Usman 2015). Therefore, a combination of such food stuffs will improve the nutritional value of the resulting blend compared to the individual components alone. Animal products such as meat, eggs, milk, and cheese are known to contain the essential amino acids that could complement this deficiency in cereal foods. However, consumption of proteins from plant sources (legumes) is encouraged (Ofuya and Akhidue 2005), since combination of legumes and grains provide high quality cheaper protein that contains all essential amino acids in proper proportion, because their amino acids complement each other (Okaka 2005). This deficiency could be complemented by others constituents, structure and colour, qualities which are locally available, under-exploited legumes that incidentally are high in protein, mainly lysine but limited in cystine and methionine and probably tryptophan. Cereals and legumes, therefore, complement each other in both traditional food preparation and for industrial uses (Nkama 1990). The relatively lower cost of vegetable protein in comparison with animal protein and preference of some people for protein of plant origin to that of animal sources has led to this increasing industrial demand for legume proteins (Arogundade et al. 2011).
However, the practice of producing flaked breakfast cereal from blends of acha, and fermented okara is a way of improving the nutritional composition of breakfast cereal. Acha (Digitaria exilis), is a cereal with very tiny seeds which poses difficulty in processing but is absolutely rich in amino acids (Vodouhè et al. 2012) and needs to be supplemented with a legume for higher nutrient-dense product. Acha (Digitaria exilis), a traditional cereal crop from West Africa, is popular because it is well adapted to local conditions and has good nutritional and culinary properties (Cruz 2012). Acha, one of the oldest and richest cereals of West Africa, is unknown to many people and neglected by research and extension services. Adapted to poor soils and limited water supply, acha is an excellent dry areas crop which grows and produces where other crops fail (Vodouhè et al. 2012). Like other millets, fonio is widely reported to be rich in amino acids but particularly in the amino acids methionine and cystine (Belton and Nuttall 2002) which supply sulphur and other compounds required by the body for normal metabolism and growth. It has potential to improve nutrition, boost food security, foster rural development and support sustainable use of the land (Anon 2012). The grains are used in porridge and couscous, for bread, and for beer. For a long time, research activities in the region have been oriented towards major cereals such as maize, rice and sorghum. The interest in fonio is relatively recent (Vodouhè et al. 2012). Fonio is also regarded as a grain with medicinal and healing properties. It is recommended for lactating women and diabetic people and is often used in diets of sick people (Vodouhè et al. 2012).
“Okara” is the residue obtained from ground soybean (Glycine max) after removing the water-extractable fraction used to produce tofu or soymilk. After soymilk extraction, much of that fat, fibre and protein remains in okara (O’Toole 1999; Riaz 2006). It contains most of the carbohydrates, some of the protein and a small portion of the oil from soybeans. It also contains minerals like calcium, iron, copper and zinc. Generally, okara contains about 50 % dietary fibre, 25 % protein, 10 % lipid, 4 % low molecular weight carbohydrates and 4 % ash (Li et al. 2008). It is well known that dietary fibre plays an important role in many physiological processes and in the prevention of diseases of different origin. Furthermore, okara contains much protein and considerable isoflavone and mineral elements, so it possesses high nutritional value and various prebiotic effects. This is consistent with the findings from other researchers.
Okara has the potential to be used as a fermentation substrate for ethanol and methane production. It can be utilized for animal feed, fertilizer and pet food among others. Okara can also be used for food products either wet, dried or as a paste in food products ranging from meat to baked products. Overall, the use of okara in foods is not prevalent anywhere in the world. Anecdotal evidence suggests that commercial soy beverage producers are interested in, and actively investigating, the potential to better utilize and add value to okara, but few details exist on these confidential, strategic planning efforts. Little okara is being used in the food industry today, either as baked soy products (in Ontario), fresh or freeze-dried for shipping to a production facility. Given the cost and technology required to process okara and the relatively low value of the product, no large scale primary processing of okara is occurring commercially in Canada (Anon 2005). However, certain barriers exist in the use of okara in food which include rapid degradation, high costs of drying, feasibility of freezing and alternative protein sources among others. Also, possible barriers to the use okara in food include process or use of okara as soon as it is produced, drying, develop entirely new market channels for premium priced products that add enough value to make okara economically food feasible and lastly, develop strategic alliances among companies (soy beverage producers, food processors, farmers) to reduce costs, hare expenditure and maximize value (Anon 2005). Fermentation of okara could be sterilized and dried (Rashad et al. 2011) or lyophilized (Aoki et al. 2003).
Meanwhile, the amount (yield) of cereals grown in Nigeria is high compared to its utilization. This is due to postharvest losses incurred from cereals, thus there is need to diversify the use of cereals into producing packaged foods such as breakfast cereals which can be made available all year round. The intake of unhealthy or very low quality breakfast cereal can result in the following, inability to focus on tasks, less memory capacity, decreased alertness throughout the day among others. Also, health-related effects include high risk of developing diabetes, increased cholesterol and high blood pressure among others. Therefore, it is necessary to eat a balanced breakfast cereals that can help ameliorate these health problems and this can be provided from breakfast cereals formulated from acha and fermented okara. Since acha is reported to be rich in amino acids, particularly methionine and cystine (Belton and Nuttall 2002) which supply sulphur and other compounds required for normal metabolism and growth, it is important to blend acha, a cereal and fermented okara, a legume as a way of improving the carbohydrate, proteins, vitamins and beta-carotene contents of the breakfast cereals. The composite flour produced from a combination of acha and okara is regarded as composite flour. For developing and under-developed countries, the use of composite flours has the following advantages; better supply of protein for human nutrition; saving of hard currency; Promotion of high yielding, native plant species and better overall use of domestic agriculture production (Bugusu et al. 2001).
Protein deficiency has become a dietary problem facing the world especially the underdeveloped and developing countries (Enweremadu et al. 2008). Animal protein is expensive in developing countries like Nigeria; therefore it becomes necessary to find an alternative means of compensating for low protein availability and also to fortify the staple foods with vitamins and minerals. Huge quantities of okara are produced worldwide. In Japan about 800,000 t, in Korea approximately 310,000 t and in China about 2,800,000 t of okara are produced from the tofu industry every year (Ohno et al. 1993; Ahn et al. 2010). These huge quantities of okara produced annually pose a significant disposal problem. Okara is sometimes used as animal feed but most is dumped and burned as waste. However, its nutritive composition makes it suitable for producing different fermentable edible products, like single cell protein for human food and feed. The high moisture content of okara makes it unfavorable to be utilized alone and so is incorporated into other food raw materials to produce a more shelf stable foods products.
Therefore, the major thrust of the study is to develop and evaluate nutritionally, microbiologically and organoleptically the nutrient-dense breakfast cereal produced by blending acha and fermented okara.
Materials and methods
Raw materials
The raw materials used in this work include: acha grains, soybean seeds, sugar, and salt. Acha grains were purchased from Nyanya market, Abuja whereas other ingredients were purchased from Ogige main market, Nsukka, Enugu state, Nigeria. Okara (fermented soybean residue) was produced from the processing of soybean into residue and acha flour was produced from acha grains.
Processing of acha grains to flour
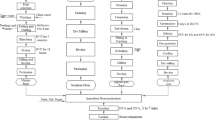
The processing of acha grains to flour is illustrated with the flowchart below (Fig. 1). Cleaning was done for the removal of unwanted particles from the grains which is done by sorting. Winnowing was done by removing the chaff from the grains. The acha grains were washed with clean water for about four (4) times. Then grains were dried with the oven trays in batches at 50 °C for six (6) hour each. The samples were then allowed to cool and milled with a hammer mill (I.G. Jurgens, Bremmer, Germany) and packaged in air-tight containers as shown in Fig. 1.
Modified flowchart for processing of acha grains to flour. Source: Ibrahim (2001)
Okara production from processing of soymilk
The various steps involved in okara production from soybeans are shown in Fig. 2. Soybean seeds were destoned for the removal of dirt which are present. The cleaned seeds are soaked in clean water for about six (6) hours or more to soften the outer coating. Washing of the beans after soaking was done as to ameliorate the unpleasant odour. The soybeans are then grinded into slurry by addition of water. The soybean slurry was sieved using a muslin cloth by constant addition of water until a residue is obtained. This residue is known as okara. The okara was then fermented by in a clean bowl by natural means under room temperature (25 ± 2 °C) for the different fermentation time (0, 12, 24, 36, 48 h). The fermented okara was then dried in an oven at a temperature of 50 °C for about six (6) hours after which it was milled into flour using hammer mill (I.G. Jurgens, Bremmer, Germany) and packaged in an airtight container for storage and used for analysis.
Formulation and processing of breakfast cereals
The stepwise process involved in the formulation of the toasted breakfast cereals is shown below (Fig. 3). The acha flour and okara flours were mixed together. Small quantity of water added to the flour mixtures as to have a binding effect; sugar and salt were added to taste. The mixture was heat treated by steaming for 10 min and then allowed to age at a temperature of 4 °C for about six (6) hours. The dough was cut into very small shapes in baking pans and flaked or toasted in an oven (Gallenkamp oven, size one, England) at a temperature of 120 °C for one (1) hour after which the flaked/ toasted breakfast cereals was allowed to cool under room temperature and packaged in rigid plastic containers for analysis.
Functional properties of the flour
Determination of bulk density
Bulk density was determined by placing 20 g of each sample flour into a 100 ml-graduated cylinder. The cylinder was taped gently about 10 times against the palm and the final volume as expressed was g/ml as described by Onimawo and Egbekun (1998). The volume of sample was recorded. All analysis was done in triplicate.
Determination of water absorption capacity
The procedure of Sathe et al. (1982) was used. Ten milliliters (10 ml) of water was added to 1.0 g of each blend samples, the suspension was then stirred using magnetic stirrer for 5 min. The suspension was transferred into centrifuge tubes and centrifuged at 3,500 rpm for 30 min. The supernatant obtained was measured using a 10 ml measuring cylinder. The density of the water was assumed to be 1 g/ml. The water absorbed was calculated as the difference between the initial water used and the volume of the supernatant obtain after centrifuging. The result was expressed as a percentage of water absorbed by the blends on %g/g basis.
Determination of swelling capacity
The method described by Leach et al. (1959) was used with slight modifications. Flour blends (1 g) was weighed and transferred into a clean dry test tube and weighed (W1). The mix was then dispersed in 50 cm3of distilled water using a magnetic stirrer. The resulting slurry was heated at desired temperatures – 40, 50, 60, 70, 80, 90 °C for 30 min in a thermo stated water bath. The mixture was cooled to room temperature and centrifuged at 2,200 rpm for 15 min. Also, 5 ml of aliquot of the supernatant was dried to a constant weight at 120 °C. The residue obtained after drying represented the amount of starch solubilized in water. The residue obtained after centrifugation with the water it retained was transferred to the clean dried test earlier and re-weighed (W2).
Determination of wettability
Wettability was determined using ISO (2011) procedure A. Two hundred fifty millilitres (250 ml) of water was measured into a dry glass beaker ensuring that the inside the beaker above the final water level remained dry. The sample (10 g) was measured into a membrane which allowed only water to penetrate into sample without the sample particles falling and was placed on top of the beaker. The stop watch was set immediately the membrane was withdrawn in a gentle continuous movement such that the test portion progressively fell onto the surface of the water. The beaker was removed immediately but gently from below the membrane (glass tubing) and allowed to stand. As the particles of the test portion was completely wetted, the stop watch was stopped and the time is in seconds was recorded (ISO 2011). Each triplicate value for the wetting time was calculated using the formula given below:
Where; W = wetting time in seconds; w = time recorded (in seconds)
Determination of viscosity
The method of Sathe and Salunkhe (1981) was used. An appropriate sample (2.5 g) was dispended in distilled/deionized water and magnetically stirred for 2 h at room temperature (28 °C) prior to viscosity measurements. Each sample was prepared at concentration of 10, 20, 30, 40, 50, 70 and 100 g/l and viscosity measured at 100 m temperature using universal Torsion viscometer (Gallenkamp Technico Compenstat, England) having corehead of 30 (standard) gauge 15/8 in. index and an approximate constant = 0.1 cP per second. All measurement was performed in triplicate and means, relative to distilled water were reported.
Determination of particle size distribution (PSD)
The sieve analysis method was used to determine the particle size distribution of acha. This method was used by Ukpabi and Ndimele (1990) to determine the particle size distribution of okara and acha flour. The upper sieve was provided with a cover and the bottom sieve with a receiver. A 25 g sample of the product was put in the top sieve, covered with the lid and the nest of sieves placed in a suitable mechanical sieve shaker (Endecott test sieve shaker, Britain). The sample was sieved continuously for 10 min after which sieving was stopped, the nest removed and the residue on each sieve transferred to a tarred weighing dish using a brush. Each dish was weighed and the percentage of the product retained on each sieve was calculated as:
Proximate analyses
Moisture, crude protein, ash, crude fibre and fat contents were determined according to the methods outline in AOAC (2010). Carbohydrates were calculated by difference according to the following equation:
Determination of micronutrients
Determination of vitamin A
The vitamin A content was determined using Kirk and Sawyer’s (1998) method. One gram (1 g) of the sample was weighed into a conical flask. Then, 2.5 ml of ethanol and 10 ml of n-heptane was added. The mixture was allowed to stand for 45 min. It was then filtered and the reading was taken using a colorimeter.
Determination of vitamin B1
Five (5) gram of sample was homogenized in 50 ml ethanoic sodium hydroxide. Then, 10 ml filtrate was added to 10 ml potassium dichromate and absorbance was recorded at 360 nm after development of color (Barkat et al. 1973).
Determination of vitamin B2
Five (5) gram of sample was extracted with 100 ml ethanol for 1 h. Then, 10 ml of this filtered extract added 10 ml 5 % potassium permanganate and 10 ml 30 % H2O2 and allowed to stand on hot water bath for 30 min. To this, 2 ml of 40 % sodium sulphate was added. The volume was made up to 50 ml and absorbance was recorded at 510 nm which was used to calculate the vitamin B2 content (Bhandange 2010).
Determination of vitamin B3
Five (5) gram of sample was treated with 50 ml of 1 N sulphuric acid for 30 min and 0.5 ml of ammonia solution was added to it. It was filtered and 10 ml of the filtrate was taken. To that 10 ml of the filtrate, 5 ml of potassium cyanide was added and the acidified with 5 ml 0.02 N H2SO4. The absorbance of the resulting solution was recorded at 420 nm (Baser et al. 2000).
Determination of calcium
Calcium was determined by titration method according to Kirk and Sawyer (1998). About 2 ml of the ash solution was diluted with 3 ml of distilled water and 1 ml of 50 % ammonium oxalate. One drop of methyl red indicator was made alkaline with ammonia drops and drops of glacial acetic acid until colour changes to pink. It was allowed to stand for 4 h and centrifuged for 5 min, followed by decantation of the supernatant. About 1 ml of hydrogen sulphate was added to the residue which was diluted with 4 ml of distilled water. The solution was boiled and titrated with 0.02 N potassium permanganate which is given as an equivalent to calcium content in the sample.
Preparation of ash solution
Five (5) gram of the ground sample was put into porcelain dish and allowed to ignite in furnace to carbon free ash. The residue was boiled in 40 ml HCl and few drops HNO3. It was transferred to 250 ml volumetric flask, cooled, diluted to volume and mixed thoroughly (AOAC 2010). This is known as the ash solution.
Determination of iron
An aliquot of ash solution (10 ml) was pipette into 250 ml volumetric flask, and 1 ml hydroxylamine hydrochloride was added. After 5 min, 5 ml buffer solution and 1 ml o-phenanthroline solution or 2 ml of dipyridyl solution was added and diluted to volume. The absorbance of solution at 510 nm was determined. The iron (Fe) content present in aliquot of ash solution was determined from absorbance reading by taking reference to standard curve (AOAC 2010). The iron content was calculated thus:
Determination of phosphorus
The already prepared ash solution was boiled with 10 ml HCl and the solution was washed into a flask with water. It was neutralised by drop wise addition of 0.88 ammonia (the volume of the solution at this stage was 50 ml). Dilute nitric acid, and 25 ml of vanadate-molybdate solution was added and the volume was made up, thereafter optical density was measured after 10 min that it was allowed to stand (Kirk and Sawyer 1998).
Determination of zinc
Two (2) drops of methyl red indicator was added aliquot of ash solution already prepared and 1 ml of CuSO4 solution and neutralised with NH4OH. Then, 5 ml HCl was added to make the solution about 0.15 N with respect to HCl. The pH of the solution was adjusted as measured with glass electrode (from 1.9 to 2.1). Stream of H2S was passed into solution until precipitation was complete. It was then filtered through fine filter paper (Whatman No. 42 or equivalent). The filtrate was collected in a beaker, washed and filtered with 3 or 4 portions of water. The filtrate was boiled until odour of H2S was no longer detected. Then, 5 ml of saturated bromine-water was added and boiling continued until Br-free. It was then cooled, neutralised to phenol red with NH4OH and made slightly acid with HCl. The resultant solution was diluted to a definite volume. To 20 ml aliquot of this prepared solution, 15 ml of ammonium citrate solution, 2 ml dimethylglyoxime solution and 10 ml of α-nitroso-β-naphthol solution were added and shaken for 2 min. Solvent layer and extract were discarded with 10 ml of CHCl3 (this procedure eliminates nickel and cobalt).
To aqueous phase following removal of nickel and cobalt, which at this point has pH 8.0 to 8.2, 2 ml of dithizone solution and 10 ml of CCl4 was added and then shaken for 2 min. The phases were allowed to separate and aqueous layer was removed as completely as possible, withdrawing liquid with pipette attached to vacuum line. About 25 ml water was used to wash down the sides of separator and without shaking again the aqueous layer drawn off. 25 ml of 0.04 N HCl was added and shaken for 1 min. The solvent was drained and discarded with care to dislodge and remove drop of solvent that floated on surface.
To acid solution, 5.0 ml of ammonium citrate solution and 10 ml of CCl4 (pH of solution at this point should be 8.8 to 9.0). At this stage, the volume of dithizone solution is determined and shaken for 2 min. Exactly 5 ml of solvent layer was pipette into clean dry test tube, and diluted with 10 ml of CCl4, well mixed and the absorbance at 540 nm was determined. The absorbance was converted to microgram of Zinc from standard curve and the Zn content of the sample was calculated.
Microbial analysis
Media preparation
Nutrient Agar powder (7 g) was dissolved in distilled water (250 ml). Sabouraud Dextrose Agar (13 g) was dissolved in distilled water (200 ml). The mixtures were homogenized by bringing them to boiling while homogenizing by shaking in a whorl motion. The mixtures were sterilized by autoclaving for 15 min at a temperature of 121 °C according to Harrigan and McCance (1981). The medium was allowed to cool after sterilization to about 40–47 °C in a desiccator.
Preparation of ringer solution
One Ringer tablet was dissolved in distilled water (500 ml). The clear solution formed was sterilized by autoclaving for 15 min at the temperature of 121 °C. The Ringer solution was allowed to cool completely to a temperature of about 28 °C.
Determination of total viable count
The Total Viable Count was determined by the method Pour Plate Count as described by Prescott et al. (2005). The method involved weighing sample (1 g) into a sterile test tube. A ¼. strength Ringer`s solution (9 ml) was poured into it and also into other test tube arranged for serial dilution. The sample with the solution was homogenised by shaking. Pipette (1 ml) was used for the serial dilution into the test tube containing Ringer`s solution (9 ml). Then, 1 ml solution at different dilution factor was transferred into the sterile Petri dishes and sterile nutrient agar (20 ml) was poured into the same Petri dish and was mixed by rocking. When they solidified, they were turned upside down and cultured by incubation for 24 h at the temperature of 37 °C. At the end of the incubation period, the colonies were counted using the colony counter (Gallenkamp colony counter, CNW 330-010X) and the number of colonies recorded appropriately.
Determination of mould count
Serial dilutions of sample were cultured by pour plate method as described by Harrigan and McCance (1981) on acidified malt extract agar for mould count and the media was prepared. Samples were plated in triplicates and incubated for 1 week for mould growth. Enumeration of the discrete colonies was carried out using a Digital electronic colony counter (Gallenkamp colony counter, CNW 330-010X).
Sensory evaluation
Sensory properties of the samples were evaluated by 20 semi-trained panelists from the Department of Food Science and Technology for various sensory qualities (appearance, taste, flavor, texture, after taste and overall acceptability). A 9-point Hedonic scale questionnaire was used where “9” represents extremely like and “1” represents extremely dislike (Ihekoronye and Ngoddy 1985).
Statistical analysis
Mean values were subjected to analysis of variance (ANOVA) using Duncan’s Multiple Range test (DMRT) and SPSS (Statistical Package for Social Science) version 20 computer was used. Significance was accepted at p < 0.05 according to Steel and Torrie (1980).
Results and discussion
Functional Properties of acha, fermented and unfermented okara flour
Table 1 shows the functional properties of acha, fermented and unfermented okara flour. The bulk density of the unfermented okara (UFOK) sample was 0.59 g/cm3 while that of the fermented okara, FEOK 1 to FEOK 4 samples ranged from 0.60 to 0.63 g/cm3 in which sample FEOK 4 had the highest value (0.63 g/cm3) and sample FEOK 1 was 0.60 g/cm3 (Table 1). There was no significant (p > 0.05) difference between the fermented samples. However, fermentation increased the bulk density slightly. This could possibly be that fermentation increased the mass of the sample. The bulk density of acha was 0.97 g/cm3. Thus, acha when compared to okara was found to be denser. This is probably due to the high starch content of acha flour more than that of legume (okara) which agreed with the results of Chukwu and Abdul-kadir (2008). The low values of bulk densities make the flour suitable for high nutrient density formulation of foods. Bulk density is generally affected by the particle size and density of the flour and it is very important in determining the packaging requirement, material handling and application in wet processing in food industry (Karuna et al. 1996). The lower the bulk density, the higher the amount of flour particles that can bind together leading to higher energy values (Onimawo and Egbekun 1998). However, a weaning food should have low water absorption capacity and bulk density in order to produce a more nutritious and suitable weaning food (Mbata et al. 2009). This could be achieved by reducing the viscosity of the starchy components by malting (Malleshi and Desikachar 1982).
The water absorption capacity (WAC) of the unfermented okara, UFAC, was 314.17 % and that of the fermented okara samples, FEOK 1 to 4 ranged from 301.20 to 250.33 % (Table 3). Sample FEOK1 had the highest value which was 301.20 % and FEOK 4 had the lowest value 250.33 %. Sample UFAC had a WAC value of 119.36 %. There was significant (p < 0.05) difference between the samples. The difference in water absorption is mainly caused by the greater number of hydroxyl group which exists in the fiber structure and allows more water interaction through hydrogen bonding (Nassar et al. 2008). As fermentation increased the rate at which the samples absorbed water decreased. Thus, the difference in the water absorption capacities may be explained by their respective content of hydrophilic constituent such as carbohydrates which bind more water than either protein or lipids. Both carbohydrates and protein are more soluble in water probably due to the fact that water (as a medium) aids in the breakdown of complexes of starch and protein to their simpler forms (that is simple sugars and amino acids). Similarly, high water absorption had been attributed to either high protein content or more hydrophilic polysaccharides during the course of fermentation (Onweluzo and Nnabuchi 2009). However, fermentation increases the acidity of a sample which then increases the water absorption capacity of okara. Also, the water absorption capacity of the breakfast cereals may equally be associated with the nature of starch granules after toasting (Okafor and Usman 2015). Considering the temperature of toasting that was 120 °C for 1 h (Fig. 3) and variation of starch content of each formulation with increasing addition of okara, damaged starch is likely to increase and will require more fluid such as water or milk on reconstitution. Similar values were recorded from treated and untreated sorghum and pigeon pea breakfast cereals (Mbaeyi 2005).
Sample UFAC had a water absorption capacity of 119.36 % which was the lower than that of all the okara samples. The difference in water absorption might be due to the quantity of damaged and undamaged starch present with the flour sample (Asiedu 1989). Water absorption in flour correlate positively with the amylase content and also particle size of the okara flour (Adeyemi and Beckley 1986). Water absorption capacity is an indication of the extent to which protein can be incorporated into food formulation. Increase in water absorption capacity implies high digestibility of the starch. The water absorption characteristic represent the ability of a product to associate with water under condition where water is limiting, in order to improve its handling characteristics and dough making potentials (Iwe and Onalope 2001). The lower water absorption capacity of the wheat and other flours could be attributed to the presence of lower amount of hydrophilic constituents in wheat (Akubor and Badifu 2001). Thus, the flour would be useful in food systems that need water in preparation such as bakery foods (Igbabul et al. 2014).
Swelling capacity is an indication of the water absorption index of the granules during heating (Loos et al. 1981). The swelling capacity of the samples ranged from 399.51 to 131.32 % with sample UFOK having the highest value of 399.51 % and sample UFAC with the lowest value of 131.32 % as shown in Table 3. There was significant (p < 0.05) difference in the samples. Fermentation decreased the swelling capacity of okara. Acha had less swelling capacity to okara possibly because protein which is more abundant in okara is hydrophilic.
Table 1 showed that wettability ranged from 290.28 to 341.26 % where sample FEOK 4 had a highest wettability of 341.26 % and sample UFAC had the lowest wettability of 290.28 %. Wettability is the relating to the ability of a solid to absorb a liquid, such as water, as it spreads over the surface of the solid (Merryweather et al. 2005). Therefore, it took a lesser time for the particles of a more dense food sample (acha) to completely wet in water to the time of wetting of okara. This could probably be attributed to the particle size of the samples in which the acha has larger surface area that allowed easy wetting.
The viscosity of the samples ranged from 1.00 to 1.08 Cp where sample UFOK had the least value of 1.00 Cp and sample UFOK 4 had the highest viscosity value of 1.08 Cp as presented in Table 1. There was no significant (p > 0.05) difference between the samples. The fermented okara samples, FEOK 1 to 4 had a higher viscosity value to the unfermented samples. This could possibly be attributed to the enzymic breakdown of the higher molecular weight polysaccharide and polypeptide to lower molecular weight dextrins and peptides during fermentation. This similar enzymic breakdown was observed by Onweluzo and Nnabuchi (2009) and was attributed to the inherent starch in acha possessing its viscofying properties since it was modified during fermentation. The difference in viscosity might also be due to the weakness of intermolecular network which may cause the flour granules to fall apart when gelatinized in hot water forming a paste of relativity low viscosity. Swelling of the granules was observed to be slight in cold water, compared to when placed in hot water. The breakfast cereals would be consumed depending on preferences or situations in various ways such as in cold water, hot water, cold and hot milk (Okafor and Usman 2015).
The results of the sieve analysis for particle size distribution of okara and acha flour samples are presented in Table 2. The sieves were ranked from seven to one: the largest sieve was ranked as number seven while the sieve with the smallest aperture was ranked as number one as suggested by Henderson and Perry (1976). From the data shown in the Table 2, all the samples passed through the sieves with diameter sizes of 8, 6, 4, and 2 which was evidence as no particle was retained in the sieve. The sieve with pore size 0.5 mm had lesser fine particles to that of 0.1 and 0.01 mm which were finer. This was evident in the increase of their “PR, percentage retained” particles. The different okara flour samples had PR values ranging from 1.4896 to 2.3166 % whereas the acha flour had less PR value of 0.3994 %, probably due to the nature of the samples. Similar trend was found in the sieve with pore size 0.1 mm but the sieve with pore size 0.01 mm showed that the percentage retained of acha (97.9528 %) was higher than that of the okara samples which ranged from 92.7985 to 94.4803 %. Thus, the smaller the pore sizes of the sieves, the larger the proportion of the “very fine” particles. The fine fractions are responsible for most of the water uptake and viscosity development during mixing or blending while the major functions of the coarse particles is to disrupt the mixture which reduces the extent of pillowing or the formation of large pockets during baking or drying (Mbaeyi-Nwaoha and Onweluzo 2013).
Microbial Count of acha flour, fermented and unfermented okara flour
The microbial count of acha flour, fermented and unfermented okara flour samples is shown in the Table 3. The total viable count for the unfermented okara sample was 8.9 × 104 cfu/g and the fermented okara samples ranged from 3.8 × 104 to 7.7 × 104 cfu/g as shown in Table 3. Sample UFAC had the lowest microbial load, (3.8 × 104 cfu/g). This result differed from that obtained by Samadrita et al. (2012) who compared the microbial load of different okara samples based on processing (drying methods). From the research, it could be inferred that cereals (acha) have lesser microbial load to legumes (soybean residue, okara) which are given the same treatment. The results from the study suggest that the dried okara should be used in further applications as soon as possible since prolonged storage may negatively impact on their proximate composition (the feature that made them to be highly valued for food and feed formulations in the first place). This information would be valuable especially where these okara powders are being consumed as protein supplements. High bacterial and fungal counts might also be attributed to the fact that the food handling must have been done held the foods at temperatures lower than 46 °C for more than 4 h. There were few microbial counts in the samples which could be because the processing of the samples were carried out in a clean environment, they were dried in an oven drier and clean containers were used to package the flour samples after processing.
In the mould count, only the sample UFOK was identified to have a growth of 1.0 × 10 cfu/g which is quiet low as to be cause harm to consumers whereas there was no detection of microorganisms in all other samples. The acceptable level for mould in breakfast cereals is 10 cfu/g whereas the level which when exceeded in one or more samples would cause the lot to be rejected as this indicate potential health hazard or imminent spoilage is 103 cfu/g (FDA 2013). The acceptable level for mould in soy flour is 103 cfu/g whereas the level which when exceeded in one or more samples would cause the lot to be rejected as this indicate potential health hazard or imminent spoilage is 105 cfu/g (FDA 2013).
Proximate Composition of the flours prepared from acha and fermented okara
The proximate composition of the flours prepared from acha and fermented okara is shown in Table 4.
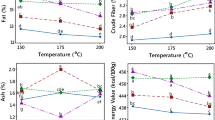
The fat content ranged from 1.57 to 16.29 % where sample UFOK, unfermented or freshly prepared okara was 16.29 % and sample UFAC, unfermented acha was 1.57 % (Table 6). The fat content of sample UFOK was lower than that of Vishwanathan et al. (2011) which was 23.2 %. The fat content of fermented okara ranged from 13.72 to 14.82 % where sample FEOK 1 (14.82 %) had the highest mean value and sample FEOK 4 (13.27 %) had the lowest mean value. This revealed that as fermentation increased the fat content decreased. This agreed to the research of Rashad et al. (2011) which revealed that fermentation of okara decreased the fat content. Previous studies have shown reduction in the lipid content of different substrates fermented with different microorganisms (Das and Weeks 1979; Iluyemi et al. 2006; Lateef et al. 2008). They attributed that to the accumulation of lipids by the fungal strains and during fungal processing, some lipolytic strains assimilate lipids from substrates for biomass production leading to a general reduction of the overall lipid content of the substrate. Perhaps, this was not in agreement with the result provided by Ugwuona et al. (2012) which showed that fermentation increased the fat content of soybean flour. The fat content of unfermented acha, UFAC, was 1.57 % and was in agreement to that the result given by FAO (1998) which was 1.8 %.
The ash content of unfermented okara was 6.36 % which was higher than the result given by Mateos-Aparicio et al. (2010) which is 4 % and also higher than the result provided by Fei et al. (2013) which was 3.9 %. This could be as a result of the processing method in which Mateos-Aparicio et al. (2010) cooked the rehydrated soybeans before grinding and filtering which was different from the processing method used. The ash content of samples FEOK 1 to FEOK 4 decreased as fermentation increased and was significantly (p < 0.05) different. This was not in agreement to the finding of Ugwuona et al. (2012) which stated that fermentation increased the ash content of soybean. This could probably be due to the difference in the processing methods where Ugwuona et al. (2012) hot-blanched soybeans seeds before processing into flour. Ugwuona et al. (2012) processed by boiling the raw soybean seeds whereas there was boiling adopted. The ash content of acha was 4.04 % which was close to the result of FAO (1998) which gave 3.4 %.
Protein content of the samples ranged from 9.00 to 33.53 %. Sample UFOK had a protein content of 33.53 % which agreed to the result of Vishwanathan et al. (2011) which was 34.7 % and was higher than the findings of Lescano et al. (2005) which was 25 %. The protein content of samples FEOK 1–4 ranged from 32.34 to 30.32 % where sample FEOK 1 had the highest value (32.34 %) and sample FEOK 4 (30.32 %) had the lowest value. This showed that as fermentation increased, the protein content decreased. There was a significant (p < 0.05) difference between the unfermented okara (UFOK), fermented okara samples, (FEOK 1 to 4) and unfermented acha (UFAC) flours. This could be due to the high percentage of fat in dry okara which agreed with the result of Vishwanathan et al. (2011). The protein content of UFAC was 9.0 % which agreed to the result given by FAO (1998) and close to the result of Temple and Bassa (1991) which was 7 % in acha flour.
Table 4 showed that moisture content ranged from 9.88 to 4.71 % where sample UFAC had the highest moisture content and sample UFOK had the lowest value. The moisture content of sample UFOK was 4.71 % which was lower than the results obtained by Matsumoto et al. (2007) that was 8.9 %. This could be from the difference in processing (drying time). This moisture content obtained was very much below the maximum 12.5 % water content for shelf-stable storage and viability of food grains (Chakraverty 2004) thus, a more shelf stable product will be produced. The samples FEOK 1 and 2 had no significant (p < 0.05) difference as well as sample FEOK 3 and 4. This implied that fermentation did not have much influence on moisture. Sample UFAC had a moisture content of 9.88 % and corresponded to the result obtained by other researchers (FAO 1998; Ugwuona et al. 2012) which revealed 10 % and 10.04 % in acha flour.
The crude fibre content ranged from 2.49 to 46.18 % where sample UFOK had the highest value and sample UFAC had the lowest value as presented in Table 4. There was significant (p < 0.05) difference between the samples. This could be due to the high fibre content of okara which agreed to the findings of Jiménez- Escrig et al. (2008) which ranged from 14.5 to 55.4 %. As fermentation of okara increased, the fibre content increased. This was not in agreement to the findings of Lateef et al. (2008) which showed that fermentation of okara with yeast decreased its crude fibre content. Sample UFAC had a fibre content of 2.49 % which was close to the findings of FAO (1998) and Ugwuona et al. (2012) which was 3.3 and 1.86 % respectively.
This ranged from 2.50 to 73.08 % and revealed that sample UFAC had the highest value whereas sample UFOK had the lowest the lowest value as shown in Table 4. There was a significant (p < 0.05) difference between the samples. This could be due to the high starch content of acha than that of okara. Sample UFOK had a carbohydrate content of 2.50 % which corresponded to the result given by Recundo-Cuenca et al. (2008) which was 2.56 %. Samples FEOK 1 to 4 had a carbohydrate content that increased as the fermentation increased. The 73.08 % of sample UFAC agreed with the findings of Chukwu and Abdul-kadir (2008) and also the result of FAO (1998) which stated that acha has a high carbohydrate content of between 70 and 89.5 %.
Sensory Scores of toasted breakfast cereals from acha-okara blends fermented at 0, 12, 24, 36, 48 h
The sensory scores of the toasted breakfast cereals are shown in the Tables 5 to 9. Fermentation causes changes in food quality including texture, flavour, appearance, nutrition and safety. The benefits of fermentation may include improvement in palatability and acceptability by developing improved flavours and textures (Sahana and Hafiz 2003).
Colour
The values for colour toasted breakfast cereals produced from blends of acha and fermented “okara”(0-h) flours ranged from 6.90 to 7.80 where samples UFAC (7.80), UFAC: UFOK A (7.80), and UFAC: UFOK B (7.75) had the highest values followed by sample UFAC: UFOK C (7.40), UFAC: UFOK D (7.00) and UFAC: UFOK E (6.90) which were least as shown in Table 7. There was no significant (p < 0.05) difference in samples UFAC, UFAC: UFOK A, UFAC: UFOK B and UFAC: UFOK C which had highest addition of acha. However, as the addition of acha decreased the colour of the toasted breakfast cereals decreased. This is in agreement to the findings of Chukwu and Abdul-kadir (2008) which showed that acha flour has smooth and bright colour, thus increased addition acha caused an increase in the acceptability of the colour of the breakfast cereals.
Table 8 shows the sensory scores of toasted breakfast cereals formulated from blends of acha and 12 h-fermented “okara” flours. The values ranged from 7.25 to 7.95 in which sample UFAC: FEOK 1E (7.95) had the highest value and sample UFAC: FEOK 1B (7.25) had the lowest value. As the addition of acha increase the color also increased (Table 8). This agreed with the findings of Chukwu and Abdul-kadir (2008) which showed that acha flour has smooth and bright colour, thus increased addition acha caused an increase in the acceptability of the colour of the breakfast cereals. There was no significant (p < 0.05) difference between the samples probably because addition of the 12 h fermented okara flour did not have much effect on the colour of the product.
Table 9 shows the sensory scores of toasted breakfast cereals formulated from blends of acha and 24 h-fermented “okara” flours. It hat the colour values ranged from 7.00 to 8.25 where sample UFAC: FEOK 2C (8.25) has the highest value and sample UFAC: FEOK 2A (7.00) has the lowest value. There was no significant (p < 0.05) difference in samples UFAC: FEOK 2A, UFAC: FEOK 2B, UFAC: FEOK 2D, and UFAC: FEOK 2E but there was significant difference between sample UFAC: FEOK 2C and UFAC: FEOK 2A, UFAC: FEOK 2B, UFAC: FEOK 2D and UFAC: FEOK 2E. This could be due to the inability of the panelists to difference in the colour variation.
Table 10 shows the sensory scores of toasted breakfast cereals formulated from blends of acha and 36 h-fermented “okara” flours. There was no significant (p < 0.05) difference for all the samples as regards the colour as shown. The value for all the sample UFAC: FEOK 3A are (7.60), sample UFAC: FEOK 3B (7.95), samples UFAC: FEOK 3C (7.60), samples UFAC: FEOK 3D (7.90) and samples UFAC: FEOK 3E (8.00). The samples had acceptable colour. This could be that the panelists liked the products in terms of these sensory evaluations or inability of the panelists to observe the difference in the sensory parameters. This result is similar to that of Chukwu and Abdul-kadir (2008) which revealed that acha contributed to good colour, appearance taste, mouthfeel and after taste to breakfast cereals, Fermentation improved the sensory (organoleptic), functionality and nutritional properties of food products (Hutkins 2006).
The colour was not significantly (p < 0.05) different as shown in Table 11. This could be that the panelists liked the products in terms of these sensory evaluations or inability of the panelists to observe the difference in the different proportions of acha:48 h FEOK product.
Flavour
Table 7 showed that flavour values ranged from 7.55 to 8.35. Sample UFAC: UFOK C (8.35) had the highest the value, whereas samples UFAC: UFOK D (8.10), UFAC: UFOK E (8.10) and UFAC: UFOK B (7.75), were the second highest, and samples UFAC (7.55) and UFAC: UFOK A (7.55) were the least. There was no significant (p < 0.05) in samples UFAC and UFAC: UFOK A, and UFAC: UFOK B, UFAC: UFOK D and UFAC: UFOK E. The products were highly rated, thus signifies that they had good flavour. The higher the addition of okara, the greater the acceptability of the products. This could possibly be due to the soy flavor of the product which has good aroma.
The flavor values ranged from 7.15 to 8.15. Sample UFAC: FEOK 1E (8.15) as shown in Table 8 had the highest value whereas sample UFAC: FEOK 1A (7.15) had the lowest value. Although there was significant difference between the samples, an increase in the quantity of fermented okara added caused an evident increase in the acceptability of the products. This could be attributed to the flavour component of fermented okara which agreed to the findings of Fei et al. (2013) who stated that increased addition of okara to noodles improved the flavour component of the product. Thus, fermentation improved the flavour of the products.
The range of values for flavour was 8.10 to 8.45 where the value of the samples are UFAC: FEOK 2A (8.30), UFAC: FEOK 2B (8.10), UFAC: FEOK 2C (8.45), UFAC: FEOK 2D (8.20), and UFAC: FEOK 2E (8.20) as shown in Table 8. These values were quiet high. There was no significant difference (p < 0.05) in all the samples possibly because the panelists liked the products or the inability of the panelists to observe the differences.
There was no significant (p < 0.05) difference for all the samples as regards the flavour as shown in Table 10. The value for all the samples; UFAC: FEOK 3A are (7.30), sample UFAC: FEOK 3B (7.65), samples UFAC: FEOK 3C (7.60), sample UFAC: FEOK 3D (7.65) and samples UFAC: FEOK 3E (7.95). The samples had acceptable flavour. This could be that the panelists liked the products in terms of these sensory evaluations or inability of the panelists to observe the difference in the sensory parameters. This result is similar to that of Chukwu and Abdul-kadir (2008) which revealed that acha contributed to good flavour of the breakfast cereals, Fermentation improved the sensory (organoleptic), functionality and nutritional properties of food products (Hutkins 2006).
The value for flavor ranged from 7.55 to 8.60 where the highest value was sample UFAC: FEOK 4D (8.60) and the lowest value was sample UFAC: FEOK 4E (7.55) as shown in Table 11. There was significant (p ˂ 0.05) difference between samples UFAC: FEOK 4B, UFAC: FEOK 4C, UFAC: FEOK 4E and UFAC: FEOK 4A, and UFAC: FEOK 4D. This could probably be due to the different proportion of acha and okara incorporated into the different products and the perception of the panelists.
Mouthfeel and aftertaste
Table 7 shows the sensory scores for both mouthfeel and aftertaste. There was no significant (p < 0.05) difference in mouthfeel and after taste for all the samples. The values for mouthfeel ranged from 7.20 to 8.05 where sample UFAC was 7.20, UFAC: UFOK A (7.40), UFAC: UFOK B (7.55), UFAC: UFOK C (8.05), UFAC: UFOK D (7.80) and UFAC: UFOK E (7.85), whereas the values for after taste ranged from 7.35 to 7.90 where sample UFAC was 7.90, UFAC: UFOK A (7.75), UFAC: UFOK B (7.85), UFAC: UFOK C (7.75), UFAC: UFOK D (7.45) and UFAC: UFOK E (7.35). This could possibly be that the panelists liked the products or there was no much effect of okara on the mouthfeel and aftertaste.
The mouthfeel had range of values from 7.40 to 8.15 where sample UFAC: FEOK 1A was 7.40, UFAC: FEOK 1B (7.50), UFAC: FEOK 1C (7.60), UFAC: FEOK 1D (8.15), and UFAC: FEOK 1E (7.90) as shown in Table 8. There was no significant (p < 0.05) difference in all the samples as regards mouthfeel. This could be due to inability of the panelists to differentiate the proportion of acha:fermented okara added as regards mouthfeel. The range of values for the after taste was 6.90 to 7.50 where sample UFAC: FEOK 1D (7.50) had the highest value and sample UFAC: FEOK 1B (6.90) had the lowest value. There was significant (p < 0.05) difference between the samples. Increased addition of fermented okara increased the acceptability of the products and gave a well after taste to the products. This was in agreement with the result obtained by Fei et al. (2013) which stated that addition of okara to noodles influenced its taste and quality. Due to this positive influence, he stated that “optimum addition of okara should be selected”.
Table 9 showed that the scores for mouthfeel ranged from 6.65 to 8.10 where sample UFAC: FEOK 2B (6.55) was the lowest and sample UFAC: FEOK 2A (8.10) was the highest. There was significant difference between samples UFAC: FEOK 2A, UFAC: FEOK 2C, UFAC: FEOK 2D and samples UFAC: FEOK 2B, UFAC: FEOK 2E. The sample with highest value (70:30 acha:fermented okara) had higher amount of acha and this agreed to the findings of Chukwu and Abdul-kadir (2008) which showed that acha has a good mouthfeel. The after taste ranged from 7.20 to 8.10. Sample UFAC: FEOK 2E (8.10) had the highest value whereas sample UFAC: FEOK 2C (7.20) had the lowest value (table 9). There was significant (p ˂ 0.05) difference between samples UFAC: FEOK 2C and UFAC: FEOK 2D and samples UFAC: FEOK 2A, UFAC: FEOK 2B, UFAC: FEOK 2E. This could have been in the ability of the consumers to detect the after taste. The after taste was liked in the increase of okara.
There was no significant (p < 0.05) difference for all the samples as regards the mouthfeel as shown in Table 10. The value for all the sample UFAC: FEOK 3A are (7.45), sample UFAC: FEOK 3B (7.80), sample UFAC: FEOK 3C (8.20), sample UFAC: FEOK 3D (8.10) and sample UFAC: FEOK 3E (8.25). The samples had acceptable mouthfeel. This could be that the panelists liked the products in terms of these sensory evaluations or inability of the panelists to observe the difference in the sensory parameters. This result is similar to that of Chukwu and Abdul-kadir (2008) which revealed that acha contributed to good flavor, texture, colour, appearance taste, and after taste to breakfast cereals, Fermentation improved the sensory (organoleptic), functionality and nutritional properties of food products (Hutkins 2006).
The values for aftertaste ranged from 6.70 to 7.55 where sample UFAC: FEOK 3E had the highest value and sample UFAC: FEOK 3D had the lowest value (Table 10). Although there was significant (p < 0.05) difference between the samples, they were above the mean value (4.5) for a 9-point Hedonic scale used for sensory evaluation. Therefore, all the products are acceptable in their after taste.
The mouthfeel ranged from 7.35 to 8.40 as presented in Table 11. Sample UFAC: FEOK 4D had the highest value whereas sample UFAC: FEOK 4B was the lowest. This was similar to the result obtained by Fei et al. (2013) who stated that increased addition of okara increased the hardness and chewiness of noodles produced with okara powder and wheat flour. Although there was significant difference between the samples, they were highly rated probably the consumer liked the mouthfeel of the product. Table 11 showed that the after taste scores ranged from 7.50 to 8.20. Sample UFAC: FEOK 4D had the highest value whereas sample UFAC: FEOK 4E had the lowest value. Although there was significant (p < 0.05) difference between the samples, they were highly rated and this suggests that the products had acceptable after taste.
Texture
The texture of the products ranged from 6.55 to 7.85 where sample UFAC: UFOK C had the highest value (7.85) and sample UFAC: UFOK D had the lowest value (6.55) as shown in Table 7. There was no significant difference in samples UFAC (6.95), UFAC: UFOK A (6.85), UFAC: UFOK B (6.90), UFAC: UFOK D (6.55) and UFAC: UFOK E (6.75). The texture of the products was highly rated for samples that had more of acha to okara protein. This could be due the smooth or fine texture of acha. Similar result was obtained by Chukwu and Abdul-kadir (2008) which showed that acha flour had a smooth texture when used to produce breakfast cereals.
The values for texture ranged from 7.05 to 7.60 where sample UFAC: FEOK 1A had a value of 7.05, UFAC: FEOK 1B (7.40), UFAC: FEOK 1C (7.25), UFAC: FEOK 1D (7.60), and UFAC: FEOK 1E (7.50) as shown in Table 8. There was no significant (p < 0.05) difference in all the samples as regards texture. This could be due to inability of the panelists to differentiate the proportion of acha:fermented okara.
The texture had a range of values 7.25 to 8.10. Sample UFAC: FEOK 3E (8.10) had the highest value whereas sample UFAC: FEOK 3B (7.25) had the lowest value (Table 10). There was no significant (p > 0.05) difference in samples UFAC: FEOK 3A, UFAC: FEOK 3B, and UFAC: FEOK 3C, but there was significant (p < 0.05) difference in samples UFAC: FEOK 3A, UFAC: FEOK 3B, UFAC: FEOK 3C and UFAC: FEOK 3D, and UFAC: FEOK 3E. Increase in addition of acha, increased the acceptability of the product.
The texture ranged from 6.75 to 7.40 where sample UFAC: FEOK 4A had the highest value and sample UFAC: FEOK 4E was the lowest (Table 11). There was significant (p ˂ 0.05) difference between the samples. It was discovered that sample UFAC: FEOK 4A which had 90 % acha was most preferred and the preference decreased as the addition of acha decrease. This agreed to the findings of Chukwu and Abdul-kadir (2008) which showed that acha has good texture.
Taste
The values for taste ranged from 7.00 to 8.10 where samples UFAC: UFOK B (8.10) and UFAC: UFOK C (8.10) have the highest values and sample UFAC: UFAC (7.00) with the lowest value (Table 7). There was no significant difference (p < 0.05) in sample UFAC: UFOK B, UFAC: UFOK C, UFAC: UFOK D and UFAC: UFOK E but this differed from sample UFAC: UFAC and UFAC: UFOK A. The samples with increased addition of okara were highly rated. This could be attributed to increase in the soy flavor of okara which impart a desirable taste to breakfast cereals.
Table 8 showed that the taste had values ranging from 6.55 to 7.30 where sample UFAC: FEOK 1A was 6.55, UFAC: FEOK 1B (6.55), UFAC: FEOK 1C (6.70), UFAC: FEOK 1D (6.80) and UFAC: FEOK 1E (7.30). There was no significant (p < 0.05) difference in all the samples as regards taste. This could be due to inability of the panelists to differentiate the proportion of acha:fermented okara with respect to taste.
The taste ranged from 7.35 to 7.90 where sample UFAC: FEOK 2A was 7.40, UFAC: FEOK 2B (7.40), UFAC: FEOK 2C (7.55), UFAC: FEOK 2D (7.90), and sample UFAC: FEOK 2E was 7.35 as shown in Table 9. There was no significant difference (p < 0.05) in all the samples possibly because the panelists liked the products or the inability of the panelists to observe the differences.
The value for all the samples UFAC: FEOK 3A are 7.60, 7.30, 7.45, 7.20, samples UFAC: FEOK 3B (7.95), (7.65), (7.80), (6.75), samples UFAC: FEOK 3C (7.60), (7.60), (8.20), (6.65), samples UFAC: FEOK 3D (7.90), (7.65), (8.10), (6.75) and samples UFAC: FEOK 3E (8.00), (7.95), (8.25) and (7.25). There was no significant (p < 0.05) difference for all the samples as regards the taste as shown in Table 10. The samples had acceptable taste. This could be that the panelists liked the products in terms of these sensory evaluations or inability of the panelists to observe the difference in the taste. This result is similar to that of Chukwu and Abdul-Kadir (2008) which revealed that acha contributed to a good taste to breakfast cereals. Fermentation improved the sensory (organoleptic), functionality and nutritional properties of food products (Hutkins 2006).
Overall acceptability
The overall acceptability of the products ranged from 6.70 to 8.05 where sample UFAC was 6.70 and sample UFAC: UFOK C (8.05) as seen in Table 7. There was significant difference (p < 0.05) in all the samples. All the samples had good rating for all the sensory attributes since they were high than the mean of 4.5 for a 9-point hedonic scale used for the sensory. Therefore, this agreed with the research of Chukwu and Abdul-kadir (2008) which showed that acha as a cereal crop impart good flavor, texture, color, appearance, taste, mouthfeel and after tate to the breakfast cereal products. Sample UFAC, (control, containing 100 % acha) had the least acceptability whereas sample UFAC: UFOK C (70:30 acha:okara) was most preferred. This signifies that breakfast cereals formulated with 70:30 acha:okara is highly preferred by consumers and agrees to the fact that cereals and legumes complement each other in both traditional food preparation and for industrial uses (Oke, 1975; Nkama 1990).
The overall acceptability (Table 8) of the products had a range of values from 6.75 to 7.70. Sample UFAC: FEOK 1B (6.75) had the lowest value whereas sample UFAC: FEOK 1D (7.70) had the highest values (Table 8). Sample UFAC: FEOK 1D (60:40 acha:fermented okara) was most preferred. This probably may be due to the higher quantity of acha to okara which imparted a smooth texture to the product. Although there was significant (p < 0.05) difference between the samples, they all passed the mean value (4.5) for a 9-point Hedonic scale used for sensory evaluation. Therefore, all the products are acceptable.
The overall acceptability values ranged from 7.30 to 8.15 where sample UFAC: FEOK 2C (8.15) was the highest and sample UFAC: FEOK 2D (7.30) was the lowest as shown in Table 9. There was no significant (p > 0.05) difference in samples UFAC: FEOK 2A, UFAC: FEOK 2B and UFAC: FEOK 2D, UFAC: FEOK 2E. This showed that almost all the samples are liked. Although all the samples were highly rated, sample UFAC: FEOK 2C (70:30 acha:fermented okara) was most preferred.
The range of values for overall acceptability was 6.70 to 7.85. Sample UFAC: FEOK 3E had the highest value and sample UFAC: FEOK 3A had the lowest value as shown in Table 10. There was significant (p < 0.05) difference among the samples. This could be in the difference in consumers’ choice or preference. The rate of acceptability increased as fermented okara (36 hrs) increased, probably due to the influence of the okara. Sample UFAC: FEOK 3C (50:50) acha:fermented okara blend was highly rated, thus mostly accepted.
The values for overall acceptability ranged from 6.85 to 7.90 where sample UFAC: FEOK 4C (70:30 acha:fermented okara) had the highest value and sample UFAC: FEOK 4E (50:50) was the least rated (Table 11). This implies that sample UFAC: FEOK 4C (70:30) acha:48 h fermented okara was most preferred. From the sensory evaluation of the toasted breakfast cereals with blends of acha:unfermented okara, and acha:fermented okara (12, 24, 36 and 48 h), the result showed that all the products were highly rated (Table 11). Thus formulation of breakfast cereals can be made with proportion of 100 % acha, 70:30, 60:40, or 50:50 blends of acha:fermented or unfermented okara. Fermentation improved the texture, flavour, appearance, nutrition and safety, palatability and acceptability according to Sahana and Hafiz (2003).
Micronutrient Compositions of toasted breakfast cereals formulated for blends of acha and fermented okara
Table 10 shows the micronutrient compositions of toasted breakfast cereals formulated from blends of acha and fermented okara. The vitamin B2 and B3 composition of the products increased whereas the vitamin A and B1 decreased. The values for the vitamin A ranged from 11.13 to 83.05 IU where sample UFAC, (83.05 IU) had the highest value, followed by sample UFAC: FEOK 1C (34.55 IU) and then sample UFAC: FEOK 4C (11.13 IU). There was significant difference in the samples. This could possibly be due to the different treatment given to the raw materials used in formulating the products. Vitamin B1 content ranged from 8.00 to 10.04 mg/100 g where sample UFAC, 100 % acha (10.04) was the highest, sample UFAC: FEOK 1C was 8.03 mg/100 g and sample UFAC: FEOK 4C was 8.00 mg/100 g. There was significant (p < 0.05) difference between samples UFAC: FEOK 1C, UFAC: FEOK 4C and UFAC but there was no significant (p < 0.05) difference in samples UFAC: FEOK 1C and UFAC: FEOK 4C. The result showed that acha is likely to contain more vitamin B1 to okara.
Vitamin B2 increased from 0.04 to 0.55 where sample UFAC: FEOK 4C (0.55 mg/100 g) was the highest, followed by sample UFAC: FEOK 1C (0.40 mg/100 g) and sample UFAC (0.04 mg/100 g) was the least. This showed that fermented okara is more abundant in vitamin B2 to acha. Thus, fermentation improved the vitamin B2 content of the products. Also, there was an increase in the vitamin B3 content of the product. This ranged from 0.53 to 2.85 mg/100 g where sample UFAC: FEOK 4C (2.85 mg/100 g) was the highest, sample UFAC: FEOK 1C was 1.72 mg/100 g and sample UFAC (0.53 mg/100 g) was the lowest. This might be attributed to the presence of phytochemicals, such as isoflavones (Wang and Wixon 1999) which are significantly enhanced after fermentation. The highly fermented okara had the highest vitamin B3 content, thus fermentation improved the vitamin B3 content of the products. Incorporating legumes to cereals helps to improve the vitamin content of foods.
The mineral contents of all the products were improved (Table 10). Sample UFAC was 0.45 mg/100 g, UFAC: FEOK 1C was 0.65 mg/100 g while sample UFAC: FEOK 4C was 1.10 mg/100 g for the iron content. The calcium content ranged from 1.97 to 3.23 mg/100 g where sample A was 1.97 mg/100 g, UFAC: FEOK 1C was 1.99 mg/100 g and sample UFAC: FEOK 4C was 3.23 mg/100 g. There was also an increase in the phosphorus content of the products and Zinc content of the products. The phosphorus content ranged from 188.30 to 289.75 mg/100 g where sample UFAC: FEOK 4C (289.75 mg/100 g) was the highest, followed by UFAC: FEOK 1C (192.80 mg/100 g) and lastly sample UFAC which was 188.30 mg/100 g. The zinc content was relatively minimal in its amount contained in the product. Sample UFAC: FEOK 4C (0.29 mg/100 g) was the highest, followed by sample UFAC: FEOK 1C (0.14 mg/100 g) and finally sample UFAC (0.09 mg/100 g) which was the lowest. The result showed that fermentation improved the nutritional composition (mineral composition) of the toasted breakfast cereals when compared to the control (100 % acha) or sample UFAC: FEOK 1C (70:30 acha:unfermented okara breakfast cereals). Also, since the ash content determination is a measure of mineral levels in the substrates, it can be inferred that fermentation contributed to the higher levels of the minerals obtained. Similar improved levels of ash content, following fermentation have been reported by O’Toole (1999) using okara as a substrate and Rashad et al. (2011). Calcium and phosphorus are important for the development of strong bones and teeth while iron is important for heme iron in red blood formation and healthy living of the children Ugwuona et al. (2012).
Proximate Composition of toasted breakfast cereals formulated for blends of acha and fermented okara (48-h)
Table 11 shows the proximate composition of toasted breakfast cereals formulated from blends of acha, acha:unfermented and acha:fermented okara (48 h). The random selection of these products was based on proximate analysis of okara (unfermented and fermented) flour, acha flour, functional properties of the different flour, and sensory evaluation of products and were found to be of highest quality and consumers’ rating. The optimal fermentation time was 48 h whereas the minimal fermentation time was 0 h which served as the basis for selection.
The protein content of the products ranged from 17.16 to 21.30 % where sample UFAC which was the control had a protein content of 17.16 % whereas sample UFAC: FEOK 1C had a protein content of 17.86 % (Table 11). Sample UFAC: FEOK 4C had a protein value of 21.86 %. There was significant (p < 0.05) difference between the samples. This is due to fermentation and was in agreement to the findings of Ugwuona et al. (2012) who stated that fermentation increased the protein content of okara which was incorporated into acha for infant feeding. The protein content of the acha toasted breakfast cereals was significantly (p < 0.05) lower than those of other samples which had either unfermented okara or fermented okara. Also the increase in protein could possibly be due addition of okara (legume) and was in agreement with the report of Temple and Bassa (1991) which revealed that addition of legume to cereals improves the level of protein.
The fat content of the toasted breakfast cereals ranged from 0.70 to 2.21 % (Table 11). Sample UFAC had a fat content of 0.70 %, sample UFAC: FEOK 1C was 0.83 % whereas sample UFAC: FEOK 4C was 2.21 %. There was significant difference between the products possibly due to the different treatment given to them during processing of flour used in the formulation. There was an increase in the fat content of the products probably fermentation increased the fat content. This was similar to the result obtained by Ugwuona et al. (2012). This trend of increase in fat is in line with the report of Temple and Bassa (1991) which revealed that addition of legume to cereals improves the level of protein.
Table 11 showed that the ash content ranged from 1.80 to 2.21 % where sample UFAC (1.80 %) had the least value and sample UFAC: FEOK 4C (2.21 %) had the highest value. There was significant (p < 0.05) difference between the samples probably due to processing technique, heat treatment, and difference in cultivar. The ash contents of all the samples were improved probably due to fermentation. This agreed with the result obtained by Ugwuona et al. (2012) which showed that fermentation increased the fat content of infant food formulated from fermented soybean flour and acha.
The values for the crude fibre ranged from 3.55 to 4.24 % with sample UFAC: FEOK 1C (4.24 %) having the highest value and sample UFAC (3.55 %) with the lowest value as presented in Table 11. Addition of fermented okara to the cereals (acha) increased the fibre content of the toasted breakfast cereals. This was probably due the fact that okara has high fibre content and also when fermented increases its fibre content. This increase was expected due to the complementation of acha flour with okara (unfermented or fermented) that contain higher amount of most nutrients.
The moisture content 7.62 to 10.25 %. Sample UFAC (10.25) was the highest, sample UFAC: FEOK 1C (11.99 %) whereas sample UFAC: FEOK 4C (7.62 %) as shown in Table 11. The 70:30 acha:unfermented okara toasted breakfast cereal had the highest moisture content whereas the 70:30 acha:fermented okara toasted breakfast cereal had the lowest moisture content. This implied that highly fermented sample has decreased moisture content and was more nutrient-dense since it contains less moisture.
Carbohydrate content ranged from 62.44 to 66.48 % where sample UFAC (66.44 %) had the highest value, followed by sample UFAC: FEOK 1C (63.16 %) and then sample UFAC: FEOK 4C (62.44 %) as shown in Table 11. There was significant difference between the samples. The carbohydrate content decreased as okara was added. This agreed with the findings of Ugwuona et al. (2012) which stated that carbohydrate decreased as fermented soy flour was added to acha. Also, Rashad et al. (2011) reported that there was a significant decrease in the carbohydrate contents of all the fermented okara samples by microorganisms. This however was expected to lower the carbohydrate content of the toasted breakfast cereals.
Microbial count of toasted breakfast cereals formulated for blends of acha and fermented okara (48-h)
Table 12 shows the total viable and mould counts of the toasted breakfast cereals formulated from blends of acha and fermented okara (48 h). The total viable count of the product ranged from 0 to 1.4 × 10 cfu/g, where sample UFAC which was unfermented okara had the highest value and sample FEOK 4C which was 48 h fermented had the lowest value. As fermentation increased, the microbial load increased. This could be that the relative humidity of the environment used for storage which was transparent plastic container was favorable for the micro-organisms to have gained entry and thrived at the first few weeks and probably due to improper handling. The total viable counts of microorganisms in the food samples was not high when assessed using the guidelines for microbiological quality of ready-to-eat foods (Anon 2001) which indicates (104 cfu/g) for satisfactory, (104 to 105 cfu/g) acceptable and unsatisfactory quality being (105). The total bacterial and fungal counts in a food product may be a consequence of the low level of hygiene maintained after post processing preservation, storage of the products and formulation. Previous studies had revealed that viable counts of foods prepared in advance and kept at ambient temperatures (20 to 46 °C) for a long period of time (4 h or more) reach critical levels (Abdulla and Nick 1995; Francina and Von Alexander 1999).
Microorganisms play significant role in the determination of shelf life of food products. They are usually responsible for spoilage of many food items. A high aerobic plate count (APC) could indicate the presence mixed population of microorganisms, which may consist of spoilage types. There was no mould growth detected. Limits of microbial counts have been recommended in most foods to keep them safe for consumption (Porter 1978). Thus, there is need to properly store product with packaging best suitable for it and in the best environment in order to prevent contamination and more microbes from gaining entrance. However, it is preferable to utilize these flours as soon as possible especially okara flour because of the high protein content which may cause easy spoilage.
Conclusion
Fermentation of okara increased the nutritional composition of the products. The mineral contents (calcium, iron, phosphorus and zinc) increased significantly (p < 0.05) as a result of the increase in the ash content which is a measure of mineral levels in the products. Thus, it can be inferred that fermentation contributed to the higher levels of the mineral obtained. Fermentation increased the functional properties, proximate composition and particle size distribution of the products. The high nutrient density and low bulk of the flours could serve as good base ingredients to be used especially in complementary foods, for young children feeding breakfast cereals to enhance dietary diversification. The study showed that fermentation increased moisture (4.71 to 6.11 %), crude fibre (36.62 to 46.18 %), and carbohydrate (2.50 to 2.71 %) contents of the flour samples, whereas there was a decrease in the fat (16.29 to 13.27 %), ash (6.36 to 1.41 %) and crude protein (33.53 to 30.32 %) contents. The proximate composition of the selected products revealed that protein (17.86 to 21.30 %), fat (0.83 to 2.21 %), ash (2.02 to 2.21 %) and crude fibre (4.16 to 4.24 %) contents increased, whereas the moisture (11.99 to 7.62 %) and carbohydrate (63.16 to 62.44 %) content decreased. There were few microbial counts in the flour samples owing to the processing which was carried out in a clean environment, the oven drying and packaging. Fermentation also influenced the vitamin content. Vitamin A and B1 content of the products decreased while the vitamin B2 and B3 contents increased. At the end of the research, the consumer’s preferred sample 70:30 UFAC: UFOK TBFC and 70:30 UFAC: FEOK 4C TBFC. Thus, breakfast cereal formulated from unfermented and fermented okara (0 to 48 h fermentation) would serve as a good dietary nutrient-dense breakfast cereals for both children and adults. Fermentation caused appreciable changes in texture, flavour, appearance, nutrition, safety, palatability and acceptability by developing improved flavours and textures.
It is recommended that further study should be carried out on the health benefits of consuming acha and okara. It is also recommended that consumers should be introduced to acha and okara as they are both not popularly known, especially in the eastern part of Nigeria where this work was carried out. It is recommended that further study be carried out on the following: Nutritional composition of other fermented products; Shelf-stability studies on acha and okara (fermented and unfermented); Isolation and characterization of the fermenting microflora in the products; Feeding trials using bioassay.
References
Abdulla AA, Nick JS (1995) Hazard Analysis and critical control point evaluation of school food programmes in Bahrain. J Food Prot 59:282–286
Adeyemi IA, Beckley O (1986) Effect of period of maize fermentation and souring on chemical properties and amyl grapy on millet biscuit characteristics. Plant Foods Hum Nutr 56:195–202
Ahn SH, Oh SC, Choi I, Han G, Jeong H, Kim K, Yoon Y, Yang I (2010) Environmentally friendly wood preservatives formulated with enzymatic-hydrolyzed okara, copper and/or boron salts. J Hazard Mater 178:604–611
Akubor PI, Badifu GIO (2001) Plant Foods for human nutrition 56: 105–115 influence of pH and sodium chloride on selected functional and physical properties of African breadfruit (Treculia Africana Decne) kernel flour. Kluwer Academic Publishers, The Netherlands
Anon (2001) Microbiological guideline for ready-to-eat food. Recommendations for food safety monitoring in Hong Kong by the expert panel on microbiological safety of food, Hong Kong, p 7
Anon (2005) Okara: Overview of Current Utilization. Retrieved on 17th April, 2013, pp 1–6.
Anon (2012) Fonio www.fonio.cirad.fr/en/index.html. Retrieved on December 1, 2012
AOAC (2010) Official method of analysis, 18th edn. Association of Official Analytical Chemistry, Washington
Aoki H, Uda I, Tagami K, Furuya Y, Endo Y, Fujimoto K (2003) The production of a new tempeh-like fermented soybean containing a high level of γ-aminobutyric acid by anaerobic incubation with Rhizopus. Biosci Biotechnol Biochem 67:1018–1023
Arogundade LA, Eromosele IC, Ademuyiwa O (2011) Rheological properties of African yam bean (Sphenostylis stenocarpa Hochst. Ex A. Rich.) calcium proteinate and isoelectric protein isolates. LWT Food Sci Technol 44:524–534
Asiedu JJ (1989) Processing of tropical and subtropical crops. Macmillam Education publisher Ltd., London
Barkat MZ, Shehab SK, Darwish N, Zahermy EI (1973) Determination of ascorbic acid from plants. Anal Biochem 53:225–245
Baser KHC, Kirimer N, Kurkcuogi M, Demirci B (2000) Chemistry of natural compounds. J Food Chem 36:356–359
Belton PS, Nuttall JR (2002) Pseudocereals and less common cereals: grains properties and utilisation potential. Springer, Berlin Heidelberg
Bhandange DG (2010) Phytochemical and antimicrobial studies of some ethnomedicinal plants from Akola and washim district. A thesis submitted to Amravati University, Amravati
Bugusu BA, Campanella O, Hamaker BR (2001) Improvement of sorghum-wheat composite dough rheological properties and bread making quality through Zein addition. J Cereal Chem 78(1):31–35
Chakraverty A (2004) Post harvest of oil seeds. Oxford Publishing Company, Private Limited, New Delhi, p 339
Chukwu O, Abdul-Kadir JA (2008) Proximate (Chemical) composition of Acha (Digitaria exilis and Digitaria iburua) grains. J Food Technol 6:214–216
Cruz JF (2012) Fonio: a small grain with potential. Ileia publishers CIRAD-CA, Breaton, France
Das DVM, Weeks G (1979) Effects of polyunsaturated fatty acids on the growth and differentiation of the cellular slime mold, Dictyostelium discoideum. Exp Cell Res 118:237–243
Duranti M (2006) Grain legume proteins and nutraceutical properties- review. Fitoterapia 77:67–82
Ensminger AH (1994) Foods and nutrition encyclopedia, 2nd edn. CRC Press, Boca Ratton, p 78
Enweremadu CC, Waheed MA, Adeala A (2008) The energy potential of brewers spent grains for breweries in Nigeria. J Eng Appl Sci 3:175–177
FAO (1998) Food and nutrition in the management of group feeding programmes. FAO and nutrition, paper 23. Review 1:150–157
FDA (2013) Revised guidelines for the assessment of microbiological quality of processed foods. Department of Health, Food and Drug Administration, Republic of Philippines, pp 5–6
Fei L, Zhenkun C, Yang L, Bo L (2013) The Effect of okara on the qualities of noodle and steamed bread. Advanced J Food Scie Technol 5(7):960–968
Francina MM, Von Alexander H (1999) Microbiological quality and safety of ready-to- eat street vendor foods in Johannesburg, south africa. J Food Prot 62:1278–1284
Harrigan WF, McCance ME (1981) Laboratory methods in food and dairy microbiology. Academic Press Inc. Ltd, London, pp 106–303
Henderson SM, Perry RL (1976) Agricultural process engineering. AVI Publishing Co. Inc., Westport, Connecticut
Hutkins RW (2006) Microbiology and Technology of fermented foods. Blackwell publishing, pp 12–13
Ibrahim A (2001) Hungry Rice (Acha): a neglected cereal crop. NAQAS Newslet 1(4):4–5
Igbabul BD, Bello FA, Ekeh CN (2014) Proximate composition and functional properties of wheat, sweet potato and hamburger bean. Global Advanced Res J Food Scie Technol 3(4):118–124
Ihekoronye AI, Ngoddy PO (1985) Integrated food science and technology for the tropics. Macmillan publishers Ltd., London, pp 345–360
Iluyemi FB, Hanafi MM, Radziah O, Kamarudin MS (2006) Fungal solid state culture of palm kernel cake. Bioresour Technol 97:477–482
ISO (2011) Determination of wettability (wetting time) of instant dried milk, pp 8–10
Iwe MO, Onalope OO (2001) Effect of extruded full fat soy flour into sweet potato flour on functional properties of the mixture. J Sustain Agric Environ 3:109–117
Jiménez- Escrig A, Tenorio MD, Espinosa-Martos I, Ruperéz P (2008) Health promoting effects of a dietary fiber concentrate from the soybean byproduct okara in rats. J Agric Food Chem 56:7495–7501
Kanu PJ, Sandy EH, Kandeh BA, Behsoon JZ, Huiming Z (2009) Production and evaluation of breakfast cereal-based porridge mixed with sesame and pigeon peas for adults. Pak J Nutr 8(9):1335–1343
Karuna D, Noel D, Dilip K (1996) Food and nutrition bulletin. United Nation 17(2)
Kirk RS, Sawyer R (1998) Pearson’s composition and analysis of foods, 9th edn. Longman group Ltd., London, p 37
Lateef A, Oloke JK, Gueguim KEB, Oyeniyi SO, Onifade OR, Oyeleye AO, Oladosu OC, Oyelami AO (2008) Improving the quality of agro-wastes by solid-state fermentation: enhanced antioxidant activities and nutritional qualities. World J Microbiol Biotechnol 24:2369–2374
Leach HW, McCowen LD, Scoch TJ (1959) Structure of starch granule 1. Swelling and solubility patterns of various starches. Cereal Chem 36:534–544
Lescano CAA, Rocha SCS and Fraile V (2005) Drying of the soymilk residue “okara” in spouted bed. 2nd Mercosur Congress on Chemical Engineering held in Brazil
Li B, Zhang Y, Yang H, Li R (2008) Effect of drying methods on functional properties of bean curd dregs. J Henan Ins Scie Technol 36:64–66
Loos PJ, Hood LF, Graham AJ (1981) Isolation and characterization of starch from breadfruit. Cereal Chem 58:282–286
Malleshi NG, Desikachar HSR (1982) Formulation weaning food with low hot paste viscosity based on malted ragi and green gram flour. J Food Sci 19:193–197
Mateos-Aparicio I, Redondo-Cuenca A, Villanueva-Suárez MJ, Zapata-Revilla M, Tenorio-Sanz M (2010) Pea pod, broad bean pod and okara, potential sources of functional compounds. LWT-Food Scie Technol 43:1467–1470
Matsumoto K, Watanabe Y, Yokoyama S (2007) Okara, soybean residue, prevents obesity in a diet-induced murine obesity model. J Bioscie Biotechnol Biochem 71:720–727
Mbaeyi IE (2005) Production and evaluation of breakfast cereal using pigeon-pea (Cajanus cajan) and sorghum (Sorghum bicolor L.). An M.Sc. Dissertation Department of Food Science and Technology, University of Nigeria, Nsukka
Mbaeyi-Nwaoha IE, Onweluzo JC (2013) Functional properties of sorghum (S. bicolor L.) – pigeon pea (Cajanus cajan) flour blends and storage stability of a flaked breakfast formulated from blends. Pak J Nutr 12(4):382–397
Mbata TI, Ikenebomeh MJ, Ezeibe S (2009) Evaluation of mineral content and functional properties of fermented maize (Generic and specific) flour blended with bambara groundnut (Vigna subterranean L). African J Food Scie 3(4):107–112
Merryweather LM, Hill SHA, Robinson AL, Spencer HA, Rudge KA, Taylor GRJ, Wheatcroft AJD, Weeks JJ, Sahibdeen SS, Flynn PJ, Blowers M, Murphy LI, Murray JT, Waites BA, Goodchild ME, Morgan C, Hogarth SL, Buckley DS, Rogers FC (2005) Dictionary of food science and technology. Blackwell Publishing, Oxford, p 392
Nassar AG, Abdel-Hamied AA, El-Naggar EA (2008) Effects of citrus by product on the chemical, rheological and organoleptic characteristics of biscuits. World J Agricul Scie 4(5):612–616
Nkama I (1990) Strategies for Industrial Development of the Indigenous Traditional Cereals Grains and Grain Legume Based Foods of the North Eastern States of Nigeria. In: Strategies for Agro-based Industry: Raw Materials Supply, Processing and Sanitation (Nkama I. and Sopada, P.A. Ed). Proceedings of a 2-day workshop organized by Food Science and Technology (NIFST) of University of Maiduguri, Nigeria, 31 May- 1 June, 1990, p 58
O’Toole DK (1999) Characteristics and use of okara, the soybean residue from soy milk production. A rev J Agricul Food Chem 47:363–371
Ofuya ZM, Akhidue V (2005) The role of pulses in human nutrition: a review. J Appl Sci Environ Manag 9:99–104
Ohno A, Ano T, Shoda M (1993) Production of the antifungal peptide antibiotic, iturin by NB22 in solid state fermentation. J Ferment Bioeng 75:23–27
Okafor GI, Usman G (2015) Physical and functional properties of breakfast cereals from blends of maize, African Yam bean. Defatted Coconut Cake and Sorghum Extract Food Scie Quality Manag 40:25–34, www.iiste.org
Okaka JC (2005) Handling, storage and processing of plant foods. OJC Academic Publishers, Enugu, pp 270–285
Onimawo IA, Egbekun NM (1998) Comprehensive food science and nutrition revised edition. Ambik press, Benin City
Onweluzo JC, Nnabuchi OM (2009) Production and evaluation of porridge-type breakfast product from Treculia Africana and Sorghum bicolor flours. Pak J Nutr 8:731–736
Porter NN (1978) Food science, 3rd edn. AVI Publishers, Westport, pp 406–415
Prescott LM, Harley JP, Klein OA (2005) Microbial nutrition, types of media in microbiology 6th edition. McGraw Hill publishers, New York, pp 93–105
Rashad MM, Mahmoud AE, Abdou HM, Nooman MU (2011) Improvement of nutritional quality and antioxidant activities of yeast fermented soybean curd residue. Afr J Biotechnol 10(28):5504–5513
Recundo-Cuenca A, Villaneuva-Suárez MJ, Mateos-Aparicio I (2008) Soybean seeds and its by-product okara as sources of dietary fibre. Measurement by AOAC and englyst methods. Food Chem 108:1099–1105
Riaz M (2006) Soy application in food. CRC Press, Boca Raton
Sahana P, Hafiz F (2003) Fermented cereal from indigenous Raw materials. Pak J Nutr 2(5):289–291
Samadrita S, Minakshi C, Jayati B, Bhattacharya DK (2012) Microbial load of different okara samples based on processing, international. J Sci Environ Technol 1(4):319–330
Sathe SK, Salunkhe DK (1981) Functional properties of the great northern bean (Phaseolus vulgaris L.) proteins-emulsion foaming, viscosity and gelation properties. J Food Sci 46:71–81
Sathe SK, Desphande SS, Salunkhe DK (1982) Functional properties of lupin seed (Lupinus mutabilis) protein and protein concentrates. J Food Sci 47:491–497
Steel RG and Torrie JH (1980) Principles and procedures of statistics: A biometrical approach. McGraw-Hill, New York, 2nd edition
Temple VJ, Bassa JD (1991) Proximate chemical composition of acha (Digitaria exilis) grain. J Food Agric 56:561–563
Tribelhorn RE (1991) Breakfast Cereals. In: Handbook of Cereal Science and Technology, Lorenz, K. J. and Kulp, K, (eds). Published by Marcel Dekker, Incorporated
Ugwuona FU, Awogbenja MD, Ogara JI (2012) Quality evaluation of soy-acha mix for infant feeding. Indian J Scie Res 3(1):43–50
Ukpabi J, Ndimele C (1990) Evaluation of the quality of garri produced in Imo State. Niger Food J 8:105–110
Vishwanathan KH, Singh V, Subramanian R (2011) Influence of particle size on protein extractability from soybean and okara. J Food Eng 102:240–246
Vodouhè SR, Achigan DE, Dansi A and Adoukonou SH (2012) Fonio: A treasure for West Africa Briefing by the International Plant Genetic Resources Institute (West and Central Africa Sub-Office) and Crop, Aromatic and Medicinal plant Biodiversity Research and Development Institute (IRDCAM). Retrieved from www.underutilizedspecies.org/documents/publications/rv. pdf on December 1, 2013
Wang C, Wixon R (1999) Phytochemicals in soybeans: their potential health benefits. Information 10:315–321
Williams PG (2014) The Benefits of Breakfast Cereal Consumption: A Systematic Review of the Evidence Base1–4. Advances in Nutrition 5: 636S–673S; doi:10.3945/an.114.006247. Downloaded from advances.nutrition.org at Nigeria: American Society for Nutrition (ASNA) Sponsored on July 3, 2015
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mbaeyi-Nwaoha, I.E., Uchendu, N.O. Production and evaluation of breakfast cereals from blends of acha and fermented soybean paste (okara). J Food Sci Technol 53, 50–70 (2016). https://doi.org/10.1007/s13197-015-2032-8
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-015-2032-8