Abstract
Antioxidant activities of butylatedhydroxyanisole (BHA) and orange peel powder extract in ghee stored at different storage temperatures (T1:6 ± 2 °C; T2: 32 ± 2 °C; T3:60 ± 2 °C) were evaluated during storage period of 21 days. Peroxide value (PV), thiobarbituric acid (TBA), radical scavenging activity (RSA) and free fatty acids (FFA) of ghee samples were analyzed during the study. PV, TBA and FFA of ghee samples increased significantly while radical scavenging activity (RSA) of ghee samples decreased significantly at accelerated temperature (T3) as compared to the temperatures at T1 and T2. Effect of storage temperature on development of peroxides and TBA of ghee samples was significantly higher than the effect of treatment and storage period while treatment had more significant effect on the change in FFA and RSA as compared to storage temperature and storage period. Ghee incorporated with orange peel extract (OPE) showed stronger activity in quenching DPPH radicals and least development of PV, TBA and FFA than ghee incorporated with BHA and control. The study revealed that orange peel could be a good natural source of antioxidants which can be used in fat rich food products like ghee to retard oxidative deterioration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ghee, anhydrous milk fat (clarified butterfat or butter oil), is usually prepared from cow milk or buffalo milk. Ghee has got typical pleasing and appetizing aroma. It contributes colour and flavor richness to foods. Clarified ghee contains not less than 99.5 % fat and fat is the most valued milk component. Ghee is one of the most widely used milk products in numerous foods for various purposes. In fact, it is considered as the supreme cooking and frying medium. Ghee undergoes oxidative degradation during storage (Shende et al. 2014; Gandhi et al. 2013; Pawar et al. 2012) and the extent of degradation depends mainly on storage temperature (controlling factor for maintaining ghee state either in liquid or solid state), oxygen availability (in unpacked, packed, type of package etc.) and ghee condition (liquid or solid state). Oxidative deterioration degrades color, flavor, aroma and nutritive value of ghee affecting suitability for consumption and reducing shelf-life of the product (Choe and Min 2006; Gandhi et al. 2013; Pawar et al. 2012; Shende et al. 2014). There are numerous evidences correlating oxidized lipids with negative health implications (Pukalskas et al. 2005). Oxidized oils cause cancer, heart disease and early aging in consumers who consume oxidized oils and fats. The use of antioxidants is the most appropriate way to stabilize oils, prevent lipids oxidation and to protect oils from the damages caused by oxidized products such as free radicals (Yassari and Yasari 2013). Hertog et al. (1993) reported that the oxidation of lipids is substantially reduced by adding antioxidants to oils and fats. Propyl gallate (PG), butylatedhydroxyanisole (BHA), tertiary butylhydroquinone (TBHQ) and butylatedhydroxytoluene (BHT) are some of the widely used synthetic antioxidants to retard oxidation and to prolong shelf-life of foods. However, use of synthetic antioxidants has been limited in many countries because of their possible toxic properties for human health and the environment (Ito et al. 1986; Stich 1991); adverse side effects of their use (Yassari and Yasari 2013); use of such substances has been questioned due to their potential health risks and toxicity (Kahl and Kappus 1993). Although synthetic antioxidants are used at low concentrations, there is a need for having antioxidants without side effects because the complications resulting from the long term use of these compounds in man cannot be ignored (Yassari and Yasari 2013). Therefore, the quest for developing antioxidants of natural origin has been increased in recent years and consequently many related studies have been reported (Beddows et al. 2001). Oxidative stability of vidarikand (Pueraria tuberosa), rosemary, green tea (Gandhi et al. 2013); tulsi (Ocimum sanctum L.) leaves (Merai et al. 2003); shatavari (Asparagus racemosus), ashwagandha (Withania somnifera) extracts (Pawar et al. 2012) and clove extracts (Shende et al. 2014) in ghee have been demonstrated. However, these commodities (herbs, spices and medicinal crops), when used as main crop, are costly, not easily accessible/available everywhere at all the time. Hence, there is a need to search for an alternative natural source which is more economical, safe and rich in antioxidant compounds.
Orange fruit, contributes up to 70 % of citrus fruits production, is widely cultivated citrus fruit in tropical and subtropical climates spreading over 130 countries. It is available both in winter and summer season. Sweet orange (Citrus sinensis) is the major citrus fruit produced worldwide (Rouseff 2007) and processed commercially for orange juice in the industries generating huge quantity of peel (peel is the primary by-product range from 20 to 50 % of total fruit weight) which can efficiently be utilized for food supplements, food preservatives and food preparations (Kumar et al. 2011; Anagnostopoulou et al. 2006). Several researchers reported that orange peel is a good natural source of phytoconstituents which exhibit antioxidant activities by reducing the concentration of local free radicals, neutralizing free radicals and by chelating metals (Bombardelli and Morazzoni 1993). Orange peels contain high amount of total phenolics (a phytoconstituents) than edible portions of the fruits (Yassari and Yasari 2013). Antioxidant activities of citrus were due to the presence of total phenolic compounds (Mour et al. 2001). Antioxidant compounds of citrus peel not only play an important and physiological role but are also of commercial interest because of their multitude of applications in the food and pharmaceutical industries activities of the world. Therefore, nowadays, considerable interest is focused on the development and evaluation of natural antioxidants and radical scavengers from plant materials which are rich in polyphenolic compounds (Dubey et al. 2011; Mathur et al. 2011).
Orange peel extract can be used as natural antioxidant (Bombardelli and Morazzoni 1993) in corn oil (Rehman 2006); in soybean oil (Gharahkhani et al. 2010; Abdelaal and Halaweish 2010); in canola oil (Yassari and Yasari 2013) for exhibiting antioxidant activities. The rapid increase in use of natural antioxidants in food industry (Beddows et al. 2001) has attracted the researchers to extract natural antioxidants from citrus peels and the use of those natural and safe substances in foods for the purpose of preventing rancidity and inhibiting lipid oxidation (Anagnostopoulou et al. 2006; Peschel et al. 2006; Rehman 2006; Yassari and Yasari 2013).
Addition of natural extracts in dairy products is a newly emerging area (Rowan 2000) and has a vast potential (Shende et al. 2014). The research on use of orange peel extract as a natural antioxidant in ghee was not reported in the available scientific literature. Therefore, the present study was carried out to investigate the antioxidant activities of orange peel extract in ghee stored at different storage temperatures. The antioxidant activities of orange peel extract in ghee were compared with the antioxidant activities of ghee incorporated with BHA in the present study.
Materials and methods
Chemicals and reagents
All the chemicals used during the investigation were AR grade and procured from standard suppliers (HI MEDIA® and SIGMA-ALDRICH).
Raw materials
Fresh, good quality and well ripened sweet orange fruits were procured from the local fruit market. Freshly prepared milk cream was obtained from the experimental dairy plant of the institute, SRS, ICAR-NDRI, Bengaluru. The synthetic antioxidant Butylated hydroxyanisole (BHA) was obtained from HI MEDIA®, Mumbai, India.
Preparation of orange peel powder
The orange fruits were sorted manually to remove damaged fruits. The sorted fruits were washed twice under running tap water followed by distilled water wash to remove dirt, dust, microorganisms and other foreign matter. The fruits were peeled using stainless steel kitchen knife to obtain peels which were sliced to pieces approximately 1.75 × 2.0 cm. The peel pieces were dried in a laboratory hot air oven attached with blower at 40 °C for about 8 h to remove moisture from the peel. The dried peel slices were ground to powder using laboratory mixer grinder and powder was sieved through 500 microns mesh (I.S.S test sieve).
Preparation of orange peel powder extract and its incorporation in to ghee
Extracts from orange peel powder (OPP) extracted at different extraction parameters i.e., solvent to sample ratio (10:1, 13.5:1 and 17:1, mL/g), extraction temperature (30, 40 and 50 °C) and extraction time (6, 12 and 24 h). Total phenol content (TPC) and radical scavenging activity (RSA) by DPPH assay of orange peel extracts were analyzed (data not shown). The extract which showed highest TPC and RSA was selected for the study and it was prepared using ethanol and orange peel powder (10:1 v/w) in amber coloured stoppered conical flask covered with alumium foil and extracted at 30 °C for 12 h. After 12 h extraction time, the extract was filtered using muslin cloth and then solvent from the filtered extract was expelled under nitrogen at 80 °C. The concentrated extract free of solvent was immediately incorporated into ghee and designated it as 1.0 % OPP extract incorporated ghee sample. The ghee incorporated with BHA (0.02 %) and control without any additive was also prepared.
Storage of ghee
The ghee samples viz. control, ghee incorporated with BHA (0.02 %) and orange peel powder extract (1.0 %) were stored at three temperatures (6 ± 2 °C: T1; 32 ± 2 °C: T2; 60 ± 2 °C: T3) for 21 days storage study. The ghee samples were analyzed (triplicate) at intervals of 3 days for peroxide value (PV), thiobarbituric acid value (TBA) and free fatty acids (FFA). and Radical scavenging activity (RSA) by DPPH assay of ghee samples were analyzed (triplicate) on 0 day, 3rd, 9th, 15th and 21st day.
Total phenol content and radical scavenging activity
The orange peel powder extract (extraction process is discussed above) was analyzed for total phenol content and radical scavenging activity. Total polyphenolic content (TPC) of the extracts was analyzed by Folin–Ciocalteu colorimetric method (Kahkonen et al. 1999). Total phenolic content was estimated using a standard curve (400 μL of 10–100 μg/ mL) of caffeic acid and presented as caffeic acid equivalents (CAE) per gram of extract. The radical scavenging activity (RSA) of the extracts was determined according to the procedure discussed by Blois (1958), with a minor modification, ethanol was used instead of methanol for sample preparation. Radical-scavenging activity was expressed as the inhibition percentage and was calculated using the following formula.
Sensory evaluation
Sensory evaluation of ghee samples were evaluated for colour and flavour using nine point hedonic scale. Sensory panel members were requested to score the samples from 1 to 9 (1 = dislike extremely; 2 = dislike very much; 3 = dislike moderately; 4 = dislike slightly; 5 = neither like nor dislike; 6 = like slightly; 7 = like moderately; 8 = like very much; 9 = like extremely).
Peroxide value
Peroxide value of ghee samples were determined by the method as described in IS:3508 (1966).
Thiobarbituric acid value
Thiobarbituric acid (TBA) value of ghee samples were determined by the method of Patton and Kurtz (1951). 0.1 g of molten ghee samples were accurately weighed into a 15 mL stoppered centrifuge tubes and to this 1 mL of trichloroacetic acid and 2 mL of TBA reagent were added. The contents were then incubated in a boiling water bath for 15 min. The tubes were then cooled under running tap water. To this 1 mL of glacial acetic acid and 2 mL of chloroform was added. The contents were mixed well using vortex mixer. Then it was centrifuged at 3000 rpm for 4 min. Two clearly separated layers were formed. The supernatant layer was used for taking readings as OD at 532 nm using spectrophotometer. Blank was also prepared simultaneously except adding ghee.
Radical scavenging activity (RSA) of ghee by DPPH (2,2-diphenyl-1-picrylhydrazyl) assay
The capacity of antioxidants to quench DPPH radicals in ghee was determined before and after accelerated oxidation tests (Espin et al. 2000). Ethyl acetate was used as a better solvent for hydrophobic compounds. 0.2 mL of the ghee sample was added to 3.8 ml of ethyl acetate to obtain 4 mL of the mixture followed by addition of 1 mL of DPPH (6.09 × 10−5 mol/L) solution in ethyl acetate (total volume, 5 mL). After 10 min had elapsed the absorbance was measured at 520 nm wavelength. The reference sample used was 1 mL of DPPH solution and 4 mL ethyl acetate. Radical scavenging activity was expressed as percentage inhibition and was calculated using the following formula.
Free fatty acids
Free fatty acid levels of ghee samples were determined by the method as described in IS:3508 (1966).
Statistical analysis
The experimental data were analysed using statistical package for social sciences (SPSS, version 15. 0) to obtain ANOVA. The effect of storage temperature, storage period, treatments and their combined effect on peroxide value, thiobarbituric acid, free fatty acid and radical scavenging activity of ghee were analysed using factorial design of SPSS.
Results and discussion
Total phenol content and radical scavenging activity of orange peel extract
Extracts from orange peel powder (OPP) were obtained at different extraction parameters i.e., solvent to sample ratio (10:1, 13.5:1 and 17:1, mL/g), extraction temperature (30, 40 and 50 °C) and extraction time (6, 12 and 24 h). Total phenol content (TPC) and radical scavenging activity (RSA) of orange peel extracts were analyzed (all the data not shown). The OPP extract extracted using 1 g OPP in 10 mL ethanol at 30 °C for 12 h was found to be highest TPC (110.77 mg of CAE/g) and radical scavenging activity (93.13 % inhibition).
Sensory evaluation
Ghee colour and flavour are important sensory attributes. Consumer acceptance of ghee mainly depends on colour and flavour attributes. Initially, in the preliminary sensory study, colour and flavour of ghee samples incorporated with 0.5, 1.0, 2.0, 4.0, 6.0 and 8.0 % orange peel powder extract (OPPE) were evaluated and compared with the control (ghee without any additive). It was observed that the sensory scores of the ghee samples incorporated with 4.0, 6.0 and 8.0 % orange peel powder extract (OPPE) were not in the acceptable range (data not shown). Based on the preliminary sensory study, 0.5, 1.0 and 2.0 % OPPE were selected and evaluated further. Colour and flavour of ghee incorporated with 0.5, 1.0 and 2.0 % orange peel powder extract (OPPE) were evaluated sensorily and compared with the control. Mean values of colour and flavour score of ghee incorporated with OPP extracts and control are shown in the Table 1. The sensory panel liked colour and flavour of control very much as their score was just above 8 (8 = like very much). Among treated ghee samples, the ghee incorporated with 2.0 % OPPE scored least colour and flavour score. There was no difference in colour of the ghee samples incorporated 0.5 and 1.0 % OPPE while the flavour score of the ghee incorporated with 1.0 % OPPE was fell between 8 (like very much) and 7.0 (like moderately). The ghee incorporated with 1.0 % OPPE was selected for the storage study as there was no difference in colour between the ghee samples incorporated with 0.5 and 1.0 % OPPE with little compromise with the flavour score.
Development of peroxides in ghee samples stored at different temperatures
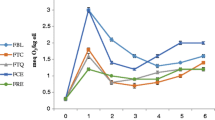
Peroxide value (PV) is an indicator of the extent of primary oxidation products in oils and lipids (Anwar et al. 2007; Chatha et al. 2006), which can be measured by their ability to liberate iodine from potassium iodide. It represents the quantity (mg) of active oxygen contained in 1 g of lipid. The development of peroxides in treated ghee samples and control stored at different storage temperatures (6 ± 2 °C: T1; 32 ± 2 °C: T2; 60 ± 2 °C: T3) for 21 days is presented in the Fig. 1. All the parameters individually and their interaction effected the PV significantly at 99 % confidence level with p-value < 0.01 (Table 2). On zero day of storage, there was no development of peroxides in all the ghee samples. Slight development of peroxides on the 3rd was observed in all the samples while least PV was observed in treated samples stored at T1 which could be due to the presence of antioxidant compounds and stability of antioxidant compounds at lower temperature. Thereafter, up to 9th day of storage, there was significant (p < 0.01) increase in peroxides in the samples with highest PV observed to be in control stored at T3 and least development of peroxides was observed in ghee incorporated with orange peel extract followed by BHA at lower temperature (T1). After 9th day of storage till 21st day of storage, there was drastic increase in peroxides in control at accelerated temperature of T3 followed by the control (stored at T2) and BHA treated ghee stored at T3. It can be seen that ghee incorporated with orange peel extract and stored at T1 and T2 found to be least development of peroxides than the development of peroxides in ghee incorporated with synthetic BHA at the same temperatures throughout the storage period. ANOVA indicating the effect of different parameters on PV in ghee samples is shown in the Table 2. The temperature had highly significant effect on increase in PV followed by treatment and storage period among individual effects while the interaction of storage period with temperature had more significant effect on increase in PV. The ghee incorporated with orange peel extract was more effective than BHA treated ghee in retarding the primary oxidation. The variation in peroxides development in BHA and orange peel extract treated ghee could be due to the presence of antioxidant compounds. Rehman (2006); Abdelaal and Halaweish (2010) demonstrated that a rapid decline in the peroxide value of corn oil and soybean oil stabilized with citrus peel extracts. The highest increase in peroxides may be due to the development of more hydroperoxides (initial products of oxidation) in the absence of antioxidant compounds in the control. Incorporation of the orange peel extract into ghee resulted in increased resistance against peroxides development. Similar conclusions were drawn when the Thompson orange peel extract added to canola oil Yassari and Yasari (2013), extracts of herbs (Shatavari and vidarikand) incorporated into ghee (Pawar et al. 2012); clove extracts incorporated into ghee (Shende et al. 2014). The antioxidant property of the orange peels may be due to presence of phenols, including numerous flavanones, flavone glycocides, poly-methoxyylated flavanones, hydryoxyl cinnamates and other miscellaneous phenolic gycocides and amines as reported by John (2004).
Development of thiobarbituric acid in ghee samples stored at different temperatures
Thiobarbituric Acid (TBA) indicates quantity of malondialdehyde (in mg) present in 1 kg of sample. TBA test measures the secondary products of lipid oxidation. It involves reacting thioabarbituric acid with malondialdehyde produced by lipid hydroperoxide decomposition to form a red chromophore with peak absorbance at 532 nm. This coloured complex results from the condensation of 2 moles of TBA and 1 mole of malondialdehyde, under the effect of the temperature and pH (Dahle et al. 1962; Janero 1969).
The experimental data of TBA in ghee stored at temperatures (6 ± 2 °C: T1; 32 ± 2 °C: T2; 60 ± 2 °C: T3) for 21 days are depicted in the Fig. 2. The storage temperature had more significant (p < 0.01) effect on the increase in TBA followed by treatment and storage period. The interaction effect of temperature, treatment and storage period on the TBA development was significant at 99 % confidence level with p-value < 0.01 (Table 2). The development of TBA was significantly higher in control than treated ghee which is in agreement with the results reported by Shende et al. 2014. There was drastic increase in TBA after 9th day of storage in the control and the ghee incorporated with orange peel extract at 60 °C (T3) than other samples. It evident from the Fig. 2 that accelerated temperature (T3) significantly increased the TBA in control (CT3) and in ghee incorporated orange peel extract (ET3) this was followed by the control stored at T2 (32 °C). Orange peel extract was more effective in reducing the increase in TBA than BHA at lower temperatures while BHA was more effective in reducing the increase in TBA than orange peel extract at higher temperature (60 °C). The more TBA value in the ghee incorporated orange peel extract at accelerated temperature could be attributed to the effect of multiple factors such as temperature, pH, chemical changes, colour compounds of orange peel extract and interaction of malondialdehyde with multiple compounds rather than development of secondary lipid oxidation products alone (Dahle et al. 1962; Janero 1969; Gilbertson 1971). TBA test has been the focus of much criticism. The first is that malondialdehyde only forms from fatty acid chains containing at least three double bonds, like linolenic acid, to the exclusion of linoleic and oleic acid peroxide decomposition products (Dahle et al. 1962). Secondly, TBA is not specific to malondialdehyde because it can react with other aldehydes, browning reaction products, protein and sugar degradation products, amino acids and nucleic acids (Janero 1969).
Free fatty acids
Free fatty acids (FFA) are formed due to hydrolysis as well as by cleavage and oxidation of double bonds (Paul and Mittal 1997). Significant (p < 0.01) increase in FFA was observed in all the ghee samples while least increase in FFA in ghee incorporated with orange peel extract and BHA throughout storage period (Fig. 3). The formation of FFA was significantly higher in control at temperatures (32 and 60 °C) than in the ghee incorporated with orange peel extract and BHA at 60 °C. It can be seen from ANOVA Table 2 that treatment had more significant effect on change in FFA than temperature and storage period. The resistance against development of FFA was more in the ghee incorporated with orange peel extract than in the ghee incorporated with BHA at lower temperature which could be due to presence of more antioxidant compounds such as phenolic compounds in orange peel extract which is in agreement with results reported by Shende et al. (2014).
Radical scavenging activity (RSA) of ghee by DPPH assay
Radical scavenging activity (RSA) of ghee samples was evaluated by DPPH (2, 2-diphenyl-1-picrylhydrazyl) assay. The DPPH assay measures the potential to quench the DPPH radicals. The experimental results of RSA of ghee are depicted in the Fig. 4. RSA of control at all the storage temperatures was significantly much lower as compared to the treated samples which could be due to absence of antioxidant compounds in control. Ghee incorporated with orange peel extract showed maximum potential to quench the DPPH radicals than ghee incorporated with BHA and control throughout storage period. Presence of antioxidant compounds in ghee incorporated with orange peel extract and BHA exhibited stronger radical scavenging activity. Similar conclusions were drawn by the Pawar et al. 2012. Radical scavenging activity was decreased in all the samples as antioxidant compounds utilized in the oxidation process to quench radical as the storage period progressed. Treatment had highest significant (p < 0.01) effect on the RSA followed by storage period and temperature (Table 3). Among the interactions, the effect of storage period-temperature-treatment on RSA change was not significant.
Conclusions
Effect of storage temperature on development of peroxides and thiobarbituric acid (TBA) in ghee was significantly higher than the effect of treatment (orange peel extract and BHA) and storage period while treatment had more significant effect on the change in free fatty acids (FFA) and radical scavenging activity as compared to storage temperature and storage period. Development of peroxides, TBA and FFA in ghee was highest at accelerated temperature (60 °C). Ghee incorporated with orange peel extract (OPE) was more stable at 6 and 32 °C than at accelerated temperature (60 °C). Ghee incorporated with OPE was found to be better resistance against development of peroxides, thiobarbituric acid (TBA) and FFA than the ghee incorporated with BHA. Ghee incorporated with OPE showed stronger activity in quenching DPPH radicals. The study revealed that orange peel could be a good natural source of antioxidants which can be used in fat rich food products like ghee to retard oxidative deterioration.
References
Abdelaal HA, Halaweish FT (2010) Food preservative activity of phenol compounds in orange peel extract (Citrus sinensis L.). Lucrari Stiintifice 53(15):233–240
Anagnostopoulou MA, Kefalas P, Papageorgiou VP, Assimopoulou AN, Boskou D (2006) Radical scavenging activity of various extracts and fractions of sweet orange peel (Citrus sinensis). Food Chem 94:19–25
Anwar F, Siddiq A, Iqbal S, Asi MR (2007) Stablization of sunflower oil with Moringa oleifera leaves under ambient storage. J Food lipid 14:35–49
Beddows CG, Jagait C, Kelly MJ (2001) Effect of ascorbyl palmitate on the preservation of α-tocopherol in sunflower oil, alone and with herbs and spices. Food Chem 73:255–261
Blois MS (1958) Antioxidant determination by the use of stable free radical. Nature 181:1199–1200
Bombardelli E, Morazzoni P (1993) The flavonoids: new perspectives in biological activities and theraputics. Chem Today 11:25–28
Chatha SAS, Anwar F, Manzoor M, Bajwa JR (2006) Evaluation of the antioxidant activity of the rice bran extracts using different antioxidant assays. Grasas y Aceites 57:328–335
Choe E, Min DB (2006) Mechanisms and factors for lipid oxidation. Compreh Rev Food Sci 5:169–186
Dahle LK, Hill EG, Holman RT (1962) The thiobarbituric acid reaction and the autoxidations of polyunsaturated fatty acid methyl esters. Arch Biochem Biophys 98:253–261
Dubey D, Balamurugan K, Agrawal RC, Verma R, Jain R (2011) Evaluation of antibacterial and antioxidant activity of methanolic and hydromethanolic extract of sweet orange peels. Rec Res Sci Technol 3(11):22–25
Espin JC, Soler-Rivas C, Wichers HJ (2000) Characterization of the total free radical scavenger capacity of vegetable oils and oil fractions using 2,2-diphenyl-1-picrylhydrazyl radical. J Agric Food Chem 48:648–656
Gandhi K, Arora S, Pawar N, Kumar A (2013) Effect of vidarikand (extracts) on oxidative stability of ghee: a comparative study-research and reviews. J Dairy Sci Technol 2(1):1–11
Gharahkhani M, Ghorbani M, Gharahkhani A, SadeghiMahoonak A, Jibraeeli S, Ghassemi Y (2010) The effects of peel and fruit extracts of Thompson oranges on different stocks in preventing the oxidation of soybean oil. Quart Med Plants 2(38):55–66
Gilbertson G (1971) Oleoresin as flavor ingredients. Flavour Ind 43:403–405
Hertog MGL, Feskeens EJM, Hollman CH, Katan MB, Kromhout D (1993) Dietary antioxidant flavonoids and risk of coronary heart disease: de Zutphen elderly study. Lancet 342:1007–1011
IS: 3508 (1966) Indian standards, methods for sampling and test for ghee (butter fat). Bureau of Indian Standards, New Delhi
Ito N, Hirose M, Fukushima H et al (1986) Studies on antioxidants: their carcinogenic and modifying effects on chemical carcinogens. Food Chem Toxicol 24:1071–1092
Janero D (1969) Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Rad Biol Med 9:515–540
John IM (2004) Fractionation of orange peel phenols in ultra filtered molasses and mass balance studied of their antioxidant levels. J Agric Food Chem 18(3):7586–7592
Kahkonen MP, Hopia AI, Vuorela HJ, Rauha J, Pihlaja K, Kujala ST, Heinonen M (1999) Antioxidant activity of plant extracts containing phenolic compounds. J Agric Food Chem 47:3954–3962
Kahl R, Kappus H (1993) Toxicology of the synthetic antioxidants BHA and BHT in comparison with the natural antioxidant vitamin E. Z Lebensm Unters Forsch 196(4):329–338
Kumar AK, Narayani M, Subanthini A, Jayakumar M (2011) Antimicrobial activity and phytochemical analysis of citrus fruit peels-utilization of fruit waste. Int J Eng Sci Technol 3(6):5414–5421
Mathur A, Satish K, Verma PR, Gupta V, Dua VK, Prasad GBKS, Mathu D, Santosh K, Singh SS (2011) Evaluation of in vitro antimicrobial and antioxidant activities of peel and pulp of some citrus fruits. IJPI’s J Biotechnol Biotherap 1(2):1–17
Merai M, Boghra VR, Sharma RS (2003) Extraction of antioxigenic principles from Tulsi leaves and their effects on oxidative stability of ghee. J Food Sci Technol 40:52–57
Mour A, Sruz GM, Franco D, Dominguez J (2001) Natural antioxidant from residual sources. Food Chem 72:145–171
Patton S, Kurtz GW (1951) 2-Thiobarbituric acid as a reagent for detecting milk fat oxidation. J Dairy Sci 34:669–674
Paul G, Mittal S (1997) Regulating the use of degraded oil/fat in deep fat/oil food frying. Crit Rev Food Sci Nutr 37:635–662
Pawar N, Arora S, Bijoy RR, Wadhwa BK (2012) The effect of Asparagus racemosus (Shatavari) extract on oxidative stability of ghee, in relation to added natural and synthetic antioxidant. Int J Dairy Technol 65(2):293–299
Peschel W, Ssnchez-Rabaneda F, Diekmann W, Plescher A, Gartza I, Jimenez D (2006) An industrial approach in the search of natural antioxidants from vegetable and fruit wastes. Food Chem 97:137–150
Pukalskas A, Van Beek AT, De Waard P (2005) Development of a triple hyphenated HPLC radical scavenging detection-DAD-SPE-NMR system for the rapid identification of antioxidants in complex plant extracts. J Chromatogr A 1074:81–88
Rehman Z (2006) Citrus peel extract—a natural source of antioxidant. Food Chem 99:450–454
Rouseff R (2007) Citrus flavour. In: Berger RG (ed) Flavours and fragrances, chemistry, bioprocessing and sustainability, XVI, pp 648
Rowan C (2000) Extracting the best from herbs. Food Eng Int 25:31–34
Shende S, Patel S, Arora S, Sharma V (2014) Oxidative stability of ghee incorporated with clove extracts and BHA at elevated temperatures. Int J Food Prop 17:1599–1611. doi:10.1080/10942912.2012.752382
Stich HF (1991) The beneficial and hazardous effects of simple phenolic compounds. Mut Res 259:307–324
Yassari S, Yasari E (2013) Effects of extract of Thompson orange peels on the stability of canola oil. Int J Agric Crop Sci 5(4):450–454
Acknowledgments
This research was supported by the ICAR-National Dairy Research Institute through its institute funded project. The authors are grateful to the Indian Council of Agricultural Research and ICAR-National Dairy Research Institute for supporting the research work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Research highlights
• Antioxidant activities of orange peel extract and BHA in ghee were evaluated.
• The antioxidant activities (AOA) evaluated at 6, 32 & 60 °C for 21 days of storage.
• Temperature, treatment and storage period significantly changed AOA in ghee.
• AOA of orange peel extract in ghee more effective than AOA of BHA in ghee.
• Orange peel could be used as natural antioxidant in ghee in place of BHA.
Rights and permissions
About this article
Cite this article
Asha, A., Manjunatha, M., Rekha, R.M. et al. Antioxidant activities of orange peel extract in ghee (butter oil) stored at different storage temperatures. J Food Sci Technol 52, 8220–8227 (2015). https://doi.org/10.1007/s13197-015-1911-3
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-015-1911-3