Abstract
The effects of BHT and green tea extracts (GTE) on the physical, barrier, mechanical, thermal and antioxidant properties of potato starch films were investigated. Results showed both BHT and GTE significantly lowered solubility of films. Addition of BHT significantly decreased water vapour transmission rate. Both BHT and GTE promoted significant increase in the elastic modulus but a decrease in % EAB. Increase in glass transition temperature (Tg) and enthalphy of transition (ΔH) of films was observed with the incorporation of GTE and BHT. Scanning electron microscopy (SEM) revealed smooth surface of the films. The DPPH radical scavenging ability of both BHT and GTE films were stronger in fatty food simulant (95 % ethanol). The GTE and BHT films were individually applied to fresh beef samples and were stored at 4 °C and room temperature for 10 days. Metmyoglobin formation and lipid oxidation (TBARS) were monitored periodically. The addition of GTE extracts and BHT resulted in decreases in metmyoglobin and TBARS values.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Meat is the edible post-mortem component originating from the live animals (Hui et al. 2005). Meat contains approximately 70.62 % water, 20.78 % proteins, 6.16 % lipids and 1.02 % ash (Fennema et al. 1996). It is highly perishable due to its high nutritional value. Its rich composition in proteins, lipids etc. makes it susceptible to the various physical, chemical and microbiological changes. The important changes that occur during the storage of meat include protein oxidation, lipid oxidation and increase in the percentage of metmyoglobin. Lipid peroxidation is a major cause of deterioration of meat quality during processing, distribution and refrigeration, thereby reducing shelf stability and acceptability. Lipid oxidation can produce changes in meat quality parameters, such as organoleptic properties and nutritional value, which leads to the generation and accumulation of compounds that may pose risks to human health (Gray et al. 1996). Controlling lipid oxidation is also crucial in limiting brown colour development, as lipid oxidation products have been shown to interact directly with the protein portion of myoglobin, resulting in increased susceptibility of the heme iron to oxidation (Alderton et al. 2003). When the heme iron is oxidized, the resulting metmyoglobin form can no longer hold oxygen. This change is observed visually as a shift from the bright cherry-red colour of “bloomed” beef to a dull brown, and is often taken by consumers as an indicator of beef freshness (Greene et al. 1971). Various means are used to enhance the shelf life of meat by slowing the rate of oxidation. This involves high pressure treatment, heat treatment, canning of meat, reformulation, packaging etc. The most important packaging technologies used to increase the shelf life of meat are vacuum packaging, modified atmosphere packaging (MAP), intelligent packaging, active packaging, bioactive packaging etc. Active packaging is a novel technique used to preserve the various foods including meat by the release of the active agents (antimicrobials and antioxidants) which have been incorporated into the package during its preparation. Release of the active agents can be controlled over an extended period of time to maintain or extend the quality and shelf-life of products, without the need for direct addition of any substances to the foodstuff (Lee 2010; Zhou et al. 2010). Increasing demand for synthetic packaging materials has put tremendous pressure on the environment because of their poor biodegradability and non-renewability (Ghasemlou et al. 2011). This has resulted in the modification of active packaging into a new concept called bioactive packaging. Bioactive packaging involves the use of edible packaging material which can be protein, polysaccharide, lipid etc. with the incorporation of edible active substances. A number of studies have reported the use of purposefully added antioxidants for extending the shelf life of meat products. Garrido et al. (2011) applied red grape pomace to pork burgers. Hayes et al. (2011) applied lutein, sesamol, ellagic acid and olive leaf extract in fresh and cooked pork sausages. The oxidative stability of meat can be extended by using antioxidants and proper packaging materials (Zhou et al. 2010).
The main objective of the work was to develop potato starch based active packaging films loaded with antioxidants (BHT and Green tea extract). Characterization of films was carried out by the determination of physical, barrier, morphological, thermal, mechanical and antioxidant properties. Films were also applied to fresh beef to study their effect on lipid and myoglobin oxidation.
Material and methods
Raw materials
Potato starch was obtained from Thomas Baker pvt. Limited, India. The beef sample was obtained from local market of Hazratbal, Srinagar, Kashmir. The animal was slaughtered by halal method. Glycerol, BHT, calcium chloride, DPPH, ethanol, petroleum ether, potassium sulphate, copper sulphate, concentrated sulphuric acid, phosphate buffer, trichloroacetic acid, thiobarbituric acid were procured from Sigma- Aldrich, St. Louis, USA.
Preparation of starch film
Starch films, containing green tea extract or BHT, were obtained by the casting technique (Ashwar et al. 2014). Five grams potato starch on dry weight basis (db) was mixed with 200 mL distilled water and heated at 95 °C for 30 min on a heating plate while stirring with a magnetic stirrer, followed by addition of glycerol (1.2 mL) as plasticizer and further heating and mixing for 10 min for cross linking to occur. Green tea extract (GTE) or Butylated hydroxy Toluene (BHT) was incorporated at 5 % (w/w with respect to starch weight) during the last 5 min of mixing. Film-forming solutions (200 mL) were casted over the levelled glass plates (26 cm × 20.5 cm) and dried at 45 °C in a hot air oven for 12 h. Then, the dried films were carefully peeled from the plates and stored at room temperature. Each film was prepared in triplicates.
Physical properties of films
Film thickness and Moisture content
Thickness of the films was measured using a Vernier calliper. Six different positions of the samples were measured and average thickness was calculated.
Films were weighed before and after drying in a hot air oven (NSW-143; Narang Scientific Works Pvt. Ltd., New Delhi, India) at 105 °C for 24 h. Moisture content was calculated as follows:
Where M1 was the initial film mass (g) and M was the bone-dry mass (g).
Film solubility
The solubility of the films in water was determined by a modified method of Gontard et al. (1994). Film disks of 2 cm diameter were cut out and dried in a hot air oven at 50 °C for 10 h before weighing. The dry disks were then immersed in 50 mL water at 25 °C for 24 h. The samples were then dried again and weighed. Solubility was expressed as percent weight loss of the film strips on soaking.
Water vapour transmission rate
Water vapour transmission rate (WVTR) of the films was determined gravimetrically based on the method described by Talja et al. (2008) with some modifications. Test cups with diameter 3.7 cm and height 6.5 cm were used. The test cup contained 25 g anhydrous calcium chloride (0 % RH) that was dried in a hot air oven at 120 °C for 1 day initially. The test films were sealed on top of the test cups. These cups were then placed in a desiccator containing distilled water (100 % RH) and kept at 30 °C. Weight increments of the cups were measured at 24 h intervals. WVTR was calculated as follows:
Where “m” was the weight of water permeated through the film (g), “A” was the permeation area (m2) and “t” was the time of permeation (h). Three repetitions were performed for each film sample.
DPPH scavenging activity
Film strips (20 mm × 20 mm) were introduced into beakers containing 100 ml of distilled water, 10 % ethanol and 95 % ethanol. Then the film samples were stirred magnetically at 250 rpm with a 2 cm rod coated with Teflon. The antioxidant activity of the film extracts was tested using a stable radical 2, 2-diphenyl-1-picrylhydrazyl (DPPH). The extracts (0.1, 0.2, 0.3, 0.4, and 0.5 mL) were added to the 1 mL of DPPH solution and the final concentration was made 3 mL by the addition of methanol. After incubation at 37 °C for 30 min, the activity was monitored by absorbance at 517 nm. The radical scavenging activity was calculated as a percentage of DPPH scavenging activity
Where A sample was the absorbance of test sample (4 ml DPPH plus 1 ml sample) and A control was the absorbance of control solution (4 ml DPPH plus 1 ml distilled water).
Mechanical properties
The mechanical properties of films prepared were evaluated by conducting tensile strength (TS) and elongation-at-break (EAB) tests according to ASTM D882-00 method (ASTM 2000). Tensile strength and EAB were performed as a tension test using a Texture analyzer TA XT2 (Stable Microsystems, Surrey, UK) with a load cell of 30 kg and crosshead speed of 60 mm/min. The samples were mounted between grips with initial grip gap of 100 mm and film width of 2 cm. The results of tensile and elongation tests were expressed by MPa and percentage (%) respectively. Each test trial per film consisted of five replicate measurements.
Thermal analysis
Thermal analysis of the film samples was carried out in a Differential Scanning calorimeter (DSC Q2000, TA Instruments, New Castle, DE USA). Briefly, 50 ± 2 mg sample conditioned at 25 °C and 53 % RH for 2 days was taken in aluminium pans and submitted to a temperature program, under nitrogen atmosphere. In the first scan, after cooling the sample at 10 °C min−1 up to −60 °C, it was submitted to heating at 10 °C min−1 until 100 °C. The second scan was between −60 °C and 250 °C, at the same cooling and heating rates. The glass transition temperature (Tg) (°C) was calculated as the middle point between the onset and end temperatures caused by the discontinuity of sample specific heat. Two replicate runs were carried out for each sample.
Scanning electron microscopy
The starch films were placed on an adhesive tape attached to a circular aluminium specimen stub. After coating with gold-palladium, the samples were photographed at an accelerator potential of 5 kV using a scanning electron microscope (Hitachi Se 300H-Tokyo, Japan).
Preparation of beef sample
Leg parts from beef carcass were obtained from the 6 h post slaughter from the local market, Hazratbal, Srinagar, J&K, India. This beef was trimmed of the visible fat and was divided into the small cuts (4 cm long and 1.5 cm thick) using sterile chopping board and knife. These meat pieces were separated into four groups and were packaged into the bioactive starch films. Two Groups of beef were packaged with films incorporated with BHT and the other two were packaged with the films incorporated with green tea extract, one among both the groups was stored at 4 °C and the other at room temperature with their control samples (meat pieces without antioxidant based packaging) as well. This beef was stored for 10 days. Samples from each group were taken at alternate days and were utilized for subsequent analysis. The analysis includes quantification of TBARS and metmyoglobin.
Analysis of beef
Metmyoglobin (%)
Meat samples (5 g) were homogenized in 25 ml ice-cold 40 mM phosphate buffer (pH 6.8) for 10 s using a Virtis homogenizer (The Virtis Co., Gardiner, NY). The homogenate was allowed to stand for 1 h at 4 °C and centrifuged at 4500 × g for 30 min at 4 °C. The supernatant was filtered through Whatman No. 1 filter paper and absorbance measured at 572, 565, 545 and 525 nm using an UV–Vis scanning spectrophotometer (UV- Spectrophotometer, Model U-2900 2JI-0003, Hitachi, Japan). The percentage of metmyoglobin was determined as described by Bekhit et al. (2003) using the formula
Lipid oxidation (TBARS)
Oxidative stability was evaluated by changes in thiobarbituric acid reactive substances (TBARS). The procedure for measurement of TBARS was based on methods used by Serrano et al. (2006)). Briefly, the procedure was as follows: 5 g of each sample was homogenized in 35 ml of 7.5 % trichloroacetic acid. The blender sample was centrifuged (3000 × g, 2 min) and 5 ml of the supernatant was mixed with 5 ml of 20 mM thiobarbituric acid; finally the solution was mixed and kept in the dark for 20 h at 20 ± 1.5 °C. The pink color that formed was measured spectrophotometrically (UV- Spectrophotometer, Model U-2900 2JI-0003, Hitachi, Japan) at 532 nm. A calibration curve was plotted with 1, 1, 3, 3-tetraethoxypropane to obtain the malonaldehyde (MDA) concentration and results were expressed as mg MDA/kg of sample. The results were expressed as mg MDA/kg of sample. TBARS determinations for each sample were performed in triplicate.
Statistical analysis
The statistical analyses of the data was performed using analysis of variance (ANOVA), Duncan mean comparison test (p < 0.05), using SPSS statistics 21.
Results and discussions
Film thickness and moisture content
Thickness and moisture contents of the films are shown in Table 1. Thickness of the films varied from 0.05 to 0.143 mm, and incorporation of BHT and GTE did not significantly affect the resulting film thickness. Slight differences in thickness of the films might be due to difference in the film forming solutions, preparation methods, drying conditions, gelatinisation and aggregation of starch granules during film preparation.
Moisture contents of film A (film loaded with BHT) and B (film loaded with Green Tea Extract) were significantly low as compared to film C (Control Film). Lowest moisture content of film A may be due to the hydrophobic nature of BHT, thus reducing the number of polar groups available to interact with water molecules and decreases the moisture content of film. The difference of films in moisture contents may have a relationship with the distinction in water solubility and chemical structure of component contained (Li et al. 2014).
Film solubility
Solubility of films is shown in Table 1. It can be seen that the film A had significantly lowest solubility (%) (20.79) followed by film B (24.886) and film C (28.36). In film A, the addition of BHT decreases the affinity towards water by reducing the number of polar groups available to interact with water molecules and hence decreases the solubility of film. Lower solubility of film B might result from the stronger structure of film network through higher extent of the interactions with polyphenols of GTE. Wu et al. (2013) also reported the reduction in water solubility of the gelatin film when GTE was added. However increase in solubility of starch films with tea extract was observed by Das et al. (2013). Highest solubility value of film C might be the result of weaker interaction between network components. The plasticiser might interact with water and interrupt the network by hydrogen bonds, reducing the cohesiveness of starch matrix and increasing its solubility in water (Marana et al. 2013). An increase in the moisture content of the film can be ascribed to an increased solubility in water, which is also dependent on the higher wettability and surface free energy (Soradech et al. 2012). Mathew et al. (2006) found that the increase in film solubility was related to water diffusion, ionisation of hydroxyl and carboxyl groups, polymer relaxation and dissociation of hydrogen and ionic bonds.
Water vapour transmission rate (WVTR)
Water vapour transmission rate is an important barrier property that decides the utility of the film. Table 1 shows water vapour transmission rate values of films analyzed at 30 °C and 0–100 % RH gradient. The mean WVTR values (g/m2/day) of films A, B and C were 6.406, 7.17 and 9.58 respectively, indicating high water vapor transmission rate of films. Film A presented significantly lowest WVTR values. The addition of BHT decreases the affinity towards water by reducing the number of polar groups available to interact with water molecules and decreases the WVTR of film (Ashwar et al. 2014). Compounds with hydrophilic groups generate films that are susceptible to water vapor, while compounds with hydrophobic groups make excellent barriers to moisture (Souza et al. 2009). Jongjareonraka et al. (2008) also found greater decrease in WVTR of the fish skin gelatin films when incorporated BHT. Films with GTE showed significantly low WVTR values than that of control film. The abundant polyphenols of Green Tea Extract could be able to form hydrogen and covalent bonds with the polar groups of polypeptide (Curcio et al. 2009). It is these bonds that limited the availability of hydrogen groups to form hydrophilic bonding with water and led to a decrease in the affinity of films with water (Ubonrat and Bruce 2010). Wu et al. (2013) and Siripatrawan and Harte (2010) also found decreased WVP of gelatine and chitosan films respectively with increasing concentrations of GTE.
DPPH scavenging activity
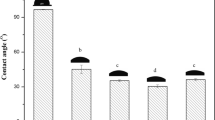
Antioxidant activity of packaging films was studied by DPPH radical scavenging activity into three food simulants: water was used as an aqueous food simulant, 10 % ethanol as an alcoholic food simulant, and 95 % ethanol as fatty food simulant. Film A showed highest % inhibition in the 95 % ethanol and least in the aqueous solvent (Fig. 1). This result can be explained by the lack of affinity and solubility of BHT in water. BHT is hydrophobic in nature which result in the lower affinity between BHT and water and hence the release of BHT in water is least expected. Ortiz-Vazquez et al. (2011) while studying the release of butylated hydroxytoluene (BHT) from Poly (lactic acid) films found that the release of the BHT in 95 % ethanol was very high and the release of the same in the water was very less. Film B also shows highest % inhibition in the 95 % ethanol and least in aqueous solvent. Green tea catechins are partially soluble in water and hence their release in water is less. Lopez-de-Dicastillo et al. (2012) while studying the active antioxidant packaging films, also found that green tea extract was highly soluble in ethanol and were released to a great extent in 95 % ethanol. The presence of 10 % alcohol slightly increased the release of green tea extract than that in the water. However, with the increase in the concentration of antioxidants (BHT and GTE), % inhibition increases in all the three solvents. Among the two films, B was having higher antioxidant activity than that of A. This might be possible because the film B (with GTE) contains more phenolic contents than film A which are responsible for the higher DPPH radical scavenging activity. Lorenzo et al. (2014) also found that the tea equivalent activities were almost twice the antioxidant power of BHT.
Mechanical properties
Tensile properties of the films are shown in Table 2. Control films (C) showed lowest tensile strength (1.656 MPa) and highest %EAB (15.903). Hence film C was more stretchable as compared to films A and B. Glycerol-containing films affected the mechanical properties and resulted in low values of tensile strength (TS) due to its hygroscopic character that tends to provide additional water into the film matrix. The film A is having significantly highest TS (6.432) and lowest EAB (2.489) followed by the film B (1.953 and 11.173 respectively). Highest TS and lowest EAB of film A might be due to the hydrogen bond formation between BHT and starch molecules which strengthened the film network. Jongjareonraka et al. (2008)) also reported increase in TS of BHT loaded gelatin films might be due to a possible interaction between BHT and gelatin in the fashion, which strengthened the film network. The improvement in TS of B films may be attributed to the interaction between starch and polyphenolic compounds from GTE. Hoque et al. (2011) reported that hydroxyl groups of polyphenolic compounds were able to combine with hydrogen acceptors of gelatin molecule by hydrogen bonds resulting in highest tensile strength. Wu et al. (2013) also reported increase in TS and decrease in % EAB in the gelatin films when GTE was added. Higher TS and lower % EAB of film A resulted in its highest Young’s modulus.
Thermal analysis
Table 2 presents a DSC thermograph of films. Film C had a glass transition temperature (Tg) value of 74.09 °C and enthalpy of transition (ΔH) value of-59.81 Jg −1. Tg and ΔH values of films was significantly increased with the incorporation of BHT (film A) and GTE (film B). The increase in Tg and ΔH values of A and B films probably may be due to the formation of hydrogen bonding between the starch and GTE or BHT which strengthened the film network and limited the molecular movement of the films. Ashwar et al. (2014) found that the incorporation of ascorbic acid and BHT significantly increased the Tg and ΔH values of rice starch films. Dicastillo et al. (2013) also found that the incorporation of green tea extract improved slightly the thermal stability of polypropylene films.
Scanning electron microscopy
The SEM pictures of A, B and C films are shown in Fig. 2. Surface of all the films was smooth and homogeneous. After adding GTE and BHT, the micrographs of the films displayed similar characteristics, smoother and more homogeneous surface was observed in film A.
Lipid oxidation analysis (TBARS)
Lipid oxidation was analyzed in raw beef sample subjected to ambient and refrigerated storage using the TBARS method. The TBARS method has been widely used to determine the degree of lipid oxidation. TBARS are produced through second stage autooxidation during which peroxides are oxidized to aldehydes and ketones (e.g., MDA). The respective treatments significantly (P < 0.05) influenced TBARS values and also impacted the change over time, as evidenced by statistically significant interactions (P < 0.05) between treatments and time (Table 3). Among the various samples, those packaged in control film and stored under ambient conditions had the highest TBARS values by the end of storage (day 10). Results show that the TBARS values of the beef sample packaged in film loaded with BHT and that of the sample loaded with GTE, both stored under refrigerated conditions increased from an initial 0.094 and 0.11 mg MDA per kg beef to 0.133 and 0.237 mg MDA per kg beef respectively. The TBARS level displayed slight changes in the sample packaged in film loaded with BHT and that of the sample loaded with GTE, both stored under ambient conditions ranging from an initial 0.164 and 0.115 mg MDA per kg beef to 0.302 and 0.517 mg MDA per kg beef respectively. The film loaded with BHT was more effective (P < 0.05) in retarding lipid oxidation than the film loaded with GTE. BHT prevents the lipid oxidation by reacting with the free radicals and chelating metal ions such as iron which act as catalysts of the oxidation process (McBride et al. 2007). Lorenzo et al. (2014) also reported that BHT was more effective against TBARS formation than GTE. Kim et al. (2013) while determining the antioxidant effect of various phenolic compounds found that the TBARS value of the positive control group with BHT was much lower than those of the control counterparts and remained almost unchanged over the storage period. The TBARS value of samples packaged in the film loaded with GTE were significantly lower than that of the control film because the addition of Green Tea Extract (phenolic-rich) protects beef against lipid oxidation. Phenolic compounds have been known to inhibit free radical formation and the propagation of free radical reactions through the chelation of transition metal ions, such as iron (McBride et al. 2007). The antioxidant activity of phenolic compounds is associated with the hydroxyl group linked to the aromatic ring, which is capable of donating hydrogen atoms with electrons and neutralizing free radicals. This mechanism blocks further degradation to more active oxidizing forms such as MDA (Baydar et al. 2004; Oliveira et al. 2011). Siripatrawan and Harte (2010) also reported that the incorporation of green tea into chitosan film enhanced the antioxidant properties of the film, as TBA values of CGT-film (Chitosan film incorporating green tea extract) wrapped samples were lower than those wrapped with C-film (chitosan-alone film).
Metmyoglobin
With storage the protein portion of myoglobin undergoes oxidation, resulting in increased susceptibility of the heme iron to oxidation. When the heme iron is oxidized, the resulting MetMb can no longer hold oxygen. % MetMb values developed during 10 days of storage for all the samples are shown in Table 4. All the raw beef samples have retained the bright red colour up to 2 days of storage and the % metmyoglobin (% MetMb) remained below 20 %. After 2nd day of storage % MetMb increased significantly (p < 0.05) for all samples reaching 26.86, 32.33 and 36 % in GFP, BFP and control samples on 6th day. On 10th day of storage, all the samples exhibited significant discoloration as indicated by more than 50 % MetMb however the discoloration of GFP being only 44.42 %. Thus the Green Tea Extract is more efficient in inhibiting (p < 0.05) oxymyoglobin oxidation and retained the red colour of buffalo meat on 10th day of storage than the BHT. Naveena et al. (2013) while studying the antioxidant capacity also found that the effect of BHT was lower than that of the natural antioxidant (Carnosic acid). In this study the GTE was more efficient in controlling myoglobin oxidation as it contains phenolic compounds which are capable of reducing both lipid radicals and ferric iron. In lipid-containing systems such as beef, this radical-quenching effect prevents subsequent myoglobin oxidation (Alderton et al. 2003).
Conclusion
Incorporation of 5 % BHT and green tea extracts (GTE) into the starch film significantly improved its physical, barrier, mechanical, thermal and antioxidant properties. Both BHT and GTE significantly improved water resistance of films. Both BHT and GTE promoted significant increase in the tensile and thermal properties. Scanning electron microscopy (SEM) revealed smooth surface of the films. The DPPH radical scavenging ability of both BHT and GTE films were stronger in fatty food stimulant (95 % ethanol). Among the two films, BHT loaded film was more effective in reducing lipid oxidation (TBARS), however GTE loaded film was more efficient in controlling % metmyglobin during storage. These active films appear promising for the development of active antioxidant packaging for use with fresh beef.
References
Alderton AL, Faustman C, Liebler DC, Hill DW (2003) Induction of redox instability of bovine myoglobin by adduction with 4-hydroxy-2-nonenal. Biochemistry 42:4398–4405
Ashwar BA, Shah A, Gani A, Shah U, Gani A, Wani IA, Wani SM, Masoodi FA (2014) Rice starch active packaging films loaded with antioxidants- development and characterization. Starch-Starke 66:1–9
ASTM (2000) Standard test methods for tensile properties of thin plastic sheeting, method D882-00. American Society for Testing and Materials, Philadelphia
Baydar H, Sagdic O, Gulcan O, Karadogan T (2004) Antimicrobial activity and composition of essential oils from Origanum, Thymbra and Satureja species with commercial importance in Turkey. Food Control 15(3):169–172
Bekhit ED, Geesink GH, Ilian MA, Morton JD, Bickerstaffe R (2003) The effects of natural antioxidants on oxidative processes and metmyoglobin reducing activity in beef patties. Food Chem 81(2):175–187
Curcio M, Puoci F, Iemma F, Parisi OI, Cirillo G, Spizzirri UG (2009) Covalent insertion of antioxidant molecules on chitosan by a free radical grafting procedure. J Agric Food Chem 57:5933–5938
Das DK, Dutta H, Mahanta CL (2013) Development of a rice starch-based coating with antioxidant and microbe-barrier properties and study of its effect on tomatoes stored at room temperature. LWT Food Sci Technol 50:272–278
Dicastillo CL, Castro-Lopez MDM, Lopez-Vilarino JM, Gonzalez-Rodriguez MV (2013) Immobilization of green tea extract on polypropylene films to control the antioxidant activity in food packaging. Food Res Int 53:522–528
Fennema OR, Damodara S, Parkin KL (1996) Fennema’s Food Chem Chap 16, p 925.
Garrido MD, Auqui M, Marti N, Linares MB (2011) Effect of two different red grape pomace extracts obtained under different extraction systems on meat quality of pork burgers. LWT Food Sci Technol 44:2238–2243
Ghasemlou M, Khodaiyan F, Oromiehie A, Yarmand MS (2011) Development and characterization of a new biodegradable edible film made from kefiran, anexopolysaccharide obtained from kefir grains. Food Chem 127:1496–1502
Gontard N, Duchez C, Cuq JL, Guilbert S (1994) Edible composite films of wheat gluten and lipids: water vapour permeability and other physical properties. Int J Food Sci Technol 29(1):39–50
Gray JI, Gomaa EA, Buckley DJ (1996) Oxidative quality and shelf life of meats. Meat Sci 43:S11–S123
Greene BE, Hsin IM, Zipser MW (1971) Retardation of oxidative colour changes in raw ground beef. J Food Sci 36:940–942
Hayes JE, Stepanyan V, Allen P, O’Grady MN, Kerry JP (2011) Evaluation of the effects of selected plant-derived nutraceuticals on the quality and shelf-life stability of raw and cooked pork sausages. LWT Food Sci Technol 44:164–172
Hoque MS, Benjakul S, Prodpran T (2011) Properties of film from cuttlefish (Sepia pharaonis) skin gelatin incorporated with cinnamon, clove and star anise extracts. Food Hydrocoll 25:1085–1097
Hui YH, Nip W, Rogers RW, Young OA (2005) Meat Science and Applications. Chap. 1, p 2
Jongjareonraka A, Benjakula S, Visessanguanb W, Tanaka M (2008) Antioxidative activity and properties of fish skin gelatin films incorporated with BHT and α-tocopherol. Food Hydrocoll 22:449–458
Kim S, Cho AR, Han J (2013) Antioxidant and antimicrobial activities of leafy green vegetable extracts and their applications to meat product preservation. Food Control 29:112–120
Lee KT (2010) Quality and safety aspects of meat products as affected by various physical manipulations of packaging materials. Meat Sci 86:138–139
Li J, Miao J, Wu J, Chen S, Zhang Q (2014) Preparation and characterization of active gelatin-based films incorporated with natural antioxidants. Food Hydrocoll 37:166–173
Lopez-de-Dicastillo C, Gomez-Estaca J, Catala R, Gavara R, Hernandez-Munoz P (2012) Active antioxidant packaging films: development and effect on lipid stability of brined sardines. Food Chem 131:1376–1384
Lorenzo JM, Sineiro J, Amado IR, Franco D (2014) Influence of natural extracts on the shelf life of modified atmosphere-packaged pork patties. Meat Sci 96:526–534
Marana JP, Sivakumara V, Thirugnanasambandhama K, Sridharb R (2013) Response surface modeling and analysis of barrier and optical properties of maize starch edible films. Int J Biol Macromol 60:412–421
Mathew S, Brahmakumar M, Abraham TE (2006) Microstructural imaging and characterization of the mechanical, chemical, thermal and swelling properties of starch–chitosan blend films. Biopolymers 82:176–187
McBride NTM, Hogan SA, Kerry JP (2007) Comparative addition of rosemary extract and additives on sensory and antioxidant properties of retail packaged beef. Int J Food Sci Technol 42(10):1201–1207
Naveena BM, Vaithiyanathan S, Muthukumar M, Sen AR, Kumar YP, Kiran M, Shaju VA, Chandran KR (2013) Relationship between the solubility, dosage and antioxidant capacity of carnosic acid in raw and cooked ground buffalo meat patties and chicken patties. Meat Sci 95:195–202
Oliveira TLC, Carvalho SM, Araújo-Soares R, Andrade MA, Graças- Cardoso M, Ramos EM et al (2011) Antioxidant effects of Satureja montata L. essential oil on TBARS and color of mortadella-type sausages formulated with different levels of sodium nitrite. LWT Food Sci Technol 45(2):204–212
Ortiz-Vazquez H, Shin J, Soto-Valdez H, Auras R (2011) Release of butylated hydroxytoluene (BHT) from Poly (lactic acid) films. Polym Test 30:463–471
Serrano, Cofrades S, Jimenez-Colmenero F (2006) Characteristics of restructured beef steak with different proportions of walnut during frozen storage. Meat Sci 72(1):108–115
Siripatrawan U, Harte BR (2010) Physical properties and antioxidant activity of an active film from chitosan incorporated with green tea extract. Food Hydrocoll 24:770–775
Soradech S, Nunthanid J, Limmatvapirat S, Luangtana-anan M (2012) An approach for the enhancement of the mechanical properties and film coating efficiency of shellac by the formation of composite films based on shellac and gelatin. J Food Eng 108:94–102
Souza BWS, Cerqueira MA, Casariego A, Lima AMP, Teixeira JA, Vicente AA (2009) Effect of moderate electric fields in the permeation properties of chitosan coatings. Food Hydrocoll 23(8):2110–2115
Talja RA, Helen H, Roos YH, Jouppila K (2008) Effect of type and content of binary polyol mixtures on physical and mechanical properties of starch-based edible films. Carbohydr Polym 71(2):269–276
Ubonrat S, Bruce R (2010) Physical properties and antioxidant activity of an active film from chitosan incorporated with green tea extract. Food Hydrocoll 24:770–775
Wu J, Chen S, Ge S, Miao J, Li J, Zhang Q (2013) Preparation, properties and antioxidant activity of an active film from silver carp (Hypophthalmichthys molitrix) skin gelatin incorporated with green tea extract. Food Hydrocoll 32:42–51
Zhou GH, Xu XL, Liu Y (2010) Preservation technologies for fresh meat — a review. Meat Sci 86:119–128
Acknowledgements
Authors are thankful to Department of Biotechnology, Govt. of India for their financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
u Nisa, I., Ashwar, B.A., Shah, A. et al. Development of potato starch based active packaging films loaded with antioxidants and its effect on shelf life of beef. J Food Sci Technol 52, 7245–7253 (2015). https://doi.org/10.1007/s13197-015-1859-3
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-015-1859-3