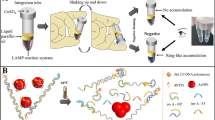
Abstract
We developed a dye-based visual detection technique for Escherichia coli, a common contaminant in raw milk and fruit juice. We used hydroxylnapthol blue (HNB) dye directly to E. coli-contaminated milk and juice samples, prior to loop-mediated isothermal amplification (LAMP) of tuf gene. The samples detected using HNB dye was further validated by running them in gel electrophoresis. LAMP was used for amplification of tuf gene as it is more specific and sensitive over conventional PCR. The detection limit of LAMP was estimated to be of 101 to 4 cfu ml−1 of E. coli, as against 102 to 5 cfu ml−1 in PCR. However, the detection limit of E. coli in milk (101 cfu ml−1) was lower compared to fruit juice (104 cfu ml−1) suggesting that the presence of fruit pulps in juice sample interfered with the detection. The developed HNB-based visual detection is superior over other dye based detection methods as HNB is added prior to LAMP reaction, and therefore the samples need not be opened for mixing dyes; thereby reducing the chances of cross contamination of the samples. The present study established a specific, easy-to-use, efficient, rapid and naked-eye based detection technique for E. coli contamination in milk and pulpy fruit juice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Raw or processed milk and fruit juices are well-known source that supports the microbial growth leading to spoilage of the product as well as infection /intoxication of consumers (Murinda et al. 2004; Oliver et al. 2005). Among the common microbial contaminants, Escherichia coli (E. coli) is the most predominant as indicated by the microbes in fecal pollution (Bonacorsi and Bingen 2005). Generally the non-pathogenic population of E. coli comprises main inhabitants of the intestinal tract of most mammalian species including humans (Marrs et al. 2005; Ochman and Selander 1984). However, very often the pathogenic population causes severe intestinal and extra intestinal diseases (such as the urinary tract, meninges, and the bloodstream) in humans (Johnson 2003; Klein et al. 2006).
The conventional culture-based diagnosis of E. coli infections requires bacterial culture to be obtained after 1–2 days of incubation in suitable growth media and subsequent confirmatory testing. The culture-based detection is thus not only laborious but time-consuming, requiring selective enrichment on different agar media followed by presumptive biochemical, serological or molecular confirmation (Romero et al. 1995). Although the DNA based diagnosis using PCR can provide fast results, its use is restricted partly because it requires considerable skill and expensive equipment. PCR based technique is quick and it remains effective for detection of pure microbial cultures. Thus, application of PCR for the direct detection of microbial contaminants in food has been limited due to the complex nature of the starting materials, including certain inhibitors of PCR amplification (Rossen et al. 1992; Wilson 1997). Moreover the sensitivity of PCR amplification of E. coli from contaminated milk samples was found to be low compared to that of bacterial cultures. Besides some false-negative PCR results were also reported (Romero et al. 1995). The variety of inhibitors including organic and inorganic substances (such as antibiotics, detergents, enzymes, fats, phenolics, polysaccharides, proteins and salts) are reported to inhibit the PCR amplification efficiency resulting in reduced sensitivity or false positive reactions (Rossen et al. 1992; Wilson 1997). This necessitates the development of a rapid and sensitive method especially for the detection of microbial contaminants in sources like milk and fruit juices.
Loop mediated isothermal amplification (LAMP) is a novel nucleic acid based amplification technique which can amplify the target DNA with high specificity, efficiency, and rapidity under isothermal condition (Nagamine et al. 2002; Notomi et al. 2000). Besides, LAMP does not involve sophisticated instruments like thermal cycler, gel electrophoresis apparatus. A water bath or heating block is sufficient to provide a constant temperature as the amplification proceeds under isothermal conditions and positive LAMP reactions can be visualized with naked eye (Mori et al. 2001). Unlike PCR, LAMP does not require a denatured template as it employs Bst DNA polymerase with strand displacement activity (Notomi et al. 2000). The LAMP reaction is more rapid generating large amounts of the target DNA in a short time. Another important advantage of the LAMP reaction is the ability to overcome the interference during amplification as caused by some inhibitory materials such as a culture medium and other biological substances that commonly affect the efficiency of the PCR (Kaneko et al. 2007). In this direction, the present study aimed to develop a dye-based quick and rapid LAMP-mediated detection of E. coli, a common bacterial contaminant in fruit juices and milk, targeting tuf gene.
Material and methods
Bacterial strains and culture conditions
Bacterial strains used in this study include Escherichia coli strain JM109, E. coli strain DH5α, Salmonella typhimurium, and S. enterica. The strains were cultured overnight in LB medium at 37 °C, at 180 rpm. Bacterial genomic DNA was prepared by lysis of bacterial pellet in 100 μl of lysis buffer (20 mM Tris–HCl, pH 8.0 and 1.2 % Triton X-100) that was boiled for 10 min.
Primers for PCR reaction
tuf gene sequences available from public databases were analyzed with GCG programs (version 8.0; Accelrys, Madison, WI, USA). Based on multiple sequence alignment and the oligo primer analysis software (Version 5.0; National Biosciences, Plymouth, MN, USA) PCR primers were designed from tuf gene, highly conserved regions of the E. coli. The chosen E. coli-specific PCR primers were TEcoI553 (5′-TGGGAGCGAAAATCCTG-3′) and TEcoI754 (5′-CAGTACSGGTAGACTTCTG-3′).
Primers for LAMP reaction
In order to design the species-specific primers for LAMP reaction, we first searched the nucleic acid sequence of E. coli deposited in the GenBank database, and used BLAST program to choose the species-specific gene sequences. The accession number of the tuf gene was J01609. The sequence was further analyzed by the Primer Explorer V1 software program (http;//venus.netlaboratory.com/partner/lamp/pevl.html) to have LAMP primers: F3 (Forward outer primer), B3 (Backward outer primer), FIP (Forward Inner primer), and BIP (Backward inner primer) (Table 1). In addition, each internal primer that recognizes both sense and anti-sense strands of the target DNA was connected by a TTTT spacer. Lamp designed LAMP primers were outsourced for synthesis.
PCR amplification conditions
Colonies of E. coli strains were resuspended in 25 ml sterile nuclease free distilled water (SNW) and denatured at 95 °C for 10 min. The samples were centrifuged at 8000 rpm for 5 min to pellet down the debris. The supernatant was used as a template. The PCR was carried out as per standard protocol. Amplification conditions were, initial denaturation at 94 °C, 5 min; denaturation at 94 °C, 30s; primer annealing at 56 °C, 30s and extension at 72 °C, 1 min. This step was repeated for 35 cycles in an automated thermal cycler. Final extension was done at 72 °C for 5 min. The PCR amplicons were run on 1.5 % agarose gel and gel stained with ethidium bromide where visualized under the UV transilluminator.
Artificial inoculation of samples
For contamination of milk and fruit juice, common E. coli strains (DH5α, JM109), Salmonella typhimurium, and S. enterica were employed. Pasteurized whole milk (Mother dairy Co.) and fruit juice (Mango Maza) was artificially inoculated with each of the bacterial strains @107 cfu ml−1 (and hereafter referred as stock samples). The inoculated stock sample (107 cfu ml−1) was serially diluted up to 7 folds (adding 1 ml of the stock to 9 ml of the sample). Thus varied levels of bacterial contamination (like 107, 106, 105, 104, 103, 102, 101, 100) in the samples were prepared. The bacterial number in the culture was subsequently estimated by standard plate counts. Bacterial cells in the samples were recovered by centrifugation at 6000 g for 20 min at 4 °C and the pellets were then treated at 95 °C for 10 min.
Sample preparation for direct visualization of E. coli
Milk
Milk samples were centrifuged at 10,000 rpm for 10 min and supernatant containing milk fat was removed. Milk protein like casein and whey were removed by washing with 0.5 M EDTA at 10,000 rpm for 7 min. A wash with mild non-ionic detergent (0.1 %) was used to remove remaining fats and hydrophobic proteins. Two subsequent sterile nuclease free water (SNW) washes (8000 rpm for 5 min) were performed to get rid of any trace EDTA or Triton X-100. Bacterial pellet was denatured in 25 ml of SNW at 95 °C for 5 min, centrifuged at 8000 rpm for 5 min. The DNA present in supernatant was used as a template for PCR and LAMP.
Mango juice
The artificially inoculated mango juice was centrifuged at 1500 rpm for 10 min to selectively pellet down the pulp. 1 ml of the supernatant obtained was centrifuged further at 7000 rpm at 10 min. The pellet was washed thrice with SNW. The pellet was dissolved in 25 ml of SNW and kept for incubation at 95 °C for 5 min. After spinning at 8000 rpm, the supernatant obtained was used as template for the PCR and LAMP.
Optimization of LAMP conditions
The LAMP reactions were optimized with respect to MgSO4, deoxynucleotide triphosphate (dNTPs), betaine and temperature. Five concentrations of MgSO4 (10 mM, 20 mM, 30 mM, 40 mM, 50 mM), six concentrations of dNTPs (2 mM, 4 mM, 4 mM, 6 mM, 8 mM, 10 mM), four concentrations of betaine (5 M, 4 M, 3 M, 2 M), and five ranges of temperature (55 °C, 57 °C, 59 °C, 61 °C, 63 °C) were used to optimize the LAMP reaction. These concentrations were chosen based on previous reports. LAMP reactions were put with each variable to optimize the best amplification conditions using a standard reaction mixture. A 25 μl of the standard reaction mixture contained 0.5 μl FIP (100 μM), 0.5 μl BIP (100 μM), 0.5 μl F3 (10 μM), 0.5 μl B3 (10 μM), 1 μl Bst DNA polymerase (8U) (large fragment; New England Biolabs Inc., USA), 2.5 μl thermopol buffer (10X), 4 μl MgSO4 (20 mM or variable tested), 3.5 μl dNTP mix (10 mM or variable tested), 5 μl betaine (5 M or variable tested), 1 μl template DNA (100 ng), and 6.0 μl water. LAMP reaction was carried out at 61 °C for 1 h.
Visual detection of LAMP products
One μl of 120 μM hydroxynapthol blue (HNB) dye was added to each tube before LAMP amplification. The non-amplified template (along with LAMP mixture) was used as control. The change of dark violet colour (control) to light blue colour indicated the positive for amplification.
Results and discussion
Microbial contaminants in raw milk and fruit juice are the major concern for health related hazards in human being. The microbe-contaminated food materials lead to infection /intoxications to the consumers (Murinda et al. 2004; Oliver et al. 2005). Escherichia coli is the most common contaminants in fruit juice and milk (Bonacorsi and Bingen 2005; Johnson 2003). The early detection of microbial contaminants is essential to reduce the toxic-food related health hazards. The conventional culture-based detection of microbial contaminants and their subsequent identification take long time and also tedious. DNA-based detection through polymerase chain reaction (PCR) is effective but issues like false positives, sensitivities are very common (Romero et al. 1995; Rossen et al. 1992; Wilson 1997) and it also requires highly expensive equipments including thermal cycler. The diagnostic technique based on loop mediated isothermal amplification (LAMP) is becoming popular as it overcomes these problems. The LAMP reaction is more sensitive as could rapidly generate large amounts of the target DNA in a short time amplifying three different regions of the target DNA (Notomi et al. 2000). Besides, the LAMP could overcome the interference caused by inhibitory materials such as a culture medium and other biological substances that commonly affect the efficiency of the PCR (Nagamine et al. 2002). Therefore, in the present study, we developed a dye-based quick and rapid detection method for E. coli in contaminated fruit juices and milk. For this purpose we used LAMP-amplified tuf gene of E. coli. Pasteurized whole milk and fruit juice was artificially contaminated with varied doses of bacterial culture. The HNB dye was preferred over the other dyes as it can be mixed directly in the sample prior to amplification and thereby the reaction tubes were not required to be opened reducing the risk of cross-contamination.
Optimization of LAMP assay condition for E. coli detection
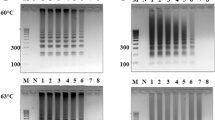
We initially optimized and standardized the LAMP assay for E. coli detection by using two outer, two inner primers and loop primers from tuf gene (Table 1) and DNA template from the E. coli strain. The tuf gene was chosen because it is highly conserved in E. coli (Maheux et al. 2009). The desired LAMP products were further confirmed through restriction digestion with Sma 1. The restricted profile of LAMP product resulted two distinct fragments, at 48 bp and 181 bp, confirming the single site-specific digestion of the desired amplicon of 209 bp (Fig. 1). The appearance of 9 visible bands starting at 209 bp in a ladder-like fashion on agarose gel confirmed the specific amplification (Fig. 2).
Optimization of the LAMP reaction for detection of E. coli. The optimized parameters are marked by underlined gel wells in the gel panel as well as its corresponding values in the legend. a. Effect of MgSO4 on the LAMP: M = 1 kb ladder, 1 = −ve control (E. coli), 2 = 10 mM, 3 = 20 mM, 4 = 30 mM, 5 = 40 mM, 6 = 50 mM. b. Effect of deoxynucleotide triphosphate on the LAMP: M = 1 kb ladder, 1 = +ve control (E. coli), 2 = −ve control, 3 = 2 mM, 4 = 4 mM, 5 = 4 mM, 6 = 6 mM, 7 = 8 mM, 3 = 10 mM. c. Effect of betaine on the LAMP: M = 1 kb ladder, 1 = 5 M 2 = 4 M, 3 = 3 M, 4 = 2 M. D. Effect of temperature on LAMP: M = 1 kb ladder, 1 = 55 °C, 2 = 57 °C, 3 = 59 °C, 4 = 61 °C, 5 = 63 °C
Effect of Mg++ concentration
Concentration of free Mg++ affects primer annealing and DNA polymerase activity (Saiki et al. 1998). In this study, the effect of Mg++ concentrations on LAMP reaction was determined. The Mg++ concentration at 30 mM gave the optimal amplification (Fig. 2a). Though the concentration ranging from 20 to 40 mM gave amplification but quality (as revealed by distinct ladder formation was observed at 30 mM. The concentration of Mg++ are reported to be varied ranging from 0 to 150 mM. The concentration determined in this study is lower than reported concentration as used in other studies (Wang et al. 2012).
Effect of deoxynucleotide triphosphate concentration
The deoxynucleotide triphosphate (dNTP) concentration largely affects the specificity of DNA polymerase amplification (Innis et al. 1988). The LAMP reaction in presence of various concentrations of dNTP was tested. The dNTP concentrations ranging from 0 to 10 mM amplified the target DNA, but 4 mM gave the maximal reaction product (Fig. 2b). The concentration of dNTP was found to be lower compared to the other reports (Wang et al. 2012).
Effect of betaine concentration
Betaine plays important role in improving the DNA amplification (Frackman et al. 1998). Thus LAMP reaction in presence of different concentrations of betaine was tested (Fig. 2c). We observed that with the increase in betaine concentrations the LAMP reaction efficiency also improved. The concentration of 5 M betaine resulted best amplification. This concentration falls within the range of other studies (Nagamine et al. 2002; Notomi et al. 2000). Contrary, there are few reports, wherein lower concentration of betaine (0.8 M) was found to be optimal for LAMP reaction (Yeh et al. 2005). The differences in the concentration of betaine are most likely due to the differences in the target DNA sequences (Henke et al. 1997). Though the exact role of betaine in improving the LAMP reaction is not fully understood, but the suggested mechanisms explain that betaine makes DNA templates accessible for DNA polymerase (Henke et al. 1997) and it destabilizes GC-rich DNA sequences (Rees et al. 1993).
Effect of temperature
Although the Bst DNA polymerase has the optimal activity at 65 °C, several reports showed this enzyme can amplify DNA templates at lower temperatures in the LAMP reaction (Iwamoto et al. 2003; Parida et al. 2004; Yoshikawa et al. 2004). The effect of temperature on the LAMP reaction was determined. In our study, LAMP reaction temperature at 61 °C generated ladder-like pattern products, but no such typical pattern product was detected at 55 °C and 57 °C (Fig. 2d).
Specificity of LAMP-based detection of E. coli targeting tuf gene
To determine the specificity of the primers to the E. coli, tuf gene from 3 species of bacteria, namely S. typhimurium, S. enterica, E. coli strain DH5α (used as positive control), that are closely associated with E. coli were tested. Each bacterial species was cultured overnight and harvested by centrifugation. The DNA templates were prepared by lysing bacterial pellets in the lysis buffer. We observed that primers only amplified the tuf gene (Fig. 3). This result suggests that these primers are specific for detection of E. coli and is consistent with other studies of specificities of LAMP assays in bacterial detection (Enosawa et al. 2003; Iwamoto et al. 2003; Maruyama et al. 2003).
Comparative sensitivity of PCR and LAMP assay for the detection of E. coli in milk
Milk, one of the most consumed dairy products, is highly susceptible to bacterial contamination. Macromolecules like proteins and fats present in milk might interfere with PCR assay. Thus, removal of theses macromolecules are required to prevent PCR inhibition. Most of the casein proteins (around 80 % of total milk protein) are incorporated in the micelles, together with a high proportion of the available calcium and inorganic phosphate (Ward et al. 1997). We added chelating agent like EDTA to sequester calcium ions and to disrupt the micelles. EDTA has shown to be used for protein denaturation and thereby increases the proportion of casein and whey protein in supernatant (Ward et al. 1997). We used triton X-100 that disperses fat flocculants to remove any residual lipids (Bringe et al. 1996). The pellet was washed with SNW to remove the left over detergent, if any, and used as template for PCR. To determine the comparative sensitivity of the developed LAMP assay with PCR detection, raw milk sample was artificially contaminated with different cell density of E. coli. The PCR technique used was able to detect as few as 103 cfu ml−1. Contrary the developed LAMP assay was found to be highly sensitive with the detection limit as low as 101 cfu ml−1 (Fig. 4). The higher sensitivity of the LAMP assay compared to the PCR was documented in several studies with various bacterial strains including E. coli, Edwardsiella ictaluri, Flavobacterium columnare, and Salmonella spp. (Hara-Kudo et al. 2008; Song et al. 2005; Teh et al. 2014; Wang et al. 2008, 2009; Yano et al. 2007; Yeh et al. 2005, 2007). However, most of these studies, particularly with reference to E. coli, were designed to detect virulence loci or markers of specific strains, such as verotoxin (Hara-Kudo et al. 2008; Song et al. 2005; Yano et al. 2007). In the present study we target tuf gene, a highly conserved gene across the E. coli strains (Maheux et al. 2009) and thus the developed LAMP method would not be limited to a particular strain rather would be more useful for detecting contamination of E. coli, irrespective of the strains.
Validation of dye-based detection with gel-based detection of E. coli in milk. a. PCR panel: M = 1 kb ladder, 1 to 6 = 107, 106, 105, 104, 103, 102 cells of E. coli ml−1. b1. LAMP panel: M = 1 kb ladder, 1 to 8 = 107, 106, 105, 104, 103, 102, 101, 100 cells of E. coli ml−1. b2. Dye panel: The presence of E. coli (as labeled in b1) were visualized directly after adding hydroxy napthol blue (1 μl of 120 μM) in the tube prior to LAMP reaction. “+” indicates amplification and “-” indicates no amplification
Comparative sensitivity of PCR and LAMP assay for the detection of E. coli in fruit juice
Fruit juices are good source of microbial contamination. The efficiency of the designed PCR technique was tested with mango juice sample (Mango maza brand). The spiked fruit juice was neutralized with NaOH to remove the acids present which might inhibit PCR. The pulp was eliminated by low-speed centrifugation which is sufficient to pellet down bacteria. Washes with SNW can remove proteins that pellet along with the bacteria. To compare the sensitivity of LAMP with conventional PCR detection, fruit juice was contaminated with E. coli at varied cell density. The result indicated that PCR was able to detect only105 cfu ml−1 while the detection limit for LAMP assay was around 104 cfu ml−1 (Fig. 5). The lower detection limit of LAMP as well as PCR with fruit juice sample compared to milk samples is thought to be due inhibition of amplification reaction by the fruit pulps (Rossen et al. 1992).
Validation of dye-based detection with gel-based detection of E. coli in fruit juice. a. PCR panel: M = 1 kb ladder, 1 to 5 = 107, 106, 10 5, 104, 103 cells of E. coli ml−1. b1. LAMP panel: M = 1 kb ladder, 1 to 5 = 107, 106,105, 10 4, 103 cells of E. coli ml−1. b2. Dye panel: The presence of E. coli (as labeled in B1) were visualized directly after adding hydroxy napthol blue (1 μl of 120 μM) in the tube prior to LAMP reaction. “+” indicates amplification and “-” indicates no amplification
Dye based visualization of LAMP products
The amplified LAMP products were directly visualized using 1 μl of 120 μM hydroxynapthol blue dye (HNB) (Figs. 4b2, 5b2). We observed the colour change in positive LAMP reaction from violet to light blue. The result indicated the simplicity of the developed LAMP technique where the amplification can be directly visualized without any aid of gel electrophoresis apparatus. Further we validated the HNB-based visualization with gel electrophoresis-based visualization. HNB has been used for visualization of LAMP reaction with λ DNA (Goto et al. 2009) and recently with human metapneumovirus (Wang et al. 2012). In this line, we demonstrate the utility of HNB for naked-eye visualization of bacterial contamination.
In conclusion, the present study established a specific, easy-to-use, efficient, rapid and naked-eye based detection technique for E. coli contamination in milk and pulpy fruit juice. The technique utilizes HNB dye-based visualization of LAMP-amplified tuf-gene. We target tuf-gene, a much conserved domain, in order to detect the E. coli irrespective of the strains contamination. We used LAMP-amplification technique as it is highly sensitive compared to conventional PCR. Thus, the developed technique would be of immense use in detecting the E. coli contamination as it overcomes the cumbersome process of gel-electrophoresis. Further, the technique is superior over other dye based detection methods as HNB is mixed with the sample prior to LAMP reaction and thereby reduces the chances of cross contamination of the samples during the process of opening of reaction tubes for mixing of dyes as needed in other cases.
References
Bonacorsi S, Bingen E (2005) Molecular epidemiology of Escherichia coli causing neonatal 10 meningitis. Int J Med Microbiol 295:73–381
Bringe NA, Howard DB, Clark DR (1996) Emulsifying properties of low-fat, low-cholesterol egg yolk prepared by supercritical CO2 extraction. J Food Sci 61:19e23
Enosawa M, Kageyama S, Sawai K, Watanabe K, Notomi T, Onoe S, Mori Y, Yokomizo Y (2003) Use of loop-mediated isothermal amplification of the IS900 sequence for rapid detection of cultured Mycobacterium avium subsp. paratuberculosis. J Clin Microbiol 41:4359–4365
Frackman S, Kobs G, Simpson D, Storts D (1998) Betaine and DMSO: enhancing agents for PCR. Promega Notes 65:27
Goto M, Honda H, Ogura A, Nomoto A, Hanaki KI (2009) Colorimetric detection of loop-mediated isothermal amplification reaction by using hydroxyl naphthol blue. BioTechniques 46:167–172
Hara-Kudo Y, Konighi N, Ohtsuka K, Hiramatsu R, Tanaka H, Takatori K (2008) Detection of verotoxigenic escherichia coli O157 and O26 in food by plating methods and LAMP method: a collaborative study. Int J Food Microbiol 122:156–161
Henke W, Herdel K, Jung K, Schnorr D, Loening SA (1997) Betaine improves the PCR amplification of GC-rich DNA sequences. Nucleic Acids Res 25:3957–3958
Innis MA, Myambo KB, Gelfand DH, Brow MAD (1988) DNA sequencing with Thermus aquaticus DNA polymerase and direct sequencing of polymerase chain reaction-amplified DNA. Proc Natl Acad Sci U S A 85:9436–9440
Iwamoto T, Sonobe T, Hayashi K (2003) Loop-mediated isothermal amplification for direct detection of Mycobacterium tuberculosis complex, M. avium, and M. intracellulare in sputum samples. J Clin Microbiol 41:2616–2622
Johnson JR (2003) Microbial virulence determinants and the pathogenesis of urinary tract infection. Infect Dis Clin N Am 17:261–278
Kaneko H, Kawana T, Fukushima E, Suzutani T (2007) Tolerance of loop-mediated isothermal amplification to a culture medium and biological substances. J Biochem Biophys Methods 70:499–501
Klein EJ, Boster DR, Stapp JR, Wells JG, Qin X, Clausen CR, Swerdlow DL, Braden CR, Tarr PI (2006) Diarrhea etiology in a Children’s hospital emergency department: a prospective cohort study. Clin Infect Dis 43:807–813
Maheux AF, Picard FJ, Boissinot M, Bissonnette L, Paradis S, Bergeron MG (2009) Analytical comparison of nine PCR primer sets designed to detect the presence of Escherichia coli/Shigella in water samples. Water Res 43:3019–3028
Marrs CF, Zhang L, Foxman B (2005) Escherichia coli mediated urinary tract infections: are there distinct uropathogenic E. coli (UPEC) pathotypes? FEMS Microbiol Lett 252:183–190
Maruyama F, Kenzaka T, Yamaguchi N, Tani K, Nasu M (2003) Detection of bacteria carrying the stx2 gene by in situ loop-mediated isothermal amplification. Appl Environ Microbiol 69:5023–5028
Mori Y, Nagamine K, Tomita N, Notomi T (2001) Detection of loop-mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochem Biophys Res Commun 289:150–154
Murinda SE, Nguyen LT, Man HM, Almedia RA (2004) Detection of sorbitol negative andsorbitol-positive shiga toxin-producing Escherichia coli, Listeria monocytogenes, Campylobacter jejuni and Salmonella species in dairy farm environments. Food-borne Pathog Dis 1:97–104
Nagamine K, Hase T, Notomi T (2002) Accelerated reaction by loop-mediated isothermal amplification using loop primers. Mol Cell Probes 16:223–229
Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T (2000) Loop mediated isothermal amplification of DNA. Nucleic Acids Res 28:e63
Ochman H, Selander RK (1984) Standard reference strains of Escherichia coli from natural populations. J Bacteriol 157:690–693
Oliver SP, Jayarao BM, Almedia R (2005) Food borne pathogens in milk and the dairy environment food safety and public health implications. Food-borne Pathog Dis 2:1115–1129
Parida M, Posadas G, Inoue S, Hasebe F, Morita K (2004) Real-time reverse transcription loop-mediated isothermal amplification for rapid detection of West Nile virus. J Clin Microbiol 42:257–263
Rees WA, Yager TD, Korte J, von Hippel PH (1993) Betaine can eliminate the base pair composition dependence of DNA melting. Biochemistry 32:137–144
Romero C, Pardo M, Grillo MJ, Diaz R, Blasco JM, Lopez G (1995) Evaluation of PCR and indirect-ELISA on milk samples for the diagnosis of brucellosis in dairy cattle. J Microbiol 33:3198–3200
Rossen L, Norskov P, Holmstrom K, Rasmussen OF (1992) Inhibition of PCR by components of food samples, microbial diagnostic assays and DNA extraction solutions. Int J Food Microbiol 17:37–45
Saiki RK, Gelfand DH, Stoffel S, Scharf SJ, Higuchi R, Horn GT, Mullis KB, Erlich HA (1998) Primer-directedenzymatic amplification of DNA with a thermostable DNA polymerase. Science 239:487–491
Song T, Toma C, Nakasone N, Iwanaga M (2005) Sensitive and rapid detection of Shigella and enteroinvasive Escherichia coli by a loop-mediated isothermal amplification method. FEMS Microbiol Lett 243:259–263
Teh CSJ, Chua KH, Lim YAL, Lee SC, Thong KL (2014) Loop-mediated isothermal amplification assay for detection of generic and verocytotoxin-producing Escherichia coli among indigenous individuals in Malaysia. Sci World J. doi:10.1155/2014/457839
Wang L, Shi L, Alam MJ, Geng Y, Li L (2008) Specific and rapid detection of foodborne Salmonella by loop-mediated isothermal amplification method. Food Res Int 41:69–74
Wang D, Liu F, Huo G, Ren D, Li Y (2009) Development and evaluation of a loop-mediated isothermal amplification method for detecting Escherichia coli O157 in raw milk. J Rapid Methods Autom Microbiol 17:55–66
Wang X, Zhang Q, Zhang F, Ma F, Zheng W, Zhao Z, Bai Y, Zheng L (2012) Visual detection of the human metapneumovirus using reverse transcription loop-mediated isothermal amplification with hydroxynaphthol blue dye. Virol J 9:138
Ward BR, Goddard SJ, Augustin MA, McKinnon IR (1997) EDTA-induced dissociation of casein micelles and its effect on foaming properties of milk. J Dairy Res 64:495e–504
Wilson IG (1997) Inhibition and facilitation of nucleic acid amplification. Appl Environ Microbiol 63:3741–3751
Yano A, Ishimaru R, Hujikata R (2007) Rapid and sensitive detection of heat-labile I and heat-stable I enterotoxin genes of enterotoxigenic Escherichia coli by loop-mediated isothermal amplification. J Microbiol Methods 68:414–420
Yeh HY, Shoemaker CA, Klesius PH (2005) Evaluation of a loop-mediated isothermal amplification method for rapid detection of channel catfish Ictalurus punctatus important bacterial pathogen Edwardsiella ictaluri. J Microbiol Methods 63:36–44
Yeh HY, Shoemaker CA, Klesius PH (2007) Sensitive and rapid detection of Flavobacterium columnare in channel catfish Ictalurus punctatus by a loop-mediated isothermal amplification method. J Appl Microbiol 100:919–925
Yoshikawa T, Ihira M, Akimoto S, Usui C, Miyake F, Suga S, Enomoto Y, Suzuki R, Nishiyama Y, Asano Y (2004) Detection of human herpesvirus 7 DNA by loop-mediated isothermal amplification. J Clin Microbiol 42:1348–1352
Acknowledgments
This study was supported by the grant of NFBSFARA (20–17/ TG-2814) from Indian Council of Agricultural Research, New Delhi. Authors thanks to Dr. S.N. Jha and Dr. Pranita Jaiswal for providing E. coli strain JM109. We thank Head, Division of Plant Pathology for providing necessary support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, S., Mondal, K.K. Visual detection of Escherichia coli contamination in milk and fruit juice using loop-mediated isothermal amplification. J Food Sci Technol 52, 7417–7424 (2015). https://doi.org/10.1007/s13197-015-1779-2
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-015-1779-2