Abstract
Since a decade, there has been a strong consumer demand for more natural products. This has augmented inclination towards substitution of synthetic colorants with natural pigments. Natural pigments not only have the capacity to increase the marketability of products, they also demonstrate valuable biological activities as antioxidants and anticancer agents. There is a long history of exploitation of natural products produced by bacteria as sources of pharmaceutically important, bioactive compounds. Among natural pigments, pigments from microbial sources are potentially suitable alternatives to synthetic pigments. The red pigment prodigiosin (PG) has unusual properties, which have long been documented. The red-pigmented prodiginines are bioactive secondary metabolites produced by both Gram-negative and Gram-positive bacteria. Prodigiosins are characterized by a common pyrrolyl pyrromethene skeleton, and the biological role of these pigments in the producer organisms remains unclear. Bacterial prodigiosins and their synthetic derivatives are effective proapoptotic agents against various cancer cell lines, with multiple cellular targets including multi-drug resistant cells with little or no toxicity towards normal cell lines. However, research into the biology of pigment production will stimulate interest in the bioengineering of strains to synthesize useful prodiginine derivatives. This review article highlights the characteristics and potential applications of prodigiosin pigment from Serratia as prodigiosins are real potential therapeutic drugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Good aesthetic value of food material is the colour that the food exhibits. Colour of a food material is vital to show its freshness and safety which also indicate good aesthetic and sensorial values of food. Pigments have been identified with properties essential to many industries. In the food industry, pigments are used as additives, colour intensifiers, antioxidants, etc. Pigments represent extensive variety of colours, some of which are water-soluble (Cserháti 2006). Recently, colouring of food with pigments produced from natural sources is gaining worldwide interest and importance. With undesirable and unsafe market of synthetic pigments, natural dyes are considered safe replacements for food use. Also, awareness of positive health benefits out of natural pigments, the demand for natural sources is increasing linearly Thus it is imperative to explore diverse natural sources of food grade colorants and their potentials. Microbial colorants play a momentous role as food colorants, because of their production and down streaming processing is easy. Industrial production of natural food colorants by microbial fermentation is economical, extraction is simple, higher yields can be obtained through strain improvement.
Sources of pigments
Natural pigments can be obtained from two primary sources, plants (Mizukami et al. 1978) and microorganisms (Cross et al. 1972) The natural pigments from plants have several drawbacks such as instability to light, heat or adverse pH, low water solubility and are often not accessible throughout the year. But the microbial pigments are of great interest due to the stability of the pigments produced and the ease of cultivation (Parekh et al. 2000). The microorganisms have easy and fast growth in the cheap culture medium, independent from environmental conditions and different shades of colours are produced. Hence, microbial pigment production has now been one of the emerging fields of research to display its potential for a variety of industrial applications.
Microbial pigments
Microorganisms are known to elaborate a variety of pigments and hence they are a promising source of food colorants (Ahmad et al. 2012). The majority of the bacteria and fungi are considered for their prospective as a source of food colorants. Natural pigments contain pro-vitamin A, possess many beneficial properties such as anticancer activity, and have additional valuable properties such as stability to light, heat and pH (Joshi et al. 2003). The pigments from microbial sources can be a good alternative to synthetic colorants. Microorganisms are known to produce an array of pigments. Therefore, they are potential sources of food colorants (Ahmad et al. 2012). Thus, the food industry has been keen on adopting microbial technology to produce colours for food application. This can also facilitate the growing public apprehension over the adverse health effects of synthetic colours added to food products. In addition, production of natural colorants will also be an advantage for the preservation of biodiversity as this can prevent the release of harmful chemicals into the environment. The colours are employed in baby foods, breakfast cereals, pastas, sauces, processed cheese, fruit drinks, vitamin-enriched milk products, and some energy drinks. Thus, natural colours are proposed to be the environment friendly, visually appealing and can also have probiotic health benefits in food products (Nagpal et al. 2011). Some of the most significant natural pigments are listed (Table 1).
Classification of pigments
Pigments are either organic/inorganic or natural/synthetic. Structural affinities and natural occurrence of biological pigments has been used as basis of classification of biological pigments.
Microorganisms producing prodigiosin
Prodigiosin, a secondary metabolite has been found to be produced by Serratia marcescens, Pseudomonas magneslorubra, Vibrio psychroerythrous, Serratia rubidaea, Vibrio gazogenes, Alteromonas rubra, Rugamonas rubra and Gram positive actinomycetes, such as Streptoverticillium rubrireticuli and Streptomyces longisporus ruber forms prodigiosin and / or derivatives of this molecule (Khanafari et al. 2006). The actinomycete Streptomyces coelicolor A3(2) has been shown to produce a closely prodigiosin related linear tripyrrole, undecylprodigiosin, and a cyclic derivative, butylmeta-cycloheptylprodiginine in a 2 : 1 ratio (Harris et al. 2004).
Factors influencing prodigiosin production
Prodigiosin, a secondary metabolite appears only in the later stages of bacterial growth (Harris et al. 2004). The production of prodigiosin has been observed to be effected by several environmental factors, inorganic phosphate availability, media composition, temperature and pH (Williamson et al. 2005). Number of differential and selective media has been used for the isolation and production of pigment from Serratia spp. The liquid media presently being employed for prodigiosin biosynthesis are nutrient broth (0.52 mg mL-1) (Pryce and Terry 2000) peptone glycerol broth (0.302 mg mL-1).
A comparative study was done using powdered seed of sesame in water, nutrient broth and peptone glycerol broth on growth of S. marcescens (Giri et al. 2004). Sesame seed was observed yield better prodigiosin. Further readily accessible cheaper sources like. Sesame oil, peanut oil and coconut oil were also evaluated with the rest of the media. In nutrient broth, the maximum prodigiosin production was obtained at 28 and 30 °C. However, at 37 °C S. marcescens did not produce any pigment in nutrient broth and the culture broth was white in colour. In case of the powdered peanut broth pigment production was observed, even at 37 °C, which was equal quantitatively to the amount produced in nutrient broth at 30 °C (Giri et al. 2004). The pigment production was enhanced in sesame seed broth even in the absence of any sugars, Glucose and maltose in powdered sesame seed medium inhibited the prodigiosin production at both 28 and 30 °C. This reduction could be due to catabolite repression.
Purification of prodigiosin
Prodigiosin was extracted from the bacterial biomass using acidified ethanol, until complete discolouration of the biomass. The ethanol extract was evaporated to dryness at 45–50°С, and the residue was redissolved in chloroform. The resulting solution was mixed with an equal volume of a water–ethanol mixture (4:1) and emulsified on a magnetic stirrer for 1 h at room temperature. The water soluble contaminants from water–ethanol mixture were separated using a separating funnel. Then, the pigment obtained was dried in a vacuum drier and redissolved in ethanol. Liquid chromatography was carried out on a glass column using silica gel. The pigment dissolved in ethanol was applied onto the surface of the sorbent and was eluted from the column successively with hexane–acetone 3:1 and then with acetone. Then the pigment was further purified using preparative thin layer chromatography on glass silica gel 60 F254 plates using hexane–ethyl ether–acetic acid (70:30:1). Single pigment bands were scraped off from plates, and pigment was eluted from silica gel using the following solvents successively: 96 % ethanol, acetone and chloroform (Guryanov et al. 2013).
Chemical/Spectral analysis of prodigiosin
Prodigiosin from Serratia was subjected to chemical analysis. It has been reported to be having different spectral curves at acid, alkaline and neutral pH, Comparison of the acid and alkaline curves with Hubbard and Ramington (Hubbard and Rimington 1950) indicated to be similar to that of acetone extracted pigment. Prodigiosin can exist in two distinct forms depending upon the hydrogen ion concentration of the solution. In an acid medium, the red pigment exhibits a sharp spectral peak at 535 mμ. The shoulder on the low wavelength limb of the acid curve at about 510 mμ was observed to be persistent in the whole pigment. In an alkaline medium, the pigment is coloured orange-yellow and possesses a broader spectral curve centred at 470 mμ (Williams et al. 1956).
Chemistry of prodigiosin
Prodiginines group of pigments are characterized by a common pyrrolyl dipyrromethene skeleton that contains a common 4-methoxy, 2–2 bi pyrrole ring system. Bacterial prodiginines have been divided into linear and cyclic derivatives (Table 2). Examples of the linear derivatives include prodigiosin and undecylprodigiosin, and examples of cyclic derivatives include streptorubin B, cycloprodigiosin, and cyclononylprodigiosin (Williamson et al. 2006; Mo et al. 2008).
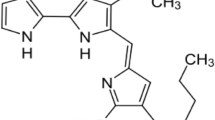
The structure of prodigiosin, the archetypal prodiginine, was elucidated in the early 1960s by partial and total chemical synthesis revealing a pyrrolyl dipyrromethene core skeleton (Wasserman et al. 1960; Rapoport and Holden 1962). The three pyrrolic rings of prodigiosin are conventionally labelled as pyrrolic ring A, ring B, and ring C (Fig. 1). Prodigiosin exists in two interconverting rotamers, cis (or β) and trans (or α) (Fig. 1). The balance between these forms is dependent on the pH of the solution as the trans form protonates more easily (Fürstner et al. 2001).
The structure of the archetypal prodiginine, prodigiosin. Prodigiosinexists in solution as a mixture of cis (or β) and trans (or α) rotamers in a ratio that is dependent on the pH of the solution (Fürstner et al. 2001). The three pyrrole rings of prodigiosin are labeled as rings a, b, and c. The lower structure shows the binding of all three pyrrolic nitrogens of prodigiosin with chloride ion when it is acting as a H + /Cl − symporter
Biosynthesis of prodiginines
The biosynthesis of prodiginines has been found to proceed via a bifurcated pathway culminating in the enzyme - catalyzed condensation of 4 - methoxy - 2–2 ′ - bipyrrole - 5 -carbaldehyde (MBC) with a monopyrrole. MBC is either condensed with 2 - methyl - 3 - pentylpyrrole (MPP) to form prodigiosin. Biosynthesis of the prodiginines proceeds via a bifurcated pathway culminating in the enzymic condensation of the bipyrrole, 4 - methoxy-2-2′ - bipyrrole −5 - carbaldehyde (MBC) with either 2 - methyl - 3 - pentylpyrrole (MPP) to form prodigiosin. The prodiginine biosynthetic clusters of several prodiginine producing organisms have been sequenced. These include two Serratia sp. (pig clusters) (Harris et al. 2004), S. coelicolor (red cluster) (Cerdeño et al. 2001), Hahella chejuensis (hap cluster) (Kim et al. 2007), the tambjamine cluster of Pseudoalteromonas tunicata (tam cluster) (Burke et al. 2007), and the prodigiosin cluster of the roseophilin producer, Str. griseoviridis (rph cluster) (Kawasaki et al. 2009). Tambjamines, of which the anticancer compound BE18591 is a member, are bipyrrole compounds that are structurally similar to the prodiginines. All clusters contain a conserved set of genes which are homologous to each of the MBC biosynthetic enzymes of Serratia, suggesting a common route to the biosynthesis of MBC. An exception to this is the absence of a pigN / redF homologue in both the rph and tam clusters of P. tunicata and S. griseoviridis. pigN mutants of Serratia sp. ATCC 39006 produce a mixture of prodigiosin and norprodigiosin. This suggested that PigN is not essential, but may facilitate PigF -catalyzed the methylation of 4 - hydroxy - 2,2 ′ - bipyrrole - 5 – carbaldehyde (HBC) (Williamson et al. 2005). Both P. tunicata and S. griseoviridis contain pigF homologues, tamP, and rphI, suggesting that these enzymes alone are sufficient for methylation of MBC in these two pathways.
The biosynthesis of MBC has been studied in Serratia sp. and Streptomyces using genetic approaches, complementation, and in vitro analysis of the purified enzymes involved in the early steps of this pathway (Thomas et al. 2002; Williamson et al. 2005; Walsh et al. 2006; Stanley et al. 2006; Mo et al. 2008) The degree of conservation of the prodiginine biosynthetic clusters suggested that biosynthesis of MBC proceeded via a common route requiring proline, acetate/malonate, serine and methionine (Fig. 2) (Williams 1973; Williamson et al. 2005; Stanley et al. 2006) Proline was incorporated to form pyrrole by a mechanism common to other pyrrole – containing compounds such as chlorobiocin, coumermycin A1, novobiocin, and pyoluteorin (Thomas et al. 2002; Garneau-Tsodikova et al. 2006). In prodiginine biosynthesis, proline would get incorporated to form pyrrolic ring A in a sequence of reactions catalyzed by PigA, PigG, and PigI and their homologues (Thomas et al. 2002; Garneau-Tsodikova et al. 2006) In the following steps, catalyzed by PigJ and PigH (and homologues), a C2 unit from malonyl CoA and a C2N unit from serine would be incorporated (both with concomitant decarboxylation) forming 4 - hydroxy −2,2 ′ - bipyrrole - 5 - methanol (HBM) (Williamson et al. 2005; Stanley et al. 2006). The final steps in MBC biosynthesis would be catalyzed by PigM and would involve the oxidation of an alcohol group of HBM forming 4 - hydroxy - 2,2′ - bipyrrole - 5- - carbaldehyde (Fig. 2) (HBC). The hydroxyl group of HBC would be then methylated to form MBC (Williamson et al. 2005). This final methylation step would be catalyzed by homologues of PigF, which, in some prodiginine producers, would be facilitated by homologues of PigN.
Str. coelicolor and Serratia sp. condensed MBC with different monopyrroles. These were synthesized using completely different enzymes, substrates, and pathways (Fig. 2) (Cerdeño et al. 2001; Harris et al. 2004; Mo et al. 2008; Williamson et al. 2005; Mo et al. 2008). The pathways and predicted functions of the enzymes have been based entirely on homology with other enzymes, gene deletions, liquid chromatography – mass spectrometry (LC - MS) analysis of accumulating intermediates, and complementation experiments (Williamson et al. 2005; Mo et al. 2008). The biosynthesis of MPP in Serratia has been found to be catalyzed by PigD, PigE, and PigB (Fig. 2). However, the precise substrates required for MPP biosynthesis are uncertain. The biosynthetic intermediate H2MPP has been shown to accumulate in a Δ pigB mutant. Hence, PigB has been proposed to catalyze the oxidation of H2MPP to form the terminal product of this pathway, MPP (Williamson et al. 2005).
Biosynthesis of prodiginines and related compounds was shown to culminate in the condensation of MBC with either a monopyrrole, MPP (for prodigiosin) (Williamson et al. 2005) This condensation reaction was proposed to be catalyzed by a novel family of pyrrole - condensing enzymes, characterized by PigC (Fig. 2) (Williamson et al. 2005). All of the biosynthetic clusters for prodiginines and related compounds sequenced to date have been found to contain a PigC homologue. PigC homologues was shown to include RedH (undecylprodigiosin), RphH (prodigiosin R1), HapC (prodigiosin), TamQ (tambjamine YP1), and a PigBC fusion enzyme from Janthinobacterium lividum. The exact nature of the compound produced by J. lividum has not been identified. However, the PigBC fusion enzyme has been the only known example of a multifunctional enzyme that appeared to catalyze both the final step of monopyrrole biosynthesis and the terminal condensation reaction (Schloss et al. 2010). As more PigC homologues have been identified, sequence alignments, site - directed mutagenesis, and crystallography must be done to identify the critically involved conserved residues. Knowledge of key residues of the active sites of the condensing enzymes, and enzymes involved in the earlier steps of the biosynthetic pathways, may allow directed evolution studies of the biosynthetic enzymes to generate novel prodiginines which might then be produced cheaply in large - scale fermentations. The prodiginine biosynthetic clusters of bacteria that produce cyclic prodiginine derivatives all contain at least one RedG homologue.
Genes responsible for prodigiosin production
The biosynthesis and complex regulation of prodigiosin in Serratia sp. ATCC 39006 involves over 30 genes, suggesting some selective ecological advantage in the production of prodigiosin by the bacteria. As with many secondary metabolites, the physiological function of prodiginines in the producing organism is unclear. However, proposed physiological functions are numerous, and include functions associated with prodigiosin’s antibacterial, antifungal, or antiprotozoal activity, a role as a metabolic sink, involvement in surface adherence, and enhancing bacterial dispersal (Gerber 1969).
The red cluster was observed to consist of 23 genes organised into four transcription units (Fig. 3). The left limit of the red cluster has been defined by the trkA operon that has been shown to encode a potassium uptake system, which is not vital for prodiginine production but which can influence titres in liquid media. Evaluation of the restriction map for pIJ941, which caused copious prodiginine production when introduced into S. parvulus (Malpartida et al. 1990) with the map generated from the sequence data limiting the right-hand end of the cluster. Two of the 23 genes in the cluster (redD and redZ, orange in Fig. 3) have been shown previously to encode pathway-specific regulators (Narva and Feitelson 1990; White and Bibb 1997; Guthrie et al. 1998). Of the remaining 21 genes, six have been assigned to 4-methoxy-2,2 P-bipyrrole-5-carboxaldehyde biosynthesis (red in Fig. 3), eight have been assigned to 2-undecylpyrrole biosynthesis (blue in Fig. 3), and two have been assigned as housekeeping genes (green in Fig. 3). Comparison of the chromosomal position of the genes assigned to 4-methoxy-2, 2P-bipyrrole-5-carboxaldehyde and 2-undecylpyrrole biosynthesis with the regions of the cluster presumed to be involved in the biosynthesis of these two moieties by complementation and co synthesis tests (Coco et al. 1991) showed excellent agreement. The proteins encoded by the remaining five genes in the cluster (white in Fig. 3) showed no likeness to proteins of known function and their accountability, in prodiginine biosynthesis has been shown to be unknown.
Organisation of the prodiginine biosynthetic gene cluster in S. coelicolor A3(2). Genes deduced to be involved in 2-undecylpyrrole biosynthesis are blue, genes deduced to be involved in 4-methoxy-2,2P-bipyrrole-5-carboxaldehyde biosynthesis are red, putative housekeeping genes are green, regulatory genes are in orange and genes of unknown function are white. Black arrows illustrate the four mRNA molecules likely to be generated by transcription of the cluster. The regions of the cluster spanned by the cosmids SC2E9, SCF7 and SC10A5 from the S. coelicolor ordered cosmid library are indicated in grey (nucleotide sequence accession numbers: SC2E9: AL021530; SC3F7: AL021409; SC10A5: AL021529)
Toxicological studies with prodigiosin
Prodigiosin has been found to have bacteriostatic activity. Agar disc diffusion sensitivity studies were done against E. coli, E. aerogenes, S. aureus, B. subtilis and P. aeruginosa with prodigiosin and its fractions dissolved in 100 % DMSO. There was no diffusible bacteriostatic activity in vitro with the solvent DMSO indicating the ineffectiveness of DMSO. Prodigiosin and the fractions, the ethanol and methanol fractions produced inhibition zones with all the organisms tested. Prodigiosin has been shown to have effect on embryogenesis. Prodigiosin and its fractions isolated in five organic solvents, petroleum ether, chloroform, acetone, ethanol and methanol, tested on chick embryogenesis showed that the whole pigment and Chloroform fraction were highly toxigenic while other fractions demonstrated toxicities with higher LD50 values of 26 to 30 μg egg−1 when dissolved in 100 % DMSO. The Ethanol fraction in DMSO was very slightly toxic. 95 % ethanol established to be highly toxic at a level of 0.1 ml egg−1 demonstrating that it was an inappropriate solvent for studies of this nature. Prodigiosin extracts have been observed to have toxigenic effects on chick embryos and inhibited the growth of several species of bacteria (Kalesperis et al. 1975). The Ames test was carried out with S. typhimurium TA 100. The toxic effect of prodigiosin was established from the survival of Salmonella typhimurium TA 100 in experimental variants as compared with the control (in the absence of prodigiosin). The study of the genotoxicity of prodigiosin using the micronucleus test showed an insignificant induction of micronuclei in polychromic erythrocytes of animals at all prodigiosin concentrations. Thus, it was shown for the first time in the Ames test and in the micronucleus test in vivo that purified prodigiosin produces no genotoxic effect (Guryanov et al. 2013).
Pharmacological activity
Antimalarial
The antimalarial action of natural and synthetic prodiginines has been quantitatively estimated in terms of chemometric descriptors. The statistically authenticated quantitative structure-activity relationship (QSAR) models offered rationale to clarify the activity against Plasmodium falciparum D6 strain of these compounds. Through combinatorial protocol in multiple linear regression (CP-MLR) analysis the participation of various chemometric 2D-descriptors has been highlighted. The major contributing descriptors were the information content index of 5-order neighbourhood symmetry (IC5), the mean topological charge indices of order five (JGI5), the Moran autocorrelation – lag 6/weighted by atomic masses (MATS6m) and the Geary autocorrelation–lag 5/weighted by atomic Sanderson electronegativities (GATS5e). The higher values of the descriptors IC5 and JGI5 and lower values of the descriptors MATS6m and GATS5e are required further to improve the antimalarial activity of a compound (Singh and Shekhawat 2012). 53 synthetic prodiginines were assessed for in vitro anti-malarial activity against P. falciparum pansensitive D6 in comparison with chloroquine (CQ).
Antibacterial
Prodigiosin was inactive against Pseudomonas aeruginosa. It had no activity against Escherichia coli, Proteus vulgaris, Candida albicans, Trichoderma koningi or Penicillium notatum (Gerber 1969). Serratia marcescens IBRL USM 84 produced intracellular antibacterial red pigment Prodigiosin (Darah Ibrahim 2014). The inhibitory effect of prodigiosin on Gram positive and Gram negative bacteria was studied and it was observed that it has a more inhibitory effect on Gram positive bacteria including Staphylococcus aureus, Staphylococcus saprophyticus, Bacillus subtilus, Enterococcus avium and Streptococcus pyogenes than Gram negative bacteria such as Echerichia coli, Pseudomonas aeruginosa, Aeromonas hydrophila, Proteus mirabilis and Klebsiella pneumonia. In addition, Prodigiosin pigment was observed to be a good curing agent on plasmids of E. coli HB101 and S. aureas but failed to cure plasmids of Proteus mirabilis and Enterococcus avium. (Mekhael and Samira 2009). The antibacterial activity of prodigiosin (PG) was the result of the ability of prodigiosin to pass through the outer membrane and inhibiting target enzymes such as DNA gyrase and topoisomerase IV, which inhibited the cell growth (Berlanga et al. 2000).
Anticancerous activity
Prodigiosin has been tested against more than 60 cancer cell lines with an average inhibitory concentration of 2.1 μM (Manderville 2001; Williamson et al. 2007). Prodigiosin has been shown to have multiple cellular targets. Therefore, the exact mode of action of prodiginines in inducing apoptosis is uncertain. Prodiginines are also attractive options because they are not affected by several multidrug resistance pumps which can confer resistance to other anticancer agents (Soto-Cerrato et al. 2004; Llagostera et al. 2005). Prodiginines have been described as proapoptotic anticancer compounds and have been shown to induce cellular stresses such as cell cycle arrest, DNA damage, and a change of intracellular pH (pHi), all of which can induce apoptosis (Fig. 4). The cellular location of prodigiosin, might give clues to its mechanism of action, because prodigiosin has been detected in the nucleus (Llagostera et al. 2005), concentrated in the cytoplasm of cells (Baldino et al. 2006), in granules near to the nucleus (Kataoka et al. 1995), and in the mitochondrial membrane (Francisco et al. 2007). Proapoptotic activity of prodigiosin in the nucleus of the cell would support the hypothesis of DNA cleavage as Proapoptotic activity. However, the hydrophobic nature of the synthetic prodiginine, Obatoclax, has been proposed to be important in the localization of the compound to the mitochondrial membrane which may promote interaction with the antiapoptotic Bcl-2 family members that reside in this membrane (Nguyen et al. 2007). Two major apoptotic pathways congregating effector caspases. The intrinsic pathway also identified as the mitochondrial cell death pathway, has been found to be activated by radiation, cytotoxic drugs, cellular stress, and withdrawal of growth factor. The extrinsic cell death pathway has been proposed to function by activating an initiator caspase, caspase - 8, Bid cleavage and activation of caspase - 3 and caspase - 7 independently of mitochondria. The extrinsic cell death pathway was induced directly by the cell death receptors Fas and the tumor necrosis factor - related apoptosis - inducing ligand (TRAIL) receptors. The lead prodiginine anticancer agent, Prodigiosin has been shown to intercalate into dsDNA. In the presence of copper, they promote oxidative cleavage of the DNA (Melvin et al. 2000; Melvin et al. 2002). This correlation between cytotoxicity and double - strand cleavage led to the hypothesis that cleavage of both strands of dsDNA is important for the proapoptotic anticancer activity of the prodiginines. Prodigiosin has also been shown to bind by intercalation, preferentially at AT sequences, from the minor groove (Melvin et al. 2000). The copper - mediated DNA cleavage of prodiginines inducing apoptosis, of cancer cells with little, or no, activity against nonmalignant cells has been explained by the availability of copper as cancer cells are reported to contain approximately 3.5 - fold higher concentrations of Cu(II) than non-malignant cells. prodigiosin and its analogue provide lead compounds to rescue deficiencies in the p53 pathway in cancer cells by up-regulating p73 and targeting mutant p53/p73 interaction there (Hong et al. 2014).
Drawbacks
The mechanism by which prodigiosin bring out the apoptosis is unknown (Campàs et al. 2003). Other prodigiosins lack apoptotic activity, Prodigiosin is less toxic than fludarabine for normal T cells but exhibited similar effect in B-cell chronic lymphocytic leukemia (B-CLL) cells. Moreover, prodigiosin induced apoptosis in B-CLL cells that were resistant to treatment with fludarabine (Campàs et al. 2003). Thus, these results suggested that inhibition of these signaling pathways could not explain the apoptotic activity of prodigiosins in B and T lymphocytes (Campàs et al. 2003). In vitro, prodigiosin was found to bind to DNA, assisting oxidative cleavage of double-strand DNA that correlated with cytotoxicity (Melvin et al. 2000). DNA damage brought about the accumulation of p53 tumor suppressor protein (Vogelstein et al. 2000), though, p53 was not induced by prodigiosin in B-CLL cells. Additionally, prodigiosin induced apoptosis in Jurkat and HL-60 cells that were deficient in p53 (Vogelstein et al. 2000). These results suggested that prodigiosin induced apoptosis independently of p53 and DNA damage (Campàs et al. 2003). Also, prodigiosins promoted H+/Cl-, a symport activity leading to acidification of the cytosol. This activity was implicated in cycloprodigiosin-induced apoptosis because imidazole inhibited both acidification and apoptosis in different human cancer cell lines (Yamamoto et al. 1999; Yamamoto et al. 2000).
Immunosuppressive activity
The ability of prodiginines to inhibit the cell cycle has been exploited, at non-apoptotic doses, as an immunosuppressant. Prodigiosin has been shown to suppresses graft versus host disease (GvHD) with no observable signs of toxicity in mouse models (Han et al. 2001). There was delayed autoimmune diabetes progression by Prodigiosin. Also, there was prevention of GvHD and collagen - induced arthritis in mouse models (Han et al. 2001). Other forms of Prodigiosin such as Undecylprodigiosin, metacycloprodigiosin, and cycloprodigiosin all selectively inhibitted T cell proliferation. However, varying levels of in vivo toxicity (Pérez-Tomás and Montaner 2003) were displayed.
Cell cycle inhibition
Prodiginines have been shown to arrest the cell cycle at multiple stages. The variety of effects has been attributed to their different structures and/or experimental systems (Pérez-Tomás and Montaner 2003). Prodigiosin inhibited proliferation of human Jurkat T cells in the G1/S phase transition, The role for prodigiosin in the late G1 phase was shown by decreased expression of cyclin E, cdk2, and cdk4, all of which are known to be expressed in mid - to - late G1 phase of cell cycle. In addition, prodiginines have been shown to inhibit phosphorylation of retinoblastoma Rb protein (Songia et al. 1997), which is an important mediator of the progression from G1 phase to S phase. Also, inhibit cyclin E, cdk2, p27, p21, and Rb phosphorylation in leukemic Jurkat cells, has been shown resulting in apoptosis (Montaner and Pérez-Tomás 2003). Prodigiosin has also been shown to affect the accumulation of p53 and induction of NAG - 1, in the human Mcf - 7 breast cancer cell line (Soto-Cerrato et al. 2007). Prodiginines have not been shown to affect expression of IL - 2, which forms a complex with its receptor (IL - 2R), which is another important checkpoint in T cell proliferation. Again, a range of effects specific to given prodiginines have been observed at this stage of the cell cycle.
Prodigiosin as food colorant
Pigments from microorganisms can serve as an alternative source to replace synthetic pigments used in the food industry, with few limitations including sensitivity, solubility, and short stability upon exposure to pH, light and high temperatures. Hence, spray-dried microcapsules containing prodigiosin was produced using kappa-carrageenan and maltodextrin as encapsulation agents after optimizing the effect of spray-drying parameters on the encapsulation yield (EY), moisture content, particle size, colour intensity of the prodigiosin microcapsules. The particles were successfully applied to yogurt, milk and carbonated drinks.(Namazkar and Ahmad 2013).
Prodigiosin as a colorant for polyolefines
The requirements for general organic pigments used for polymer coloration, namely, wide acceptable temperature range, lightness stability, fine dispersion in the carrying material, a good migratory stability are challenging and result in high cost of organic pigments. The microbial pigments may be attributed to the ecologically safe compounds in the chemical industry also. Prodigiosin, a natural bacterial pigment can be a good substitute for coloration of polyolefines. Prodigiosin from Serratia marcescens strain 9986 was used as a dye of polyolefines (polyethylene, ultratene). The pigment suspension was introduced gradually up to rolled polymer sheet for the equilibrium coloration of the dyed-stuff. The manufactured polymer sheet served as a colored concentrate. The pigment preparation prodigiosin was used in the technology dyeing as a thick suspension-concentrate without spraying in the air (Ryazantseva and Andreyeva 2014).
Prodigiosin as Potential Sunscreen
A demonstration of prodigiosin as a sunscreen has been shown by Suryawanshi et al.(Suryawanshi et al. 2014). Prodigiosin was observed to increase the sunscreen protection factors (SPF) of commercial sunscreens. Prodigiosin was used as an additive with extracts of Aloe vera leaf, and Cucumis sativus (cucumber) fruit which have photo-protective activity, and commercial sunscreen preparations. With the addition of prodigiosin, for the plant extracts, SPFs were increased by an order of magnitude (i.e., up to ~3.5) and those for the commercial sunscreens increased by 20–65 %.
Conclusions
Prodigiosins, a family of natural red pigments characterized by a common pyrrolylpyrromethane skeleton, are elaborated by a variety of bacteria. It first characterized from Serratia marcescens. This promising pigment has antifungal, immunosuppressive and antiproliferate activity. Prodigiosin could be a replacement of natural pigments to synthetic colorants for possible application in the food industry. The assortment and the application of novel polyethylene concentrates and masterbatches based on the coloration by bacterial pigment can be expanded including raw material supply and appropriate technological innovations in the chemical industry. This specific pigment induced apoptosis in different cancer cell lines including acute human T-cell leukemia, promyelocytic leukemia, and human and rat hepatocellular cancer, human breast cancer and TNF-stimulated human cervix carcinoma. Prodigiosins promote H+/Cl- a symport activity leading to acidification of the cytosol. This specific activity has been associated in cycloprodigiosin induced apoptosis Under-standing the mechanism of prodigiosin-induced apoptosis and identification of its molecular target is at infancy and this would help to design more potent agents to induce apoptosis. The prodigiosin family is noteworthy for their varied range of biological effects, even though much deeper insight into the mode of action of these compounds is needed before a completely reliable and conclusive picture of prodigiosin family is drawn. Prodigiosins represent a new group of molecules with a common mechanism of action to select molecular targets. Prodigiosins may represent a new class of anticancer drugs. Although their mechanisms of apoptosis remain to be determined, additional in vivo assays are necessary. These studies coupled with the finding that prodigiosin is non genotoxic supports the gallant application of this compound in pharmaceutical and food industry. Evaluation of cytotoxic properties of prodigiosin against epithelial cells had merit with properties like chemically inert, non-irritating, non-toxic, non-allergic, non-carcinogenic and photostable thus having lot of biotechnological potential for use in commercial sunscreens, and the need for studies of mammalian cells to determine safety.
References
Ahmad WA, Wan Ahmad WY, Zakaria ZA, Yusof NZ (2012) Appl Bact Pigments Colorant. doi:10.1007/978-3-642-24520-6
Baldino CM, Parr J, Wilson CJ et al (2006) Indoloprodigiosins from the C-10 bipyrrolic precursor: new antiproliferative prodigiosin analogs. Bioorg Med Chem Lett 16:701–4. doi:10.1016/j.bmcl.2005.10.027
Berlanga M, Ruiz N, Hernandez-Borrell J et al (2000) Role of the outer membrane in the accumulation of quinolones by Serratia marcescens. Can J Microbiol 46:716–22
Browning DF, Whitworth DE, Hodgson DA (2003) Light-induced carotenogenesis in Myxococcus xanthus: functional characterization of the ECF sigma factor CarQ and antisigma factor CarR. Mol Microbiol 48:237–51
Burke C, Thomas T, Egan S, Kjelleberg S (2007) The use of functional genomics for the identification of a gene cluster encoding for the biosynthesis of an antifungal tambjamine in the marine bacterium Pseudoalteromonas tunicata. Environ Microbiol 9:814–8. doi:10.1111/j.1462-2920.2006.01177.x
Campàs C, Dalmau M, Montaner B et al (2003) Prodigiosin induces apoptosis of B and T cells from B-cell chronic lymphocytic leukemia. Leukemia 17:746–50. doi:10.1038/sj.leu.2402860
Cerdeño AM, Bibb MJ, Challis GL (2001) Analysis of the prodiginine biosynthesis gene cluster of Streptomyces coelicolor A3(2): new mechanisms for chain initiation and termination in modular multienzymes. Chem Biol 8:817–829
Coco EA, Narva KE, Feitelson JS (1991) New classes of Streptomyces coelicolor A3(2) mutants blocked in undecylprodigiosin (Red) biosynthesis. Mol Gen Genet 227:28–32
Cross BE, Edinberry MN, Turner WB (1972) Pigments of Gnomonia erythrostoma. Part I. The structures of erythrostominone, deoxyerythrostominone, and deoxyerythrostominol. J Chem Soc Perkin Trans 1:380. doi:10.1039/p19720000380
Cserháti T (2006) Liquid chromatography of natural pigments and synthetic dyes. 602
Darah Ibrahim TFNJKS-HL (2014) Prodigiosin - an antibacterial red pigment produced by Serratia marcescens IBRL USM 84 associated with a marine sponge Xestospongia testudinaria. J Appl Pharm Sci 4:001–006
Francisco R, Pérez-Tomás R, Gimènez-Bonafé P et al (2007) Mechanisms of prodigiosin cytotoxicity in human neuroblastoma cell lines. Eur J Pharmacol 572:111–9. doi:10.1016/j.ejphar.2007.06.054
Fürstner A, Grabowski J, Lehmann CW et al (2001) Synthesis and biological evaluation of nonylprodigiosin and macrocyclic prodigiosin analogues. Chembiochem 2:60–8
Garneau-Tsodikova S, Dorrestein PC, Kelleher NL, Walsh CT (2006) Protein assembly line components in prodigiosin biosynthesis: characterization of PigA, G, H, I, J. J Am Chem Soc 128:12600–1. doi:10.1021/ja063611l
Gerber NN (1969) Prodigiosin-like pigments from Actinomadura (Nocardia) pelletieri and Actinomadura madurae. Appl Microbiol 18:1–3
Giri AV, Anandkumar N, Muthukumaran G, Pennathur G (2004) A novel medium for the enhanced cell growth and production of prodigiosin from Serratia marcescens isolated from soil. BMC Microbiol 4:11. doi:10.1186/1471-2180-4-11
Golubev WI (1995) Perfect state of Rhodomyces dendrorhous (Phaffia rhodozyma). Yeast 11:101–10. doi:10.1002/yea.320110202
Guryanov ID, Karamova NS, Yusupova D V., et al (2013) Bacterial pigment prodigiosin and its genotoxic effect. Russ J Bioorganic Chem 39:106–111. doi:10.1134/S1068162012060040
Guthrie EP, Flaxman CS, White J et al (1998) A response-regulator-like activator of antibiotic synthesis from Streptomyces coelicolor A3(2) with an amino-terminal domain that lacks a phosphorylation pocket. Microbiology 144(Pt 3):727–38
Han SB, Park SH, Jeon YJ et al (2001) Prodigiosin blocks T cell activation by inhibiting interleukin-2Ralpha expression and delays progression of autoimmune diabetes and collagen-induced arthritis. J Pharmacol Exp Ther 299:415–25
Harris AKP, Williamson NR, Slater H et al (2004) The Serratia gene cluster encoding biosynthesis of the red antibiotic, prodigiosin, shows species- and strain-dependent genome context variation. Microbiology 150:3547–60. doi:10.1099/mic. 0.27222-0
Hong B, Prabhu VV, Zhang S et al (2014) Prodigiosin rescues deficient p53 signaling and antitumor effects via upregulating p73 and disrupting its interaction with mutant p53. Cancer Res 74:1153–65. doi:10.1158/0008-5472.CAN-13-0955
Hubbard R, Rimington C (1950) The biosynthesis of prodigiosin, the tripyrrylmethene pigment from Bacillus prodigiosus (Serratia marcescens). Biochem J 46:220–5
Joshi VK, Attri D, Baja A, Bhushan S (2003) Microb Pigments 2:362–369
Kalesperis GS, Prahlad KV, Lynch DL (1975) Toxigenic studies with the antibiotic pigments from Serratia marcescens. Can J Microbiol 21:213–20
Kataoka T, Muroi M, Ohkuma S et al (1995) Prodigiosin 25-C uncouples vacuolar type H(+)-ATPase, inhibits vacuolar acidification and affects glycoprotein processing. FEBS Lett 359:53–59
Kawasaki T, Sakurai F, Nagatsuka S, Hayakawa Y (2009) Prodigiosin biosynthesis gene cluster in the roseophilin producer Streptomyces griseoviridis. J Antibiot (Tokyo) 62:271–6. doi:10.1038/ja.2009.27
Khanafari A, Assadi MM, Fakhr FA (2006) Review of prodigiosin, pigmentation in Serratia marcescens Qods Sqr ., Tajrish Sqr . Tehran, Iran Department of Forest Sciences, Faculty of Forestry, The University of British Columbia, 4th Floor Forest Sciences Centre # 4320–2424 Main Mall Vancouver. 6:1–13
Kim D, Lee JS, Park YK et al (2007) Biosynthesis of antibiotic prodiginines in the marine bacterium Hahella chejuensis KCTC 2396. J Appl Microbiol 102:937–944. doi:10.1111/j.1365-2672.2006.03172.x
Llagostera E, Soto-Cerrato V, Joshi R et al (2005) High cytotoxic sensitivity of the human small cell lung doxorubicin-resistant carcinoma (GLC4/ADR) cell line to prodigiosin through apoptosis activation. Anticancer Drugs 16:393–9
Malik K, Tokkas J, Goyal S (2012) Microbial Pigments: a review. 361–365
Malpartida F, Niemi J, Navarrete R, Hopwood DA (1990) Cloning and expression in a heterologous host of the complete set of genes for biosynthesis of the Streptomyces coelicolor antibiotic undecylprodigiosin. Gene 93:91–9
Manderville RA (2001) Synthesis, proton-affinity and anti-cancer properties of the prodigiosin-group natural products. Curr Med Chem Anticancer Agents 1:195–218
Mekhael R, Samira Y (2009) The role of red pigment produced by Serratia marcescens AS. J Duhok Univ 12(No1 (Special Issue) 12):268–274
Melvin MS, Tomlinson JT, Saluta GR et al (2000) Double-strand DNA cleavage by Copper•Prodigiosin. J Am Chem Soc 122:6333–6334. doi:10.1021/ja0000798
Melvin MS, Tomlinson JT, Park G et al (2002) Influence of the A -ring on the proton affinity and anticancer properties of the prodigiosins. Chem Res Toxicol 15:734–741. doi:10.1021/tx025507x
Mizukami H, Konoshima M, Tabata M (1978) Variation in pigment production in Lithospermum erythrorhizon callus cultures. Phytochemistry 17:95–97. doi:10.1016/S0031-9422(00)89687-9
Mo S, Sydor PK, Corre C et al (2008) Elucidation of the Streptomyces coelicolor pathway to 2-undecylpyrrole, a key intermediate in undecylprodiginine and streptorubin B biosynthesis. Chem Biol 15:137–48. doi:10.1016/j.chembiol.2007.11.015
Montaner B, Pérez-Tomás R (2003) The prodigiosins: a new family of anticancer drugs. Curr Cancer Drug Targets 3:57–65
Nagpal N, Munjal N, Chatterjee S (2011) Microbial pigments with health benefits—a mini review. Trends Biosci 4:157–160
Namazkar S, Ahmad WA (2013) Spray-dried prodigiosin from Serratia marcescens as a colorant. Biosci Biotechnol Res Asia 10:69–76. doi:10.13005/bbra/1094
Narva KE, Feitelson JS (1990) Nucleotide sequence and transcriptional analysis of the redD locus of Streptomyces coelicolor A3(2). J Bacteriol 172:326–33
Nguyen M, Marcellus RC, Roulston A et al (2007) Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci U S A 104:19512–7. doi:10.1073/pnas.0709443104
Parekh S, Vinci VA, Strobel RJ (2000) Improvement of microbial strains and fermentation processes. Appl Microbiol Biotechnol 54:287–301
Pérez-Tomás R, Montaner B (2003) Effects of the proapoptotic drug prodigiosin on cell cycle-related proteins in Jurkat T cells. Histol Histopathol 18:379–85
Pryce LH, Terry FW (2000) Spectrophotometric assay of gene expression: Serratia marcescens pigmentation. Bioscene 26:3–13
Rapoport H, Holden KG (1962) The synthesis of prodigiosin. J Am Chem Soc 84:635–642. doi:10.1021/ja00863a026
Ryazantseva I, Andreyeva I (2014) Application of prodigiosin as a colorant for polyolefines. Adv Biol Chem 04:20–25. doi:10.4236/abc.2014.41004
Schloss PD, Allen HK, Klimowicz AK et al (2010) Psychrotrophic strain of Janthinobacterium lividum from a cold Alaskan soil produces prodigiosin. DNA Cell Biol 29:533–41. doi:10.1089/dna.2010.1020
Singh P, Shekhawat N (2012) chemometric descriptors in the rationale of antimalarial activity of natural and synthetic prodiginines. 2:244–260
Songia S, Mortellaro A, Taverna S et al (1997) Characterization of the new immunosuppressive drug undecylprodigiosin in human lymphocytes: retinoblastoma protein, cyclin-dependent kinase-2, and cyclin-dependent kinase-4 as molecular targets. J Immunol 158:3987–95
Soto-Cerrato V, Llagostera E, Montaner B et al (2004) Mitochondria-mediated apoptosis operating irrespective of multidrug resistance in breast cancer cells by the anticancer agent prodigiosin. Biochem Pharmacol 68:1345–1352. doi:10.1016/j.bcp.2004.05.056
Soto-Cerrato V, Viñals F, Lambert JR et al (2007) Prodigiosin induces the proapoptotic gene NAG-1 via glycogen synthase kinase-3beta activity in human breast cancer cells. Mol Cancer Ther 6:362–9. doi:10.1158/1535-7163.MCT-06-0266
Stahmann KP, Revuelta JL, Seulberger H (2000) Three biotechnical processes using Ashbya gossypii, Candida famata, or Bacillus subtilis compete with chemical riboflavin production. Appl Microbiol Biotechnol 53:509–16
Stanley AE, Walton LJ, Kourdi Zerikly M, et al. (2006) Elucidation of the Streptomyces coelicolor pathway to 4-methoxy-2,2’-bipyrrole-5-carboxaldehyde, an intermediate in prodiginine biosynthesis. Chem Commun (Camb) 3981–3. doi: 10.1039/b609556a
Suryawanshi RK, Patil CD, Borase HP et al (2014) Studies on production and biological potential of prodigiosin by Serratia marcescens. Appl Biochem Biotechnol 173:1209–21. doi:10.1007/s12010-014-0921-3
Takano H, Obitsu S, Beppu T, Ueda K (2005) Light-induced carotenogenesis in Streptomyces coelicolor A3(2): identification of an extracytoplasmic function sigma factor that directs photodependent transcription of the carotenoid biosynthesis gene cluster. J Bacteriol 187:1825–32. doi:10.1128/JB.187.5.1825-1832.2005
Thomas MG, Burkart MD, Walsh CT (2002) Conversion of L-proline to pyrrolyl-2-carboxyl-S-PCP during undecylprodigiosin and pyoluteorin biosynthesis. Chem Biol 9:171–184
Vogelstein B, Lane D, Levine AJ (2000) Surfing the p53 network. Nature 408:307–10. doi:10.1038/35042675
Walsh CT, Garneau-Tsodikova S, Howard-Jones AR (2006) Biological formation of pyrroles: nature’s logic and enzymatic machinery. Nat Prod Rep 23:517–31. doi:10.1039/b605245m
Wasserman HH, McKeon JE, Smith L, Forgione P (1960) prodigiosin. Structure and partial synthesis 1. J Am Chem Soc 82:506–507. doi:10.1021/ja01487a075
White J, Bibb M (1997) bldA dependence of undecylprodigiosin production in Streptomyces coelicolor A3(2) involves a pathway-specific regulatory cascade. J Bacteriol 179:627–33
Williams RP (1973) Biosynthesis of prodigiosin, a secondary metabolite of Serratia marcescens. Appl Microbiol 25:396–402
Williams RP, Green JA, Rappo-Port DA (1956) Studies on pigmentation of Serratia marcescens. I. Spectral and paper chromatographic properties of prodigiosin. J Bacteriol 71:115–20
Williamson NR, Simonsen HT, Ahmed RAA et al (2005) Biosynthesis of the red antibiotic, prodigiosin, in Serratia: identification of a novel 2-methyl-3-n-amyl-pyrrole (MAP) assembly pathway, definition of the terminal condensing enzyme, and implications for undecylprodigiosin biosynthesis in Streptomyces. Mol Microbiol 56:971–89. doi:10.1111/j.1365-2958.2005.04602.x
Williamson NR, Fineran PC, Leeper FJ, Salmond GPC (2006) The biosynthesis and regulation of bacterial prodiginines. Nat Rev Microbiol 4:887–899. doi:10.1038/nrmicro1531
Williamson NR, Fineran PC, Gristwood T et al (2007) Anticancer and immunosuppressive properties of bacterial prodiginines. Future Microbiol 2:605–618
Yamamoto C, Takemoto H, Kuno K et al (1999) Cycloprodigiosin hydrochloride, a new H(+)/Cl(−) symporter, induces apoptosis in human and rat hepatocellular cancer cell lines in vitro and inhibits the growth of hepatocellular carcinoma xenografts in nude mice. Hepatology 30:894–902. doi:10.1002/hep.510300417
Yamamoto D, Uemura Y, Tanaka K et al (2000) Cycloprodigiosin hydrochloride, H(+)/CL(−) symporter, induces apoptosis and differentiation in HL-60 cells. Int J Cancer 88:121–8
Yokoyama A, Izumida H, Miki W (1994) Production of astaxanthin and 4-Ketozeaxanthin by the marine bacterium, Agrobacterium aurantiacum. Biosci Biotechnol Biochem 58:1842–1844. doi:10.1271/bbb.58.1842
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Darshan, N., Manonmani, H.K. Prodigiosin and its potential applications. J Food Sci Technol 52, 5393–5407 (2015). https://doi.org/10.1007/s13197-015-1740-4
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-015-1740-4