Abstract
Bacteriocin producing Lactobacillus spicheri G2, isolated from Gundruk - a traditional fermented vegetable product of North East India. L. spicheri G2 identified by morphological, biochemical techniques followed by 16S rRNA gene technique. The 16Sr RNA sequence of bacteriocin producer is registered in NCBI under accession no. JX481912. The bacteriocin producing potential of L. spicheri is being reported for the first time in the present investigation. Bacteriocin of L. spicheri G2 showed strong antagonism against food spoiling and pathogenic bacteria viz. Listeria monocytogenes, Staphlococcus aureus, Clostridium perfringens, Streptococcus mutans, Lactobacillus plantarum, Leuconostoc mesenteroides and Bacillus cereus. Bacteriocin production of L. spicheri G2 was enhanced by optimization of production time, pH of medium and incubation temperature by following one variable at a time method. Maximum bacteriocin activity (2000 AU/ml) was recorded in MRS broth at 34 h with an initial pH of 4.0 after incubating at 35 °C. The bacteriocin was purified by single step gel exclusion chromatography. Molecular weight of this novel bacteriocin was determined by SDS PAGE which was found to be 43 kDa. Purified bacteriocin was found resistant to high temperature and varied pH range but sensitive to proteolytic enzymes like trypsin and proteinase k, the characters desirable for food preservation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ever since the ancient times research has been going on to expedite various methods to control detrimental microorganisms in food products. Though, number of preservation techniques and various kinds of preservatives have been recommended in food preservations. Comprising a subgroup within a far larger body of commercial food preservatives, use of natural preservative i.e., bacteriocin has become a trademark approach in food safety and preservation of processed food products. Bacteriocins are ribosomally synthesized extracellular released bioactive peptides or peptide complexes that vary in spectrum of activity, mode of action, molecular weight, genetic organization and considered to be safe biopreservatives since they are assumed to be degraded by proteases in to gastrointestinal tract (Balgir et al. 2010). Production of bacteriocins, their purifications, biochemical characterization in terms of their stability, host range and mode of antimicrobial action is also an essential criteria to evaluate their possible potential as food preservatives. Although, several LAB strains have been reported as bacteriocin producers (Hata et al. 2009) but many more potential bacteriocin producing bacterial strains are still unrecognized. Therefore, microbiologists around the world got interested to isolate and identify potential bacteriocin producing microorganisms that could get commercial status and can be use to preserve food products. The objective of this study was to purify and characterize new bacteriocin produced by L. spicheri G2 which is so far not reported for bacteriocin production.
Material and methods
Isolation of lactic acid bacteria
Lactic acid bacterial strain was isolated from Gundruk which is a non-salted, fermented acidic vegetable product and is consumed as a soup and pickle by people of North East region of India. The Leaves of mustard, radish and cauliflower are used in its preparation. Isolation was carried out on De Man Ragaosa Sharpe (MRS) agar under anaerobic conditions by standard spread plate method. Anaerobic conditions were maintained under anaerobic gas jars by using gas pack system (Hi-media, make). The bacterial colonies obtained on MRS agar were purified by streaking twice on MRS agar. The pure cultures were preserved at −20 °C on MRS broth containing 40 % glycerol (v/v) in deep freezer.
Screening of isolates on the basis of morphological, physiological and biochemical characteristics (Sharpe 1979; Kandler and Weiss 1986)
Colour, form, margin, elevation and texture of each isolated strain was noted down. Gram staining, catalase test, oxidase test, citrate utilization test, gas production from glucose, casein hydrolysis, H2S production and sugar fermentation were performed with isolated strains. The isolates with characteristics LAB profile viz., catalase negative, oxidase negative and non-motile were selected to check their bacteriocin producing potential.
Screening of isolates on the basis of bacteriocin producing potential
The bit disk method (Barefoot and Klaenhammer 1983) and well diffusion method (Kimura et al. 1998) was used to screen out bacteriocin producer. Bacteriocin activity in cell free supernatants was determined by activity unit per milliliter (AU/ml). Activity unit per ml was determined as the inverse of the last dilution at which growth inhibition was still detectable following the well diffusion (Barefoot and Klaenhammer 1983).
Molecular identification of isolate exhibiting bacteriocin activity
Hyper bacteriocin producer isolate G2 was subjected to 16S rRNA gene sequencing. Genomic DNA of G2 was isolated by using standard protocol of DNA prep kit (Banglore Genei, India Pvt. Ltd. make).
PCR amplification of 16S rRNA region
The PCR analysis was carried out with following concentration of reagents i.e., Taq buffer (10X) −5.0 μl; dNTP 2 mM-2.5 μl; Primer (F) - 1.0 μl; Primer (R) - 1.0 μl; Taq Polymerase - 0.2 μl; Glycerol- 0.5 μl; Water −12.8 μl; DNA - 1 μl; MgCl2−1 μl. The procedure consisted of 35 cycles of 92 °C for 1 min, 55 °C for 1 min, 72 for 1 min. The universal primers of expected product size i.e., (1500 bp) used for amplification were BITS-1(5′AGAGTTTGATCCTGG) and BITS-4-(5′-TACCTTGTTACGACTT). The amplified PCR product were clean up using PCR clean up kit (Real Genomics Hi yieldTM Make). Eluted PCR product of G2 was sequenced by commercial available services of Genei, Bangalore, India. The sequences obtained were analyzed using the NCBI nucleotide BLASTN bioinformatics tool.
Identification
On the basis of 16S rRNA gene technique G2 identified as Lactobacillus spicheri. The sequences so obtained were submitted in National Centre for Biotechnology Information (NCBI) to get an accession number. L. spicheri G2 registered under the accession no. JX481912.
Optimization of process parameters for bacteriocin production by using classical one variable at a time method
Various growth conditions viz. growth cycle, effect of incubation temperature, pH, inoculum size were studied to monitor their effect on bacteriocin production.
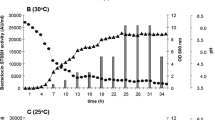
Bacteriocin production during growth cycle of L. spicheri G2 (Lade et al. 2006)
100 ml of MRS broth (pH 6.5 ± 2) was seeded with active bacterial isolate L. spicheri G2 @10 % (1.0 OD). Bacterial isolate was incubated in orbital shaker at 35 ± 2 °C with a shaking speed of 120 rpm for 60 h. OD520 and bacteriocin production of isolate was detected periodically after every 2 h. To detect bacteriocin production, the culture of L. spicheri G2 was centrifuged after every 2 h at 18,000 rpm at 4 °C for 20 min. The cell free supernatant was neutralized to pH 7.0 (with sterilized 1 N NaOH), catalase was added @ 10 mg in 100 ml and well diffusion method was repeated with this crude preparation against three selected standard indicators viz. Listeria monocytogenes, S. aureus and C. perfringens. The plates were kept for incubation at 35 ± 2 °C for 24 h and results were observed as clear halos of inhibition formed around the wells.
Effect of temperature on bacteriocin production
The optimum temperature for bacteriocin production for L. spicheri G2 was determined by inoculating 24 h old active culture (10 %) in Erlenmeyer flasks containing MRS broth followed by incubation at different temperature regime i.e., 25, 30, 35, 40, 45, 50 °C. The inhibitory activity of neutralized culture supernatant of L. spicheri G2 was determined after 34th h of growth by well diffusion assay against L. monocytogenes, S. aureus and C. perfringens.
Effect of pH on bacteriocin production
Twenty-four hours old culture of L. spicheri G2 was inoculated in MRS medium of varying pH in the range of 3.0, 3.5, 4.0, 4.5, 5.0, 5.5, 6.0, 6.5, 7.0, 7.5, 8.0 and effect of different pH on inhibitory activity of its neutralized culture supernatant was studied after 34 h of growth against three standard indicators viz. L. monocytogenes, S. aureus and C. perfringens by well diffusion method.
Effect of inoculum size
Twenty-four hours old active culture of L. spicheri G2 was inoculated with different inoculum size viz. 0.5, 1.0, 1.5 and 2.0 (O.D.) @ 10 % in MRS broth having best studied pH. The growth of bacterial strain was achieved by incubating it at best optimized temperature. The antagonistic pattern of nutrilized culture supernatant was determined after 34 h of growth by well diffusion assay against three reference targets.
Extraction and purification of bacteriocin
L. spicheri G2 having OD 1.5 was inoculated in MRS broth (pH 4.0) @ 10 %. Inoculated flasks were kept at 35 ± 2° C at 120 rpm for 34 h.
Lyophilization of bacteriocin
Culture supernatant of L. spicheri G2 (20 ml) was taken in round bottomed flask for concentrating produced bacteriocin. Supernatant was lyophilized in a lyophilizer (Allied Frost, make) for 5 h. Lyophilized supernatant was assayed to check its bacteriocin activity by well diffusion method.
Gel filtration chromatography
Sephadex G-75 (5 g) was suspended in 500 ml of distilled water for 48 h. It was packed into the glass column having dimensions of 31 × 2.5 cm avoiding entrapment of any air bubble in the gel bed as described by Kumar and Vadhera (1980). It was then equilibrated with the three bed volumes of 0.5 M phosphate buffer (pH 7.0). Lyophilized bacteriocin sample (2 ml) was loaded on the sephadex G-75 column. It was then eluted with three bed volumes of 0.5 M phosphate buffer (pH 7.0) and 3 ml fractions were collected. A flow rate of 3 ml in 7 min was maintained. The protein content of collected fractions were measured at 280 nm and fractions showing maximum absorbance were analyzed for bacteriocin activity. The most active fractions were pooled and stored at 4 °C. Purity of sample was checked out by 12 % SDS polyacrylamide slab gel electrophoresis.
Characterization of purified bacteriocin of L. spicheri G2
The purified bacteriocin of L. spicheri G2 was characterized by studying the effect of pH, heat, proteolytic enzymes.
Effect of pH on activity of purified bacteriocin (Motta and Brandelli 2002; Sharma et al. 2011)
0.5 ml of aliquot of purified bacteriocin was added to 2.5 ml of freshly prepared buffers of different pH in the range 3, 5….10, 11. (viz., Citrate phosphate buffer, 0.1 M for pH 3.0 to 7.0, Tris HCl buffer, 0.1 M for pH 8.0, and glycine- NaOH buffer 0.1 M for pH 9.0 to 11.0) These preparations were incubated for 30 min. at 35 ± 2 °C and was assayed using well diffusion method against their corresponding indicators
Effect of temperature on activity of purified bacteriocin
0.5 ml of purified bacteriocin preparation was added into 2.5 ml phosphate buffer (pH 7.0) in sterilized test tubes. Each test tube was then overlaid with paraffin oil to prevent evaporation and then treated at different temperatures of 40, 50, 60, 70, 80, 90 and 100 °C and 121 °C for 10 min. and was assayed using well diffusion method against their corresponding indicators. Purified bacteriocin was kept in autoclave for 10 min to treat it at 121 °C.
Effect of proteolytic enzyme–trypsin, proteinase k on activity of purified bacteriocin Enzyme activity
-
i)
Enzyme control I (C1): 0.3 ml phosphate buffer
-
ii)
Enzyme control II (C2): 0.15 ml purified bacteriocin + 0.15 ml of phosphate buffer
-
iii)
Enzyme reaction (ER): 0.25 mg of enzyme trypsin (ER1) and proteinase k (ER2) (Sigma chemicals) were dissolved in 1 ml of 0.5 M phosphate buffer separately. Purified bacteriocin was treated with each enzyme preparation in the ratio of 1:1. The preparations C1, C2, ER1 and ER2 were then incubated for 1 h at 37 °C. The enzyme reaction and both the enzyme control were assayed by well diffusion method against their corresponding indicators (Paik et al. 1997).
Result and discussion
Isolation and identification of bacteriocin producer
In total 8 bacteriocin producing strains were isolated from Gundruk which is a fermented leafy vegetable product consumed by the people of North East region of India and Nepal (Lucose et al. 2012). These bacteriocin producers were screened on the basis of their antagonistic pattern against food borne/spoilage causing test indicators. The serious food borne pathogens and spoilage causing microorganisms like Listeria monocytogenes MTCC 839, Staphylococcus aureus, Leuconostoc mesenteroides MTCC 107, Escherichia coli, Enterococcus faecalis MTCC 2729, Lactobacillus plantarum, Bacillus subtilis MTCC 121, Bacillus cereus MTCC 1272 and Clostridium perfringens MTCC 450 were chosen as test indicators to study the antagonistic pattern of isolates. Out of all 8 isolates, G2 was selected for further studies based upon its good activity units i.e., 2 × 103 AU/ml and excellent antagonism against maximum number of test indicators. Rest seven isolates were rejected as they show least antagonism against tested indicators or they were found to have nil activity units AU/ml. Bacteriocin positive isolate G2 was identified upto genera level by morphological and biochemical characteristics. Morphologically colonies of G2 appeared white on MRS agar having circular form with entire margin, flat elevation and smooth texture (Fig. 1). Biochemical tests viz catalase test, cytochrome oxidase, citrate utilization, gas production from glucose, casein hydrolysis, H2S production were found negative for the isolate whereas isolate was found to utilize different sugars viz. sucrose, trehalose, xylose, maltose, lactose and dextrose. On the basis of above mentioned characters G2 was identified as Lactobacillus as per Bergey’s Manual of Determinative Bacteriology Kandler and Weiss (1986). The identified Genus Lactobacillus was further identified to the species level by using 16S rRNA gene technique. The determined sequence of isolate was compared directly with the Gen bank database. The high level of homology i.e., (97 % of matches) of G2 was observed with the sequence of L. spicheri. The 16S rRNA sequence of L. spicheri G2 is registered under the accession number JX481912 in NCBI. Phylogenetic tree have been presented in Fig. 2.
Optimization of bacteriocin production by one variable at a time process
Maximum bacteriocin production was optimized through classical one variable at a time process which was observed at 34th h i.e., early stationary phase, at pH 4.0, temperature 35 °C with an inoculum size of 1.5 OD @ 10 %. Increase in bacteriocin production after optimization through one variable at a time process was 70, 0 and 41.17 % against L. monocytogenes, and C. perfringens respectively as shown in Fig. 3.
Purification of bacteriocin of L. spicheri G2
Purification of bacteriocin of L. spicheri G2 was achieved by single step gel exclusion chromatography sephadex G-75. The loaded protein sample (2 ml) was eluted using phosphate buffer pH (6.9). A total of 30 fractions of 3 ml were collected. The fractions 8 to16 having maximum protein concentration viz. 1.18, 1.80, 2.0, 2.3, 2.8, 3.3, 3.42, 2.71, 1.06, 1.06 and positive bacteriocin activity were pooled (Fig. 4). Different steps of purification have been exhibited in Table 1. The purity and molecular weight of bacteriocin of L. spicheri G2 was confirmed through SDS PAGE and was found to be 43 kDa (Fig. 5.). The activity of bacteriocin had increased several folds after purification step and exhibited increase in zone size as well as arbitrary units of purified bacteriocin as compare to zone size and arbitrary units formed by crude bacteriocin. After gel exclusion chromatography, there was nil increase in zone size but 75 % increase in AU/ml against L. monocytogenes was observed, 16.6 % increase in zone size 75 % increase in AU/ml was estimated against S. aureus while 15 % increase in zone size and 15 % in AU/ml was measured against C. perfringens as depicted in Figs. 6 and 7. The increase in antagonistic potential and bacteriocin activity after purification suggests that the targeted proteins have been concentrated while other interfering proteins have been removed from the sample which otherwise may shield the effect of bacteriocins and thus it has become more active against its sensitive indicators resulting in stronger inhibition thus enhancing its effect in a favorable direction i.e., for safer biopreservation of food. This study is in accordance with a study reported in literature, where increase in bacteriocin activity was observed after partial purification of lenticin with an increase of 46.7 and 33.3 % in inhibitory zone size (Sharma et al. 2006). To confirm the bacteriocin potential of purified single band of L. spicheri G2, it was cut from the gel and its antimicrobial activity was checked against L. monocytogenes. The antagonistic potential of purified protein band ascertained the true status of bacteriocin.
Characterization of purified bacteriocin
Pure bacteriocin of L. spicheri G2 was characterized to assess its capability to work in different environmental conditions viz temperature, pH, salt concentration etc. from food preservation point of view.
Effect of temperature
Figure 8. exhibits the inhibitory activity of heat treated bacteriocin of L. spicheri G2 at temperature range varying from 40 to 100 °C. When purified bacteriocin exposed to 40 °C for 10 min, it inhibited the activity of L. monocytogenes by forming zone of inhibition of 12 mm while it decreased to 10, 2 mm at 70 °C and finally lost its activity beyond 80 °C. In case of S. aureus the purified bacteriocin formed zones of clearance of 5, 2, 2, 2, 2 and 1 mm at temperature 40, 50, 60, 70, 80 and 90 °C respectively. Zone sizes of 5 mm were noticed against C. perfringens after exposing pure bacteriocin at temperature 40 and 50 °C afterwards exhibiting nil activity. Inhibitory potential of heat treated purified bacteriocin of L. spicheri G2 against L. monocytogenes and S. aureus turn it desirable to preserve heat processed frozen foods and salty foods as in these foods both these pathogens are main cause of spoilage. It has been well reported in literature that few bacteriocins are extremely heat stable and can withstand high heat of 100 °C and even autoclaving temperature of 121 °C. While some bacteriocins are extremely heat sensitive and start losing activity from 50 °C onwards because a modest increase in temperature results in unfolding and loss of secondary and tertiary structure of protein that may cause denaturation of protein following its destabilization to almost complete loss of native confirmation Voet and Voet (2004).
Effect of pH
Figure 9 depicts the effect of pH on activity of purified bacteriocin of L. spicheri G2. Purified bacteriocin of L. spicheri was most active at pH 7.0 as it inhibited all the three test indicators. Purified bacteriocin remained most active for L. monocytogenes at neutral pH 7.0 (10 mm zone size for this indicator). Bacteriocin activity against L. monocytogenes was receded on both sides of optimum range i.e., in acidic side (pH 6.0 to 4.0) as well as on basic side (8.0 to 11.0) with a varying zone size of 2 and 1 mm only. The bacteriocin activity was maximum at pH 6.0 and 7.0 for S. aureus as on these pH maximum inhibition of (7, 8 mm) was observed, while on acidic and alkaline side activity declined. Bacteriocin was active against C. perfringens at pH 6.0 and 7.0 with zone size of 2 mm. The bacteriocin was found sensitive against S. aureus up to pH 4.0 and up to pH 8.0 and made no halos of clearance beyond 4.0 and 8.0. Whereas against C. perfringens bacteriocin completely lost its activity on acidic pH while remained active at alkaline range without losing its activity. The overall purified bacteriocins of L. spicheri G2 have been found most active at neutral or near neutral range (6.0 and 7.0) showing strong suppression of test pathogens. The stability of bacteriocins at neutral pH made it suitable for their use in neutral food products where use of nisin (a commercial bio preservative) is limited as nisin is only active at pH 5.0 and 5.5. Apart from its use in neutral and neutral food products, purified bacteriocin L. spicheri G2 was found active at alkaline range, suggesting its use in alkaline food products. Till date many bacteriocins have been reported which have different behavior at different pH some of them are active at neutral pH, some at acidic and some are at alkaline pH. (Oh et al. 2000), studied the effect of pH in bacteriocin activity by L. acidophilus 30SC and found that bacteriocin was completely stable at pH 6.0 and 7.0 and 50 % of activity remained after subjection to the pH values between 3.0 and 10.0.
Effect of proteolytic enzymes
Bacteriocin of L. spicheri G2 showed sensitivity to proteolytic enzyme trypsin and proteinase k. Purified bacteriocin treated with trypsin (ER1) and proteinase k (ER2) showed nil inhibition against indicators, viz. L. monocytogenes, S. aureus and C. perfringens. Whereas purified bacteriocin treated with phosphate buffer (C2) formed inhibitory zones of 10, 12 and 10 mm against L. monocytogenes, S. aureus and C. perfringens (Fig. 10). The results of this experiment revealed that purified bacteriocin was proteinaceous in nature as it lost its activity after reacting with proteolytic enzymes. This further adds to the fact that bacteriocin upon consumption in the food is broken down by the digestive juices secreted in the digestive tract of human beings thus rendering it completely safe for human consumption (Cleveland et al. 2001). Due to the sensitivity of bacteriocin towards proteolytic enzymes these have been given the GRAS status by the US Food and drug administration (Federal 1988). The experiment also depicted that phosphate buffer in which enzymes were diluted had nil effect towards activity of bacteriocin of L. spicheri G2 as nil inhibition was observed in enzyme control (C1).
Conclusion
It may be concluded that bacteriocin synthesized from first time reported isolate L. spicheri has bright prospects to be used as a food biopreservatives as it poses a combination of desirable characteristics viz: bacteriocin is secreted from food grade lactic acid bacteria already existing in edible food sample, thus rendering it completely safe for consumption, strong antagonism against a broad range of serious and challenging food borne pathogens/spoilage causing microorganisms, action of bacteriocin at higher temperature and wider pH range, degradation of bacteriocin in the presence of proteolytic enzyme making it completely safe for human consumption. All the above mentioned attributes advocate strongly that bacteriocin of L. spicheri G2 isolated from Gundruk has potential to be used as effective food biopreservative in place of chemical preservatives thus keeping intact the nutritive properties of processed food as well as safer for consumption.
References
Balgir PP, Bhatia P, Kaur B (2010) Sequence analysis and homology based modeling to asses structure-function relationship of pediocin CP2 of Pediococcus acidilactici MTCC 5101. Indian J Biotechnol 9:431–434
Barefoot SF, Klaenhammer TR (1983) Detection and activity of Lactacin B, a bacteriocin produced by Lactobacillus acidophilus. Appl Environ Microbiol 45(6):1808–1815
Cleveland J, Montville TJ, Nes IF, Chikindas ML (2001) Bacteriocins: safe, natural antimicrobials for food preservation. Int J Food Microbiol 71:1–2
Hata T, Alemu M, Kobayashi M, Suzuki C, Nitisinprasert S, Ohmomo S (2009) Characterization of a bacteriocin produced by Enterococcus faecalis N1-33 and its application as a food preservative. J Food Prot 72:524–530
Kandler O, Weiss N (1986) Genus Lactobacillus beijernick. 1901. Bergey’s Manual of systematic. Bacteriology 2:1209–1234
Kimura H, Sashihara T, Matsusaki H, Sonomoto K, Ishizaki A (1998) Novel bacteriocin of Pediococcus sp. ISK-1 isolated from well – aged bed of fermented rice bran. Ann. New York Acad Sci 864:345–348
Kumar R, Vadhera DV (1980) Gel permeation and ion exchange chromatography. Analytical and purification techniques in Microbiology. Lab Manual, Department of Microbiology, Punjab University, Chandigarh, p 91
Lade HS, Chitanand MP, Gyananth G, Kadam TA (2006) Studies on some properties of bacterocins produced by Lactobacillus species isolated from agro-based waste. Int J Microbiol 2:1
Lucose F, Sundar K, Shetty PH (2012) Process improvement to enhance the nutritional quality of indian fermented food (Gundruk). Int J Hum Gen Med Biotechnol Microbiol Stud 1(2):2319–1732
Motta AS, Brandelli A (2002) Characterization of an antibacterial peptide produced by Brevibacterium lines. J Appl Microbiol 92:63–70
Oh S, Kim SH, Worobo RW (2000) Characterization and purification of bacteriocin produced by a potential probiotic culture L. acidophilus 30SC. J Dairy Sci 83(12):2747–2752
Paik HD, Bae SS, Park SH, Pan JG (1997) Identification and partial characterization of Tochicin, a bacteriocin produced by Bacillus thuringeinsis subsp. tochigiensis. J Ind Microbiol Biotechnol 19:294–298
Register F (1988) Nisin preparation affirmation of GRAS status as a direct human food ingredient. Food Reg 54:11247–11251
Sharma N, Kapoor G, Neopaney B (2006) Characterization of a new bacteriocin isolated from a novel isolated strain Bacillus lentus NG 121. Antonie Van Leeuwenhoek 89:337–343
Sharma N, Kapoor R, Gautam N, Kumari R (2011) Purification and characterization of bacteriocin produced by Lactobacillus sp. A75 isolated from fermented chunks of Phaseolus radiata. Food Technol Biotechnol 49(2):169–176
Sharpe ME (1979) Identificación of the lactic acid bacteria. In: Skimmer FA, Lovelock DW (eds) Identification methods for microbiologists. Soc. Appl Bacteriol. Technical series no Academic Press, London, pp 246–255
Voet D, Voet J (2004) Three dimensional structure of proteins. Biochemistry 3rd (ed). Wiley, pp 265–268
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gautam, N., Sharma, N. A study on characterization of new bacteriocin produced from a novel strain of Lactobacillus spicheri G2 isolated from Gundruk- a fermented vegetable product of North East India. J Food Sci Technol 52, 5808–5816 (2015). https://doi.org/10.1007/s13197-015-1710-x
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-015-1710-x