Abstract
This work is part of a large study aimed to evaluate the potential of bambara groundnut (Vigna subterranea) flour as starting raw material for the preparation of a widely cherished legume-based food product known as koki. Towards this objective, the flours from two varieties of bambara groundnut along with their respective starch and protein isolates were analyzed for some physicochemical and microstructural properties. It was observed that bambara flour contained appreciable amount of proteins (24.0–25.5 g/100 g), carbohydrates (57.9–61.7 g/100 g), fiber (3.45–3.68 g/100 g) and ash (3.65–3.85 g/100 g) with marginal differences between both varieties. The properties of starch and proteins isolated from the flours were different from one variety to another. In particular the starch granules of the white variety were larger (size range 10–35 μm) and polygonal while those from the black variety were smaller (size range 6–15 μm) and spherical in shape. In addition, the peak of gelatinization temperature was higher for white variety (81.7 °C) than for black variety (77.5 °C). The gelatinization temperature and the enthalpy of gelatinization of starch in the flours were systematically lower than for the starch isolates, suggesting an interaction of starch with other components on the gelatinization process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bambara groundnut (Vigna subterranea (L.) Verdc.) is a legume with significance as a source of proteins in many parts of Central and West Africa where it is cultivated mainly by women for the sustenance of their families. The protein content of bambara groundnut is comparable or superior to that of other legumes, making it a good complement for cereal-based diets (Linnemann & Azam-Ali, 1993; Minka & Bruneteau, 2000; Kaptso et al., 2013). Nutritionally, bambara groundnut contains 19.7 % protein, 53.1 % carbohydrates, 6.1 % fat, 6.1 % fiber, 3.4 % ash, 0.098 % calcium and 0.007 % iron (Amarteifio et al., 1997; Kaptso, 2009). In addition, lysine content is relatively high, while methionine and calcium contents are low (Doku, 1996). Despite its nutritional potential, bambara groundnut is rather under-utilized inside and outside its indigenous areas. A monograph published on the potential of some little known but promising African tropical legumes (NAS-National Academy of Sciences 1984) presented bambara groundnut as the third important crop after groundnut (Arachis hypogea) and cowpea (Vigna unguiculata) with an approximate production of 300,000 t (Linnemann & Azam-Ali, 1993). However, bambara is seen as a snack or food supplement but not a lucrative cash crop (Rachie & Silvestre, 1977; Linnemann, 1992). One of the limits to the utilization of bambara groundnut is the lack of processing techniques and this has been linked to increasing hard-to-cook defect in the nuts and reducing production (Kaptso et al., 2007). In Central Africa this legume is commonly cooked and eaten as such or often used for the preparation of a widely cherished legume-based food product known as koki (Mbofung et al., 1999). Generally the method of preparation of koki is not only laborious, but also equally time consuming and as such not convenient for individuals living in a busy urban areas. This has a limiting effect on the frequency of preparation and consumption of the product. The successful production of legume-based flours that can conveniently be used for the preparation of koki and akara has been demonstrated to be a way of improving the availability and consumption of the legume seeds (McWatters & Brantley 1982; Njintang et al., 2001). In the event of bambara groundnut flour production and utilization, a better understanding of the properties of its major components, starch and proteins, will help in designing and optimizing the process. In fact, proteins and starch exhibited varying functionalities that provide to final product a special mouth feel (Singh, 2001). In particular the physicochemical and thermal properties of flour are major determinants of their functionality in koki preparation (Mbofung et al., 1999; Njintang et al., 2001; Mbofung et al., 2002). However, little information exists on the physicochemical and microstructural properties of bambara flour as well as their starch and proteins counterparts. Therefore, the objective of the study was to study some physicochemical and microstructural properties of two varieties of bambara groundnut flour, starch and protein.
Materials and methods
Production of bambara groundnut flour
Two varieties of bambara groundnut (black and white) were purchased from a farmer in Maroua (Far-North region of Cameroon). The seeds were screened to eliminate the defective ones. Wholesome seeds were washed, rinsed and soaked for 12 h in distilled water (1:3 w/v) at 20 °C. The soaked grains were then allowed to drip-dry of excess water for 30 min before being introduced into an air-draught oven (CKA 2000, Ngaoundere, Cameroon) set at 45 ± 4 °C for 24 h to reach a moisture level of about 10 %. Following this, the seed coats were then manually dehulled and hammer-milled (Culatti, Polymix®) to pass through sieves of 500 μm mesh diameter. The resulting powders from black bambara (BB) and white bambara (WB) were packaged in polyethylene bags and kept in a refrigerator at 4 °C until use.
Bambara groundnut starch isolation
Starch was isolated from each variety of bambara flour following the modified method of Sathe et al. (1982). In the extraction procedure, 1 kg of bambara groundnut flour was added to 10 L of distilled water in a flask and the mixture stirred for 30 min at room temperature (25 °C). Following this, the flask was covered with aluminum foil and kept for 5 h at 40 °C. The resulting mixture was centrifuged at 4,500 g for 15 min at 20 °C. The pellet was solubilised in 10 L of 2 % NaCl solution and it was stirred for 10 min. The flask was again covered with foil and let to stand for 12 h before being centrifuged at 4,500 g for 15 min. The residue was repeatedly washed with distilled water, dissolved in 10 L of 0.03 M NaOH, and the homogenate allowed to stand at 4 °C for 12 h. The mixture was again centrifuged for 30 min at 4,500 g. The residue was then washed with water several times and passed through a sieve of mesh 75 μm for fiber removal. The starch obtained was centrifuged and the pellet obtained spread on an aluminum plate and dried in an oven at 30 ° ± 2 ° C for 12 h. The starch obtained was sealed in polythene bags and stored at 4 °C for the various analyzes.
Bambara groundnut proteins isolation
Protein isolates were produced from each variety of bambara flour following the procedure described by Lawal (2004). In the procedure, an amount of flour was mixed with distilled water (1:5 w/v) and the pH of the mixture adjusted to 9.5 with 1 M NaOH. The mixture was left to stand for 3 h at 30 ± 2 °C and centrifuged at 4,000 g for 30 min at 20 °C. The supernatant was collected, the pellet undergone the same operation as before and the supernatant was again collected and mixed with the first. The supernatant was then adjusted to the isoelectric point (pH 4.5) using 1 M of HCl and/or 1 M NaOH and centrifuged at 4,000 g for 20 min. The precipitated proteins were washed in three volumes of distilled water and neutralized to pH 7 with 1 M NaOH. The neutralized protein isolate was freeze-dried and the resulting powder was sealed in polyethylene bags and stored at 4 °C for further analyzes.
Chemical composition
Moisture, ash, protein, crude fiber and carbohydrates were determined on flours, starch and proteins samples by the (AOAC 2000) standard methods. Fat content was estimated by the method of Bourely (1982). Amylose content was analyzed using the complex coloration amylose/iodide methodology proposed by Juliano et al. (1981) using a UV/Visible spectrophotometer (Spectronic Genesys 2 PC) at 620 nm. The Phosphorus content was estimated using the colorimetric reaction with ammonium molybdate and ascorbic acid to give a blue color which absorbs at a maximum wavelength of 690 nm (AOAC 2000).
Particle size analysis
About 0.5 g of each flour and starch sample was dispersed in 300 mL of ethanol with the aid of water bath sonication (Starsonic 60, Italy) for 2 min. The particle size distribution was determined with a laser diffraction particle size analyzer (Malvern Mastersizer Instruments, Orsay, France) at obscuration between 0.10 and 0.15.
Thermal properties
Thermal properties of the starch were analyzed using a Differential scanning calorimeter (DSC) equipped with a thermal analysis data station (Perkin-Elmer Corp Norwalk. USA). The starch, protein isolates and flours (25–35 mg dry weight) were dispersed separately in distilled water (1:3 w/v) in a hermetically sealed aluminum pans using a Hamilton microsyringe. The pan was allowed to stand for 4 h at room temperature before being subjected to a heating scan in the DSC. The DSC was calibrated using indium, and an empty aluminum pan was used as the reference. Sample pans were freezing at −20 °C for 10 min and heating progressively up to 120 °C using a dry nitrogen flow of 20 mL/min at a rate of 5 °C/min.
Fourier transformed infrared spectroscopy (FTIR)
FT-IR spectra were recorded with a spectrophotometer (Pelkin Elmer) in the range 400–4,000 cm−1, using a resolution of 4.000 cm−1 and four scans. Samples of flours, starch and protein were diluted at 1–2 mg of samples for 150–200 mg of KBr. Microcapsules were applied on dry KBr pellets prepared using a manual hydraulic press (Perkin Elmer, Spain).
X-ray diffraction (XRD)
X-ray diffraction patterns were collected in transmission using an X-ray diffractometer (Jobin Yvon Sigma 2080) with Cu Kα radiation generated at 30 mA and 40 kV. All samples were measured in the 2θ angle range between 5° and 65° at a scan rate of 0.5 s per step and a step size of 0.2°. Triplicate scans were performed for each flour fraction sample. In this study, the degree of crystallinity of samples was quantitatively estimated following the method of Nara & Komiy, 1983. A smooth curve, which connected peak baselines, was computer- plotted on the diffractograms. The area above the smooth curve was taken as the crystalline fraction, and the lower area between smooth curve and the linear baseline, which connected the two points of the intensity 2 θ of 30° and 4° in the samples, was taken as the amorphous fraction. The ratio of upper area to the total diffraction area over the diffraction angle 4–30° 2θ were integrated using Origin Microcal 5.0 (OriginLab Corporation) and was taken as the degree of crystallinity. The relative crystallinity index was obtained by subtracting the diffractogram of the amorphous material from that one of the sample under study and integrating the total area of the crystalline peaks and finally by dividing the value of crystalline area by that one of the total area before subtraction (Van Soest et al., 1995).
Scanning electron micrograph
The morphology of unprocessed and processed particles was examined using SEM (CamScan MV 2300, England). Scanning electron micrographs (SEM) were obtained using a Hitachi S-3,000 N scanning electron microscope (Hitachi S2500, Tokyo, Japan). Starch samples were suspended in 1 % ethanol solution. One drop of the starch ethanol mixture was applied onto an aluminum stub using double-sided adhesive tape. The sample was coated with gold–palladium. An accelerating potential of 15 KV was used during electron micrography.
Statistical analysis
In order to determine whether there is a statistically significant difference among the means of variables, one-way analysis of variance (ANOVA) was performed and when significant (P < 0.05), the least significant difference (LSD) test was done to classify the means using the statistical software Statgraphic Plus 5.0.
Results and discussions
Chemical composition
The proximate composition of the flours, starch and proteins samples is presented in Table 1. The moisture content of the samples varied significantly from 3.2–4.3 % (proteins) to 12.3–12.8 % (starch). Bambara flour contained fat (5.9–6.1 %) and appreciable amount of proteins (24.0–25.3 %), carbohydrates (57.9–61.7 %), ash (3.6–3.8 %) and fiber (3.4–3.7 %). From the above data it can be seen that the variety has only marginal effect on the proximate composition of the flour, although significant differences (p < 0.05) were observed in the cases of fat and crude fibers contents. The values of the protein contents obtained were higher than that reported (20.7 %) by Adebowale et al. (2002) and 18.3 % by Amarteifio & Moholo (1998) on bambara groundnut. In addition the ash content was close to value reported by Amarteifio & Moholo (1998) but significantly (p < 0.05) higher than 0.63 % reported by Adebowale et al. (2002). Similar behaviors were observed on crude fiber contents, and the differences observed with those in literature may be due to the difference in landraces, soil and climatic conditions (Amarteifio & Moholo 1998). Irrespective of the variety, total carbohydrates was the most representative constituent in bambara flour. Kaptso et al. (2007) reported significant varietal influence on the proteins and carbohydrates of bambara groundnut. Commonly, legumes seeds with high protein values are offset by low fat contents and vice versa. As Nti (2011) showed, the black bambara with the highest protein content had the lowest fat content of 4.7 % while the bambara varieties with relatively low protein content were high in fat. Our sample seemed to not deviate from this general behavior.
The amylose content of samples is presented in Table 1. The amylose levels of the two starch extracts were within the ranges found in the literature (Hoover & Ratnayake, 2002), 25.0 % for white bambara starch and 27.8 % for yellow pea starch. This is in concordance with literature that legume starches are characterized as high amylose contents (Singh et al., 2004). Thus the amylose levels of bambara starch samples were within the ranges reported for several legumes starches: 20.9–48.7 % for cowpea, 20.7–42.2 % for chickpea, and 22.0–49.6 % for yellow pea starches (Arora & Das 1976; Saini & Knights, 1984; Won et al., 2000; Ratnayake et al., 2001; Hoover & Ratnayake, 2002). The differences in the amylose contents of the starches of different origins have been partly attributed to differences in the activities of the enzymes involved in the biosynthesis of linear and branched components within the starch granules (Krossmann & Lloyd, 2000). The amylose content of starches from different and similar plant sources has been also reported to be affected by location, soil type, starch isolation procedures as well as by analytical methods used (Kim et al., 1995; Singh et al., 2004).
Table 1 shows the phosphorus content of bambara groundnut samples. The phosphorus content of starch extract in the two varieties (18.32 mg/100 g and 19.55 mg/100 g respectively for WB starch and BB starch) were statistically (p < 0.05) different. The values found were lower than reported by Huang et al. (2007) for cowpea starch (22 mg/100 g). In potato starch, the phosphorus is mainly present as phosphate monoesters, which are covalently bounded to the amylopectin fraction of the starch (Craig et al., 1989). However, the phosphorus content in potato starch has been reported to be influenced by growing conditions, storage conditions and the amylose/amylopectin ratio (Cottrell et al., 1995). Similarly a significant (r = −0.97) correlation was observed between amylose content and phosphorus content of bambara flours and starch samples.
Particle size analysis
Particle size distributions of black and white bambara flour and starch are shown in Fig. 1. The results of the particle-size distribution of flour granules showed that the distribution curves for both flour samples were similar in shape. All flour samples displayed trimodal size distribution, with the first mode at 50–75 μm, the second and major mode at 150 μm, and the third at 600 μm. In this respect it has been suggested that trimodal distribution is characteristic of the corn flour (Hareland, 1994). It is possible that the particles size distribution is related to the friability of the seeds, with the most friable disaggregating easily to release the various components which are divided into various peaks.
From the distribution curves, the parameters d10, d50 and d90 were calculated to find out the variation in granule size distribution. These variables respectively represent the diameter below which 10, 50 and 90 % of the population of the particles are represented. The values of d10 d50 and d90 of the flours do not differ significantly for the two varieties and are on average 12, 36 and 103 μm for WB and 13, 36 and 102 μm for BB. The granule size distributions of bambara groundnut starches were unimodal, with the mode between 45 and 56 μm for the two starches varieties. The values of d10 d50 and d90 of the starches were 5.5, 25 and 67 μm for WB and 5.6, 23 and 65 μm for BB. Compared to work reported by Okechukwu & Rao (1996) on cowpea, the bambara groundnut starch granules sample was smaller than their range (range, 3–64 μm). A similar result for chickpea starch (6–31 μm) and smooth pea (14–37 μm) using microscopy has been observed by Hoover & Ratnayake (2002) and Singh et al. (2004).
Thermal analysis
Differential thermal analysis gives information on the variation of energy released during the change of state of flour, starch and protein of Bambara groundnut. All the thermograms obtained for different varieties of flour have an endothermic transition characteristic of the state change of food. Table 2 presents the thermal properties of flours, starches and proteins. It may be noted that the difference between the temperatures at the beginning and end of gelatinization is not too large, indicating a low variability of thermal properties of molecules, including starches granules that make up each variety. On the other hand, it was observed that the transition temperature of the flour does not vary significantly between varieties. Unlike the peak of gelatinization, the enthalpy representing the variation of the flow of energy released during this change of state varies significantly (p < 0.05) from one variety to another with BB (6.31 J / g) showing the lowest value and WB the highest value (9.37 J / g). The low value of ΔH for different varieties of flour could be attributed to the size of the starch granules. The values of the energy flow of bambara flour were in the range of values reported for other in literature, but low compared to that reported for corn starch (10.2 J / g) by Li & Tey (2001).
Table 2 also presents the results of DSC analysis of Bambara groundnut starches. The onset temperature (To) varied between 73.4 °C (BB) and 76.1 °C (WB), the conclusion temperature (Tc) between 83.1 °C (BB) and 84.8 °C (WB), and peak temperature of transition (Tp) between 77.5 °C (BB) and 81.7 °C (WB). The flow energy released during the gelatinization varied between 12.7 J/g (WB) and 13.9 J/g (BB). The peak temperature obtained fall within the range reported for other foodstuffs such as cowpea (67–78 °C, Okechukwu et al., 1991). In addition the values of ΔH were close to that obtained for corn starch (10.2 J / g) by (Li and Yeh 2001). However the gelatinization enthalpy values obtained for flour were higher than 2 J/g but lower than 15.2 J/g reported by Abu et al. (2006) and Huang et al. (2007) cowpea starch, respectively.
The values of the flow energy released during the endothermic transition by proteins are shown in Table 2 for different varieties. We found that the enthalpy energy of proteins was very low, generally <1 J / g for all varieties. Recent study by Li et al. (2007) revealed that soy protein isolate has no endothermic transition between 50 and 150 °C. The energy enthalpy observed for protein isolates might be due to the presence of traces of starch. This idea is reinforced by the values of peak temperature of transition which were close to that of starch samples. In overall, the thermal analysis of flours, starch and proteins showed that the main compound of the flour, starch, impose its functionality. Proteins rather played a role of dilution with little interaction. In this respect the transition temperatures and enthalpies of gelatinization were systematically lower for flours than for starches. Aboubakar et al. (2008) working on taro corms found that starches exhibited higher enthalpy values (mean 14.99 J/g) compared to flours (mean 11.42 J/g).
Fourier transform infrared spectroscopy (FT-IR)
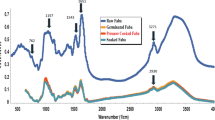
Infrared spectroscopic analysis of different bambara groundnut flours gave information on the possible difference between the structures of the flours, starch and proteins isolates. The spectra obtained for the flours WB and BB have the same shape but different level of intensity peaks (Fig. 2a). The IR spectra of flours exhibit absorption bands (1,022–1,047 cm−1) corresponding to the rotational vibration of the molecules of amylose and amylopectin. Both molecules are found responsible of the change in structure of starch chains such as conformation, helicity, crystallinity and retrogradation process (Van Soest et al., 1995). The spectra show absorption peaks at wave numbers 1,020, 1,026, 1,056, 1,151, 1,365, 1,631, 2,922 and 3,400 cm−1 that are characteristic of the presence of sugars in flours. In addition, we note the absorption peaks at wave numbers of 1,400 and 1,800 cm−1 that is characteristic amide bands. In addition, the bands between 3,500 and 3,300 cm−1; 1,649 and 1,550 cm−1 were indexed as bands corresponding to groups of primary amide NH deformation which in combination with strong vibrations located around 1,157 and 1,080 cm−1, correspond to those proteins. The bands located near 3,380 cm−1 and 1,400 cm−1 are usually due to stretching vibrations and deformation of the OH bond (Noël & Roeges, 1994). The band that lies between 1,600 and 1,700 cm−1 is attributed to the stretching vibrations of the OH group of the water content. The band 3,400 cm−1, characterizes the deformation vibrations of H2O molecules. The bands between (1,400 and 1,500 cm−1) are assigned to the deformation vibration of CH3 groups located at 1,480 cm−1. It was shown that the bands at 1,047 and 1,022 cm−1 respectively describe the indices of crystalline and amorphous of starch (Sevenou et al., 2002). In addition, Sevenou et al., (2,002) reported the ratio 1047/1022 as indicator of the degree of organization of starch. Therefore it appears that the ratio 1047/1022 for flour generally varied from 1.04 (WB)–0.97 (BB). These values were systematically higher compared to those (0.82–0.91) recently reported for taro flour (Aboubakar et al., 2008), but quite close to those reported on potato starches (Van Soest et al., 1995).
Figure 2b shows the IR spectra of Bambara groundnut starches. It is clear from the figure that high absorbance peaks were obtained between 900 and 1,200 cm−1. These peaks are characteristic of polysaccharides and are due to rotational vibration of C-O bonds. Other peaks were observed between 3,300 and 3,500 cm−1, characteristics of hydroxyl groups. Kapusniak & Siemion (2007) obtained similar spectra on potato starches. Peaks obtained in 1,710 and 1,605 cm−1 correspond to stretching vibrations of C = O of carboxyl groups (Pezolet et al., 1992). These results are confirmed by the ratio of crystalline index / index starch, which is close to unity for the two varieties of starches.
The results of the FTIR spectra obtained for Bambara groundnut proteins are presented in Fig. 2c. High vibration peaks were obtained between 3,000–3,500 cm−1, probably corresponding to the asymmetric vibrations of the amide groups of proteins with a band for CO-NH and two bands for CO-NH2. Bands at 1,649 and 1,550 cm−1 are indexed as corresponding to groups of primary amide N-H deformation which in combination with strong vibrations located around 1,157 and 1,080 cm−1, are characteristic of proteins (Noël & Roeges, 1994). These observations suggested that bambara protein isolates from both varieties have similar composition qualitatively and only the proportion of amino acids and the chemical nature of bond would be different.
X-ray diffraction (XRD)
X-ray diffractometry is widely used to reveal the characteristics of the crystalline structure of starch granules (Zobel et al., 1988). The diagrams of X-ray diffraction of the starch WB and BB are shown in Fig. 3. This figure shows that both starches exhibited peaks corresponding to reflection angle 2θ for the various starches, respectively, 17.52, 19.80 and 26.59 for WB starch, 17.49, 20.11 and 26.83 for BB starch.
All starches exhibited a peak around the reflection angle 2θ = 27, which is characteristic of type-A starches. No peak was detected at the diffraction angle 2θ = 6.5 characteristic of Type-B starches. These observations suggested that both the starch samples are Type-A. Similar starch type was reported for cowpea while a type-B was reported for chickpeas (El Faki et al., 1983). This contrasted with Donald (2004) who suggested a type-C state for most legumes starches. From all these contradictions, it can be seen that the behavior in the X-ray diffraction may depend on various factors including variety, agro-ecological conditions, pretreatment applied to the sample. Osella et al. (2005) in a study of the effect of water content on the structural modifications of starches showed that for a water content below 23 %, starches develop a structure of type-A while those with a grade water more than 49 % develop type-B structure. Studies on the crystallization of sucrose show that crystallization is largely dependent on the concentration of sucrose in water and temperature (Van Hook, 1959). In addition starch damage caused during milling because of mechanical (pressure) effect destroys the order (crystalline areas) in the starch structure converting them into amorphous and hence decreases its crystallinity (Van Soest et al., 1995).
The total relative crystallinity index of flours was calculated taking into account the proportion of all the starch milling fractions. The relative crystallinity index for both starches is shown in Table 3, where it can be seen that the relative crystallinity index for the white Bambara starch was relatively the same than those of black bambara starches. Table 3 confirms the results of infrared analysis because the degree of crystallinity of both Bambara groundnut starches were higher (41.13–40.40 %) compared to those obtained on cowpea by Kaptso (2009). The variation in the relative crystallinity index of starches implies differences in the extent of starch damage. These differences between starches can be generated in the milling process. These values are similar to those reported in the literature for many foods like yam starch which showed a degree of crystallinity ranging between 31.5 and 45.9 % (Shujun et al., 2006) while El Faki et al. (1983) reported on cowpea relative crystallinity value of 26 %.
Figure 3b shows the diffractograms of protein isolated from Bambara groundnut. The essential problem in determining protein structure by X-ray diffraction is the growth and production of a large crystal size and quality. Thus, it can be seen in this figure that intense crystallization occurs with a single peak around the reflection angle 2θ = 24.66° for WB proteins and that this peak of crystallization was relatively low for BB proteins.
Scanning electron micrograph
SEM micrographs of bambara starches are shown in Fig. 4a and b. These figures show that the starches WB granules are irregular and polygonal in form, while the spherical form characterizes the starch BB granules. The starches WB granules have sizes ranging between 10–35 μm, whereas those BB have sizes which vary between 6–15 μm. The difference between particles size evaluated by Mastersizer and that obtained by scanning electron microscope can be explained by the possible aggregation of starch granules during Mastersizer analysis. (Abu et al. 2006) obtained values of about 15–40 μm on cowpea whereas a range from 7.5–37.5 μm was reported by Blanshard (1987) on cowpea.
The microscopic characteristics of proteins are represented in Fig. 4c and d. On these photographs, proteins of WB and BB surfaces presented cracking. These observations are in agreement with the results of diffraction to X-rays and of the IR spectroscopy, which showed that the proteins of WB have a crystalline structure more intense than those of BB. These results obtained let us think that the seeds of bambara groundnut could have a different behavior during soaking and drying, operations which are the necessary stages to the production of legumes flours.
Conclusion
The thermal analysis of the flours, starch and proteins, shows that the starch is potentially the principal compound which presents a thermal transition and thus can vary the properties of the flour. The diffractogram of the starch granules shows that they are type-A and have a good crystallinity. Scanning electron microscopy showed that the starches of White bambara groundnut show the structural level by their irregular polygonal form, while that of Black bambara groundnut is characterized by its sphericity and its small size. This information illustrates that the starches and proteins bambara groundnut present different properties. It is obvious that these differences could be reflected on the properties of the flours and the koki, which will derive from it. Structural characterization and development of structure–physical property relationships are crucial for rational design of process conditions to produce flour with desired attributes and storage conditions to enhance stability. Although, the importance of structure–property relationships has long been recognized.
References
Aboubakar NYN, Scher J, Mbofung CMF (2008) Physicochemical, thermal properties and microstructure of six varieties of taro (Colocasia esculenta L. Schott) flours and starches. J Food Eng 86:294–305
Abu JO, Duodu KG, Minnaar A (2006) Effects of g-irradiation on some physicochemical and thermal properties of cowpea (Vigna unguiculata L. Walp) starch. Food Chem 95:386–393
Adebowale KO, Afolabi AT, Lawal OS (2002) Isolation, chemical modification and physicochemical characterization of Bambarra groundnut (Voandzeia subterranean) starch and flour. Food Chem 78:305–311
Amarteifio JO, Moholo D (1998) Chemical composition of four legumes consumed in Botswana. J Food Compos Anal 11:329–332
Amarteifio JO, Sawula G, Gibbons MRD (1997) Comparison of four landraces of bambara groundnuts. Trop Sci 37:143–145
AOAC (2000) Official Methods of Analysis. Official Analytical Chemists, Washington, DC
Arora SK, Das B (1976) Cowpea as potential crop for starch. Starch/Stärke 28(5):158–160
Blanshard JMV (1987) Starch granule structure and function: A physicochemical approach. In: Galliard T (ed) Starch: properties and potential. Wiley, New York, p 17
Bourely J. Observation sur le dosage de l'huile des graines de cotonnier. Cot Fib Trop 1982; 27(2): 183–196
Cottrell JE, Duffus CM, Paterson L, George RM (1995) Properties of potato starch: Effects of genotype and growing conditions. Phytochem 40:1057–1064
Craig SAS, Maningat CC, Seib PA, Hoseney RC (1989) Starch paste clarity. Cereal Chem 66:173–182
Doku VE (1996) Problems and prospects for the improvement of bambara groundnut. In Proceedings of the International Bambara Groundnut Symposium. University of Nottingham, UK, pp 19–27
Donald AM (2004) Understanding starch structure and functionality. In: Eliassion AC (ed) Starch in food: Structure, function and applications. Woodhead Publishing Limited/CRC Press LLC., Cambridge/New York, pp 156–184
El Faki HA, Desikachar HSR, Paramahans SV, Tharanathan RN (1983) Physico-chemical characteristics of starches from chickpea, cow pea and horse gram. Starch 35:118–122
Hareland GA (1994) Evaluation of flour particle size distribution by laser diffraction, sieve analysis and near-infrared reflectance spectroscopy. J Cereal Sci 20:183–190
Hoover R, Ratnayake WS (2002) Starch characteristics of black bean, chick pea, lentil, navy bean and pinto bean cultivars grown in Canada. Food Chem 78:489–498
Huang J, Schols HA, Van Soest JJG, Jin Z, Sulmann E, Voragen AGJ (2007) Physicochemical properties and amylopectin chain profiles of cowpea, chickpea and yellow pea starches. Food Chem 101:1338–1345
Juliano BO, Perez CM, Blakeney AB, Castillo T, Kongseree N, Laignelet B (1981) International cooperative testing on the amylase content of milled rice. Starch 33:157–162
Kaptso KG (2009) Potentiel technologique des farines de niébé (Vigna unguiculata) et de voandzou (Vigna subterranea) pour la préparation du koki (gâteau de pâte cuite à la vapeur). Thèse de Doctorat/PhD, ENSAI. Université de Ngaoundéré, Cameroun
Kaptso KG, Njintang NY, Hounhouigan JD, Scher J, Mbofung CMF (2007) Production of bambara groundnut (Voandzeia subterranean) flour for use in the preparation of koki (a steamed cooked paste): Effect of pH and salt concentration on the physicochemical properties of flour. Int J Food Eng. Vol 3, Iss 3, Art 5
Kaptso KG, Njintang NY, Nguemtchouin MMG, Scher J, Hounhouigan JD, Mbofung CMF (2013) Drying kinetics of two varieties of bambara groundnuts (Vigna subterranea) seeds. J Food Technol 11:30–37
Kapusniak J, Siemion P (2007) Thermal reactions of starch with long-chain unsaturated fatty acids. Part 2. Linoleic acid. J Food Eng 78:323–332
Kim SY, Wiesenborn DP, Orr PH, Grant LA (1995) Screening potato starch for novel properties using differential scanning calorimetry. J Food Sci 60:1060–1065
Krossmann J, Lloyd J (2000) Understanding and influencing starch biochemistry. Crit Rev Biochem Mol Biol 35:141–196
Lawal OS (2004) Functionality of African locust bean (Parkia biglobossa) protein isolate: effects of pH, ionic strength and various protein concentrations. Food Chem 86:345–355
Li JY, Yeh AI (2001) Relationships between thermal, rheological characteristics and swelling power for various starches. J Food Eng 50(3):141–148
Li JY, Yeh AI, Fan KL (2007) Gelation characteristics and morphology of corn starch/soy protein concentrate composites during heating. J Food Eng 78:1240–1247
Linnemann AR, Azam-Ali SN (1993) Bambara groundnut (Vigna subterranea). In: Williams JT (ed) Underutilized Crops. Pulses and Vegetables. Chapman and Hall, London, pp 13–57
Linnemann AR (1992) Bambara groundnut (Vigna subterranea) literature: a revised and updated bibliography. Tropical Crops Communication 7. Department of Tropical Crop Science. Wageningen Agricultural University, Netherlands, p 124
Mbofung CMF, Njintang YN, Waldrom WK (2002) Functional properties of Cowpea-soy-dry red beans composite flour paste and sensorial characteristics of akara (deep fat fried food): effect of whipping conditions, pH, temperature and salt concentration. J Food Eng 54:207–214
Mbofung CMF, Ribgy N, Waldron KW (1999) Use of two varieties of hard-to-cook beans (Phaseolus vulgaris) and cowpea (Vigna unguiculata) in the processing of koki (a steamed legume product). Plant Foods Hum Nutr 54:131–150
McWatters KH, Brantley BB (1982) Characteristics of akara prepared from cowpea paste and meal. Food Technol 36(1):66–69
Minka DR, Bruneteau M (2000) Partial chemical composition of bambara pea (Vigna subterranea L. Verdc.). Food Chem 68:273–276
Nara S, Komiy T (1983) Studied on the relationship between water saturated state and crystallinity by the diffraction method for moistened potato starch. Starch 35:407–410
NAS-National Academy of Sciences (1984) Tropical legumes: resources for the future. National Academy Press, Washington, DC
Njintang NY, Mbofung CMF, Waldrom KW (2001) In vitro protein digestibility and physicochemical properties of dry red beans (Phaseolus vulgaris) flour: Effect of processing and incorporation of soy beans and cowpea flour. J Agric Food Chem 49:2465–2471
Noël P, Roeges G (1994) A guide to the Complete Interpretation of Infrared Spectra of Organics Structures. John Wiley, New York
Nti CA (2011) Effects of bambara groundnut (Vigna subterranea) variety and processing on the quality and consumer appeal for its products. Int J Food Sci Technol 44:2234–2242
Okechukwu PE, Rao MA (1996) Role of granule size and size distribution in the viscosity of cowpea starch dispersions heated in excess water. J Texture Stud 27:159–173
Okechukwu PE, Rao MA, Ngoddy PO, McWatters KH (1991) Rheology of sol–gel thermal transition in cowpea flour and starch slurry. J Food Sci 56:1744–1748
Osella CA, Sanchez HD, Carrara CR, De la Torre MA, Buera MP (2005) Water redistribution and structural changes of starch during storage of a gluten-free bread. Starch 57:208–216
Pezolet M, Bonenfany S, Dousseau F, Popineau Y (1992) Conformation of wheat gluten proteins: Comparaison between functional and solution state as determined by infrared spectroscopy. Fed Euro Biochem Soc 299:247–250
Rachie KO, Silvestre P (1977) Grain legumes. In: C.L. Leakey and J.B. Wills (Eds.). Food crops of the lowland tropics. Oxford University Press, London, pp 41–47
Ratnayake WS, Hoover R, Shahidi F, Perera C, Jane J (2001) Composition, molecular structure, and physicochemical properties of starches from four field pea (Pisum sativum L.) cultivars. Food Chem 74(2):189–202
Saini HS, Knights EJ (1984) Chemical constitution of starch and oligosaccharide components of “desi” and “kabuli” chickpea (Cicer arietinum) seed types. J Agric Food Chem 32(4):940–944
Sathe PD, Deshpande SS, Salunkhe DK (1982) Isolation and partial characterisation of black gram (Phaseolus mango L.) starch. J Food Sci 47:1524–1527
Sevenou O, Hill SE, Farhat IA, Mitchell JR (2002) Organisation of the external region of the starch granule as determined by infrared spectroscopy. Int J Biol Macromol 31:79–85
Shujun W, Jinglin Y, Wenyuan G, Hongyan L, Peigen X (2006) New starches from traditional Chinese medicine (TCM)-Chinese yam (Dioscorea opposita Thunb.) cultivars. Carbohydr Res 341:289–293
Singh U (2001) Functional properties of grain legume flours. J Food Sci Technol 38:191–199
Singh N, Sandhu KS, Kaur M (2004) Characterization of starches separated from Indian chickpea (Cicer arietinum L.) cultivars. J Food Eng 63:441–449
Van Hook A (1959) Principles of Sugar Technology. in Honig P, 2nd edn. Elsevier, Amsterdam, pp 113–149
Van Soest JJG, Tournois H, De Wit D, Vliegenthart JJG (1995) Short-range structure in (partially) crystalline potato starch determined with attenuated total reflectance Fourier transform IR spectroscopy. Carbohydr Res 279:201–214
Won SY, Choi WS, Lim HS, Cho KY, Lim ST (2000) Viscoelasticity of cowpea starch gels. Cereal Chem 77:309–314
Zobel HF, Young SN, Rocca LA (1988) Starch gelatinization: An X-ray diffraction study. Cereal Chem 65:443–446
Acknowledgment
The first author would like to acknowledge funding of International Foundation for Sciences (IFS, Sweden). He equally acknowledge the Agence Universitaire de la Francophonie (AUF) for the financial support under its Programme Scientifique de Cooperation Inter-universitaire de la Francophonie (PSCI).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaptso, K.G., Njintang, Y.N., Nguemtchouin, M.M.G. et al. Physicochemical and micro-structural properties of flours, starch and proteins from two varieties of legumes: bambara groundnut (Vigna subterranea) . J Food Sci Technol 52, 4915–4924 (2015). https://doi.org/10.1007/s13197-014-1580-7
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-014-1580-7