Abstract
Kalakand, a popular traditional milk sweet of Indian sub-continent, was packaged under air and modified atmospheric packaging (MAP) conditions (98 % N2, 98 % CO2 and 50 % N2: 50 % CO2). The samples were stored at 10, 25 and 37 °C and evaluated for various physico-chemical, microbial, textural and sensory changes, in order to establish the applicability of MAP for storage of Kalakand. It could be established that the MAP conditions of 50 % N2: 50 % CO2and storage at 10 °C, were the most suitable conditions for preserving the Kalakand for upto 60 days.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Milk based sweets are an integral part of the dietary system of Indian sub-continent. These sweets are specially served on various occasions such as weddings, festivals and celebrations. Traditional milk products in India have great commercial importance as they account for over 90 % of all the milk products consumed in the country (Aneja et al. 2002). Being the largest producer in the world, India has produced about 140 million tons of milk annually in the year 2013 (Govt. of India Report 2013). It is estimated that about 50-55 % of this milk produced is converted into traditional dairy products like heat desiccated milk products viz., Khoa, Basundi, Lalpeda, Rabri, Kalakand etc., coagulated milk products viz., Dahi, Shrikhand, Paneer, Chhana and Chhana based products and heat clarified products viz., butter oil, Ghee etc., which have a strong social and cultural heritage in the Indian society.
These dairy foods have a limited shelf life as many deteriorative changes take place during storage. Milk based sweets during storage undergo several physical, biochemical and microbiological changes making them unfit for human consumption (Londhe et al. 2012). Several studies have been reported on the use of hurdle technology, water activity and increase in sugar content to increase the shelf life of these traditional dairy foods (Kumar and Srinivasan 1982; Biradar et al. 1985; Thakur et al. 1992; Kumar et al. 1997; Sharma et al. 2001), however, scientific information on the use of MAP for extending the shelf life of traditional dairy foods is still limited. Reports have been published on the application of MAP for preservation of Peda (Biradar et al. 1985), Paneer (Rai et al. 2008; Thippeswamy et al. 2011), Malai peda (Sharma et al. 2003), brown peda (Londhe et al. 2012) and Lal peda (Jha et al. 2013) which shows that this technology holds a promise for enhancing the shelf life of traditional dairy foods.
Kalakand is prepared by blending sugar with ‘danedar’ Khoa and heated for giving it a pleasant caramel flavor and granular texture. The color of Kalakand varies from off white to light caramel (Aneja et al. 2002). Kalakand is manufactured on a small scale and it is generally consumed fresh because of its limited shelf life of just a few days limited to just few days at ambient temperature.
Shelf life of the product can be increased by changing the environment to which it is exposed and/or the packaging system (Harte and Gray 1987). The deteriorating agents of the food quality are oxygen contained in the air and microorganisms (bacteria and mold). Oxygen promotes several types of deteriorative reactions in foods including oxidation of fat, browning and pigment oxidation. Oxygen is the key element required for the growth of common spoilage bacteria and fungi. Therefore, to increase the shelf life of foods, the package atmosphere should contain a low concentration of residual oxygen (Sandhya 2010). The addition of chemical additives (preservatives, antioxidants, colorants, etc.) has frequently been associated with certain health problems, including allergies, and other more serious illnesses such as the initiation of carcinogenesis (Helliwell et al. 1995).
MAP technique can be effectively used to reduce or eliminate the need of chemical preservatives while maintaining a desired shelf life for the packaged food product (Fernandez et al. 2006). The gases that are mostly used are carbon dioxide (CO2) and nitrogen (N2). CO2 is both fungistatic and bacteriostatic and prevents insect growth in packaged and stored food products. Nitrogen is an inert, tasteless gas and used as a filler gas. Generally, O2 concentration in MAP must be below atmospheric levels (i.e. < 21 % v/v) (Farber 1991; O’Conner et al. 1992). MAP can alter the respiration rate, microbial growth and oxidation reactions (Mangarj and Goswami 2009).
In the present work, Kalakand samples were packaged using MAP technique under different combinations of N2 and CO2 (98 % CO2, 98%N2 and 50:50 % CO2:N2) and stored at three different temperatures viz.10, 25 and 37 °C. The effect of MAP on the microbial, textural, sensory and physico-chemical properties of Kalakand was studied during the storage.
Materials and methods
The present work was carried out in the laboratory of Centre of Food Science and Technology, Banaras Hindu University, Varanasi, India. Kalakand was manufactured using the buffalo milk standardized to 6 % fat and 9 % SNF. Milk was procured from Dairy Farm of Banaras Hindu University, Varanasi, India. Sugar was procured from the local market of Varanasi, India.
Manufacturing of Kalakand
Kalakand was prepared by the procedure suggested by Aneja et al. (2002) with slight modifications. Standardized milk was taken in a jacketed vessel and heated to simmering temperature (85–90 °C) with continuous stirring by a wooden ladle in a circular motion with occasional scraping of the heating surface. After 10–15 min of boiling, 0.02%of citric acid (in the form of solution) was added to milk to facilitate granulation with vigorous stirring obtain a good quality product. The intensity of heating was reduced when semi-solid state was reached. To this preparation, 6 % sugar (by weight of milk) was added, while continuously stirring the product. The finished product was transferred to tray previously greased with ghee for cooling and setting at room temperature. The process diagram along with the mass balance for manufacturing Kalakand is shown in Fig. 1.
Packaging and storage of Kalakand under MAP
Kalakand samples (100 g) were packed in polyethylene pouches packed in five layered polyethylene (dimensions: 26 × 15 cm of 0.1 mm) using MAP equipment Reepack®, VAC-STAR, S 220 MP, Switzerland and MAP mix 9,000 Gas mixer, manufactured by PBI DANSENSOR A/S, Ringstead, Denmark. The five layers comprised of an alternate layer of low density polyethylene (LDPE) film with bonding layer. The oxygen transmission rate of the LDPE film was 480 cc/0.001 inch/100 inch2/24 h/ atm O2 and the water vapour transmission rate of the LDPE film 1.2 g/1002 inch/24 h at 90 % RH. The packaging material was procured from Suprabha Protective Products Pvt. Ltd., Pune, India. Two different combinations of gases i.e. 98 % CO2 (with 2 % oxygen impurity), 98 % N2 and 50 % N2:50 % CO2 were used to pack the Kalakand samples. The samples packed under air were kept as control. The samples were stored in a BOD incubator (Remi Elektrotechnik Ltd., New Delhi, India) at 10, 25 and 37 °C at 60 % relative humidity and analyzed for microbial, sensory, textural and physico-chemical changes at an interval of 10 days up to 60 days.
Microbial analysis
All the samples were subjected to total plate count (TPC), yeast and mold count (YMC) and coliform count. The TPC was determined by surface spreading the homogenate (prepared by macerating the Kalakand samples in mortar and pestle) with 10−2 dilutions on plate count agar (PCA) and incubated at 37 °C for 24 h. For mold and yeast detection, appropriate dilutions (10−2) of sample was spread on potato dextrose agar (PDA) and incubated at 25 °C for 24 h. Coliforms in the samples were estimated by plating appropriate dilutions (10−2) on Violet Red Bile Agar (VRBA) before being incubated at 37 °C for 24 h.
Texture profile analysis (TPA)
TPA on samples was performed by using the Texture Analyser TA.XT plus, Exponent Lite (Stable Micro Systems, Surrey, UK) to characterize the hardness, adhesiveness, springiness, cohesiveness and gumminess of Kalakand. The samples of Kalakand were cut into 1.5 cm3 size pieces and their temperature maintained at 25 °C during the textural analysis. The samples were subjected to mono axial compression of 5 mm height. The force distance curve was obtained for a two bite compression cycle with the test speed of 1 mm/s and trigger force of 5 g.
Sensory evaluation
The sensory analysis of samples was carried out at 25 °C by a trained panel of 9 judges drawn from the staff and students of the Department of Animal Husbandry and Dairying and the Centre of Food Science and Technology of the Banaras Hindu University, Varanasi, India. The judges were asked to score for the sensory attributes viz. color and appearance, flavor, body and texture, sweetness and overall acceptability on a 9-point Hedonic scale.
Moisture and nutritional component analysis
The moisture content and the nutritional composition in Kalakand samples were analyzed using the AOAC (2000) method.
Hydroxy methyl furfural (HMF)
The HMF content in Kalakand was determined by following the method of Keeney and Bassette (1959) with slight modifications. Kalakand (0.5 g) sample was thoroughly mixed with 9.5 mL distilled water. Then, 5.0 mL of 3 N oxalic acid was added and the tubes were kept in boiling water bath for 60 min. The contents of the tubes were cooled and 5 mL of 40 % trichloroacetic acid solution was added. The precipitated mixture was filtered through Whatman No. 42 filter paper. Filtrate (0.5 mL) was pipetted out into a 5 mL test tube and added with 3.5 mL of distilled water and 1 mL of 0.05 M thiobarbituric acid solution (aq.) and mixed well. Tubes were kept in water bath at 40 °C for 50 min. After cooling to room temperature, absorbance was measured at 443 nm (UV-1800, UV Spectrophotometer, Shimadzu Corporation, Kyoto, Japan). A blank was run in the same manner as those for the samples, substituting distilled water for Kalakand. A standard curve of HMF concentration and absorbance at 443 nm was drawn by using a range of 1.0 to 10 μmol/mL (0.5 mL aliquot) HMF concentrations.
Thiobarbituric acid (TBA)
The extent of oxidation of fat in Kalakand was measured in terms of Thiobarbituric acid-reactive substances (TBARS) value. The extraction method of Strange et al. (1977) was adopted with slight modifications. For estimation, 2 g sample was taken and blended with 50 ml of 20 % TCA (trichloroacetic acid) and 50 mL of distilled water. The contents were left undisturbed for 10 min followed by filtration. Further, 5 mL of filtrate was pipetted out in each test tube and added with 5 mL of 0.01 M 2-thiobarbituric acid. The test tubes were heated in boiling water bath for 30 min at 95 °C for colour development. The contents were then cooled to 30 °C and absorbance determined at 532 nm. Blank determinations were made using distilled water in place of sample. TBA value was expressed as absorbance at 532 nm.
Free fatty acid
The method prescribed by Deeth et al. (1975) was used to determine the free fatty acid (FFA) content in Kalakand samples.
Statistical analysis
All the data were expressed as mean ± standard deviation of mean calculated from three independent experiments. One-way analysis of variance (ANOVA) was applied and Duncan multiple range test was performed to measure the test of significance by post hoc test using SPSS 16.0 software (SPSS Italia, Bologna, Italy).
Results and discussion
Microbial analysis Table 1 depicts the TPC, YMC and coliform count in Kalakand samples.
The TPC of the Kalakand samples showed significant (P < 0.05) variations among samples packed under all three compositions of gases and different temperatures. The TPC in control (air filled) samples increased and varied significantly among samples stored at 10, 25 and 37 °C, during the storage period of 30 days, after which it was rendered unacceptable on sensory basis. However, the samples stored at MAP conditions were found to be acceptable up to 60 days and the microbial quality was better than the control samples. However, the samples were unacceptable after 60 days of storage, hence discarded. Among MAP samples, the general trend in increase in the TPC and YMC was 50:50::N2:CO2 < 98 % CO2 < 98 % N2, during the storage period of 60 days. No coliforms were detected in any of the samples. The microbiological quality of the Kalakand samples at the time of packaging was good, without working under sterile conditions of aerobic microorganisms. The control samples supported the growth of microorganisms, due to the presence of oxygen in the packets. The samples stored in 98 % N2 showed delayed microbial proliferation as nitrogen does not support microbial growth. The samples stored at 98 % CO2 showed better microbial control than 98 % N2, which could be due to the microbial inhibiting property of CO2. However, it was observed that the samples stored at 50 % N2: 50 % CO2, inhibited the microbial growth with greater efficiency than those of the former. This could be attributed to the combined benefits of N2 and CO2 as reported by several workers (Daniels et al. 1985; Banks and Annis 1990; Davis 1998; Devlieghere and Debevere 2000; Arvanitoyannis et al. 2011; Ghayal et al. 2013; Jha et al. 2013). Smith et al. (1986) reported that crusty rolls packed in MAP (40 % N2: 60 % CO2) remained mold free even for as much as 60 days, as the O2 concentration never exceeded beyond 0.05 %. Current observations are in accordance with their findings.
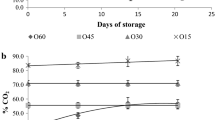
Changes in moisture content
Moisture content of any product is a critical parameter in determining the textural and organoleptic parameters of the product. The average moisture content of the Kalakand samples decreased with increase in the storage period (Fig. 2). Moisture content was also affected by the temperature of the storage. The reduction in moisture content was the least in the samples stored at 10 °C (Fig. 2a), while it was the highest in the samples stored at 37 °C (Fig. 2c). The moisture content of the Kalakand also varied with packaging conditions. The reduction of moisture content was highest in samples packed under air, while it was the lowest in the samples packed under 50 % N2: 50 % CO2, at all the temperatures under study. Ghayal et al. (2013) and Jha et al. (2013) showed similar changes in the control and the MAP packed Rabri and Lal peda samples, respectively.
Changes in textural characteristics
The textural properties of any product areimportant criteria to determine the acceptability of the product. Table 2 depicts the textural changes in Kalakand during storage.
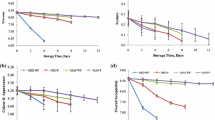
There was a gradual increase in the hardness of the Kalakand samples with an increase in the storage period for the control samples and for the MAP samples. The hardness varied significantly with variation in the gas composition and the storage temperature. Table 2 shows that the hardness of the Kalakand samples increased significantly (P < 0.05) with increase in the temperature, the hardness of the samples packed under air increased to a greater extent than those packed under the MAP conditions. The hardness of any product is directly related to its moisture content. It can be observed from Table 2, that the samples packed under 50 % N2: 50 % CO2 showed the minimum changes in hardness, which could be attributed to its better moisture retention (Fig. 2) under the MAP conditions.
Adhesiveness of a product is related to its sensory stickiness. Table 2 shows that the average adhesiveness of the Kalakand samples decreased gradually, with increasing the storage period. The average adhesiveness of the samples had no significant change with increase in temperature from 10 to 25 °C, however it varied significantly with further increase in temperature form 25 to 37 °C. This decline in the adhesiveness could be due to the decrease in free moisture during the storage. Similar trend in adhesiveness was observed in the Lal peda samples by Jha et al. (2013).
Springiness of a food refers to its ability to return to its original form after compression. It is the height that the product recovers between the first and the second compression. The springiness for Kalakand samples did not vary significantly (P > 0.05) for upto 10 days of storage at all the temperatures in the control and the MAP samples. However, the springiness increased with further increase in storage period and varied significantly (P < 0.05) with variation in the temperature and gas composition. Jha et al. (2013) reported similar observations in Lal peda samples stored under MAP conditions. The springiness values in Kalakand were lower than those reported in Lal peda (Jha et al. 2013), but higher than those reported in brown peda by Londhe et al. (2012).
Cohesiveness of a product is the ratio of the area under the second bite curve before reversal compression to under the first bite curve. It is the measure of the extent to which the structure of product was disrupted during the first compression. The average cohesiveness of the samples remained fairly constant throughout the storage period with no significant variation. Also, change in temperature had a minimal effect on the cohesiveness during 40 days of storage, but varied significantly after 40 days with temperature variation. The cohesiveness increased initially during the storage period, however it decreased marginally thereafter. These observations are similar to those reported by Londhe et al. (2012) in brown peda and Jha et al. (2013) in Lal peda. Loss in moisture content could be the reason for loss of cohesiveness with the progression of storage period. Gupta et al. (1990) also reported similar findings in Khoa with increase in total solids.
Gumminess is the product of hardness and cohesiveness. It can be seen from Table 2, that the average gumminess of Kalakand was significantly (P < 0.05) affected by variation in the temperature. It was lower in samples stored at 10 °C, which gradually increased with increase in the storage temperature from 25 to 37 °C. The variation in gas composition had a significant (P < 0.05) effect on gumminess of the Kalakand samples. However, it showed no significant (P > 0.05) variation during the storage period (Table 3).
Changes in sensory properties
The average score for all sensory attributes decreased significantly (P < 0.05) with storage period. The control samples were found to be unacceptable after 30 days, hence discarded from further sensory evaluation. The samples packed under 50 % N2: 50 % CO2, scored the maximum among all the samples for all the sensory properties at their respective storage temperatures and storage periods. The sensory scores for color and appearance, flavor, sweetness and body and texture varied significantly (P < 0.05) among MAP samples after 30 days of storage at all the temperatures. Increase in the temperature had a detrimental effect on the sensory score of the product. The color and appearance, flavor and body and texture were affected the most by an increase in the storage temperature with progression of storage period. Nonetheless, the samples stored at 10 °C packed under 50 % N2: 50 % CO2 showed the highest sensory characteristics of the samples.
Changes in HMF content
HMF is the byproduct formed during browning reactions such as Maillard reaction. The HMF content for samples are depicted in Fig. 3 (a, b and c).
The HMF content for the control samples varied with variation in the temperature. The samples stored at 10 °C, showed minimal change in the HMF content, as compared to the samples stored at 25 and 37 °C. The HMF content of the samples increased gradually with progression in the storage period. It was found to be the lowest (6.04 ± 0.66) in samples packed under 50 % N2: 50 % CO2 stored at 10 °C, while it was the highest (9.17 ± 0.81) in the samples packed under 98 % N2 stored at 37 °C at day 60. The control samples were found to be unacceptable after 30 days on sensory basis. The HMF content of the control samples reached its highest level (9.46 ± 0.85) at day 30. Thus, it could be stated that the HMF content of the Kalakand was highly affected by the alteration in the gas composition under which it is stored and the gas composition of 50 % N2: 50 % CO2 was found to be the most desirable in reducing the rate of formation of HMF. Londhe et al. (2012) and Jha et al. (2013) reported that combination of CO2 and N2 showed better results in the prevention of HMF formation in the brown peda and the Lal peda, respectively.
Changes in TBA values Kalakand
contains considerable amount of fat (17.74 %), making it highly susceptible to oxidation. Fig. 4 (a, b and c) show the TBA values for Kalakand samples stored under air and MAP at 10, 25 and 37 °C.
The TBA values in the samples increased gradually with the storage period and storage temperature was also found to be affected by the variation in the gas composition in the MAP samples. The general increasing trend of TBA observed in the MAP samples was 50 % N2: 50 % CO2 < 98 % CO2 < 98 % N2. The control samples showed high TBA values during 30 days of storage as compared to the MAP samples. The samples stored at MAP underwent slow deteriorative changes as compared to the samples packed under air. Similar observations were reported by Jha et al. (2013). (Meshref and Al-Rowaily 2008) reported that increased TBA value for milk and other dairy products were obtained when subjected to heat treatment. As Kalakand is a heat desiccated dairy product, oxidation may set in during the processing, thus increasing the TBA value. Similar observations were made by Ghayal et al. (2013).
Changes in the FFA
is a measure of the liberation of free fatty acids from the fat globules because of processing or degradation of fat content during or after manufacturing. The FFA content of the Kalakand samples is depicted in Fig. 5.
The FFA content of the samples showed an increasing trend with progression of the storage period. Several workers have reported similar increase in FFA content of Khoa during the storage period (Jha et al. 1977; Kumar et al. 2010). It is evident from Fig. 5 (a, b and c) that the FFA values were highly affected by the temperature of the storage. The values were found to be lowest for the samples stored at 10 °C (Fig. 5a) which further increased in case of samples stored at 25 (Fig. 5b) and 37 °C (Fig. 5c). It can also be seen that FFA content was also affected by the gas composition under which product is stored in MAP. The general increasing trend of FFA content observed in MAP samples was 50 % N2: 50 % CO2 < 98 % CO2 < 98 % N2. The control samples showed higher FFA values during 30 days of storage as compared with MAP samples. The delayed lipolysis in Kalakand samples stored under MAP could be attributed to the absence of oxygen in the package. Similar findings were reported by Londhe et al. (2012), Jha et al. (2013) and Ghayal et al. (2013).
Conclusions
An attempt was made to evaluate the shelf life of Kalakand stored under air and MAP with variation in gas composition viz. 98 % N2, 98 % CO2 and 50 % N2: 50 % CO2 during three different storage temperatures (10, 25 and 37 °C). The samples stored under air had a short shelf life of 30 days, while MAP samples were acceptable upto 60 days. The control samples had a comparatively higher HMF, TBA, FFA and microbial load. Therefore, MAP could be considered as a better option for storage of Kalakand. The samples packed under 50 % N2: 50 % CO2 were optimally effective in preserving the microbial, textural, sensory and physico-chemical properties of Kalakand. This study could prove to be helpful in preservation of other traditional dairy products using MAP.
References
Aneja RP, Mathur BN, Chamdan RC, Banerjee AK (2002) Technology of Indian milk products. A Dairy India Publication, New Delhi
AOAC (2000) Official methods of analysis of AOAC International, 17thedn. AOAC International, Washington, USA
Arvanitoyannis IS, Kargaki GK, Hadjichristodoulou C (2011) Effect of three map compositions on the physical and microbiological properties of a low fat greek cheese known as anthotyros. Anaerobe 17:295–297
Banks HJ, Annis PC (1990) Comparative advantages of high CO2 and low O2 types of controlled atmospheres for grain storage. In: Food preservation by modified atmospheres, Calderon M, Barkai-Golan R, eds. Boca Raton, Florida, USA: CRC Press Inc, pp 93–122
Biradar US, Dev DK, Ingle UM (1985) Shelf life extension of Pedha by packaging. J Food Sci 50(1):51–55
Daniels JA, Krishnamurthi R, Rizvi SSH (1985) A review of effect of carbon dioxide on microbial growth and food quality. J Food Prot 48:532–537
Davis HK (1998) Fish and shellfish. In: Principles and applications of modified atmosphere packaging of foods,2nd edn, Blakistone BA, ed. London, UK: Blackie Academic & Professional, pp 194–239
Deeth HC, Fitz-Gerald CH, Wood AF (1975) A Convenient method for determining the extent of Lipolysis in milk. Aust J Dairy Technol 30:109–111
Devlieghere F, Debevere J (2000) Influence of dissolved carbon dioxide on the growth of spoilage bacteria. LWT- Food SciTechnol 33:531–537
Farber JM (1991) Microbiological aspects of modified- atmosphere packaging technology: a review. J Food Prot 54:58–70
Fernandez U, Vodovotz Y, Courtney P, Pascall MA (2006) Extended shelf-life of soy bread using modified atmosphere packaging. J Food Prot 69:693–698
Ghayal G, Jha A, Arvind GAK, Rasane P (2013) Effect of modified atmospheric packaging on physico-chemical and microbial changes in dietetic Rabri during storage. J Food Sci Technol. doi:10.1007/s13197-013-1171-z
Gupta SK, Patil GR, Patel AA, Garg FC, Rajorhia GS (1990) Instron texture profile parameters of Khoa as influenced by composition. J Food Sci Technol 27:209–213
Harte BR, Gray JI (1987) The influence of packaging on product quality. In: Food product package compatibility proceeding, Gray J I, Harte B R and Miltz J, eds Lancaster, Pennsylvania: Technomic Publishing Co Inc, pp 17
Helliwell B, Murcia M, Chirico S, Aruoma OI (1995) Free radicals and antioxidants in food and in vivo: what they do and how they work? Crit Rev Food Sci Nutr 35:7–20
Jha YK, Singh S, Singh S (1977) Effect of antioxidants and antimicrobial substances on keeping quality of Khoa. Indian J Dairy Sci 30:1–6
Jha A, Arvind, Jain P, Gautam AK, Rasane P (2013) Effect of modified atmosphere packaging on the shelf life of Lalpeda. J Food Sci Technol doi 10. 1007/s13197-013-1064-1
Keeney M, Bassette R (1959) Detection of intermediate compounds in early stages of browning reaction in milk products. J Dairy Sci 42:945–960
Kumar G, Srinivasan MR (1982) Effect of packaging and storage on the sensory characteristics of Khoa samples. Indian J Dairy Sci 35:132–137
Kumar GK, Bandyopadhyay P, Punjrath JS (1997) Shelf life extension of Peda using different packaging techniques. Indian J Dairy Sci 50(1):40–49
Kumar M, Beniwal BS, Rai DC (2010) Effect of antioxidant on shelf life of Khoa under refrigerated conditions. Egypt J Dairy Sci 38:211–218
Londhe GK, Pal D, Raju PN (2012) Effect of packaging techniques on shelf life of brown peda, a milk based confection. LWT Food Sci Technol 47:117–125
Mangarj S, Goswami TK (2009) Modified atmosphere packaging-an ideal food preservation technique. J Food Sci Technol 46:399–410
Meshref A, Al-Rowaily (2008) Effect of heating treatments, processing methods and refrigerated storage of milk and some dairy products on lipids oxidation. Pakistan JNutr7: 118–125
O’Conner RE, Skarfhewski P, Phrower SJ (1992) Modified atmosphere packaging of fruits, vegetables, seafood and meat: state of the art. ASEAN Food J 7:127–136
Rai S, Goyal GK, Rai GK (2008) Effect of modified atmospheric packaging (MAP) and storage on the chemical quality of Paneer. J Dairy Foods Home Sci 27(1):33–37
Sandhya (2010) Modified atmosphere packaging of fresh produce: current status and future needs. LWT Food Sci Technol 43:381–392
Sharma HK, Singhal RS, Kulkarni PR (2001) Effect of under vacuum or under nitrogen on the keeping quality of ‘danedar’ Khoa. Int J Dairy Technol 54:107–110
Sharma HK, Singhal RS, Kulkarni PR (2003) Effect of modified atmosphere packaging on the keeping quality of Malaipeda. J Food Sci Technol 40(5):543–545
Smith JP, Ooraikul B, Koersen WJ, Jackson ED, Lawrence RA (1986) Novel approach to oxygen control in modified atmosphere packaging of bakery products. Food Microbiol 3:315–320
Strange ED, Benedict RC, Smith JL, Swift CE (1977) Evaluation of rapid tests for monitoring alterations in meat quality during storage. J Food Prot 10:843–847
Thakur BR, Semwal AD, Arya SS (1992) Packaging requirements and stability of sorbate preserved Khoa sweets (Pedas). Indian Food Packer 46:53–56
Thippeswamy L, Venkateshaiah BV, Patil SB (2011) Effect of modified atmospheric packaging on the shelf stability of Paneer prepared by adapting hurdle technology. J Food Sci Technol 48(2):230–235
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jain, V., Rasane, P., Jha, A. et al. Effect of modified atmospheric packaging on the shelf life of Kalakand and its influence on microbial, textural, sensory and physico-chemical properties. J Food Sci Technol 52, 4090–4101 (2015). https://doi.org/10.1007/s13197-014-1501-9
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-014-1501-9