Abstract
The study was conducted on seven Ficus species namely F. drupacea Thunb., F. elmeri Merr., F. hispida L.f., F. microcarpa L.f, F. nervosa B. Heyne ex Roth, F. rumphii Blume and F. virens Aiton collected from West Garo Hill district, Meghalaya with the aim to examine the detailed anatomical and physical characteristics of these species. The study revealed homogeneous structure among Ficus species. Diffuse porous wood with indistinct growth rings, vessels solitary, in radial multiple of 2–3, simple perforation plates, intervessel pits alternate, banded parenchyma, multiseriate, homocellular and heterocellular rays were common anatomical characteristics among species. However, some distinct features like vessel ray pits with much reduced border to apparently simple with vertical pits in F. hispida, scalariform pits in F. virens, thin to thick walled fibres in F. virens, F. nervosa, sheath cells in F. elmeri and F. hispida and horizontal laticifers in F. virens were observed. The fibre percentage was maximum in F. elmeri and parenchyma percentage was maximum in F. rumphii. Wood density was maximum in F. elmeri and moisture content was minimum in F. rumphii. There was significant variation in quantitative characteristics within and among species. Therefore, both qualitative and quantitative anatomical and physical characteristics can be used for reliable identification of Ficus species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The family Moraceae is widely distributed in tropical and sub-tropical regions of the world and few species also occur in temperate zone (Raturi et al. 2001). It comprises of trees, shrubs, hemiepiphytes, climbers and herbs. The presence of milky latex in parenchymatous tissue, unisexual flowers, anatropous ovules, aggregated drupes or achene type of fruits are the diagnostic features of this family (Datwyler and Wieblen 2004). The members of this family are source of many timber species, edible fruits, rubber and dye etc. Chlorophora excelsa, Brosimum oarensis, Piratinera guianensis are some of durable timbers in the world. The species of Artocarpus and Morus are used for general carpentry works in India. The commercial rubber is obtained from Ficus elastica. The leaves of Morus alba are used for rearing silkworms (Raturi et al. 2001). The living bridges from the aerial roots of Ficus elastica in Khasi and Jaintia Hills of Meghalaya to cross the rivers represent an example of unique botanical architecture (Ludwig et al. 2019).
The family is divided into five tribes namely Artocarpeae, Dorstenieae, Castilleae, Ficeae and Moreae (Berg 1977, 2001). The tribe Ficeae is represented by a single genus Ficus L. It is the largest genus with 1000 species. Syconium inflorescence and obligate pollination mutualism with fig wasps are the diagnostic features of the genus Ficus (Datwyler and Wieblen 2004; Clement and Weiblen 2009). It is the most diversified genus with evergreen, semi-evergreen and deciduous in habit and represented by trees, shrubs, hemi epiphytes, climbers, creepers, rheophytes and lithophytes with wide distribution in tropical and sub-tropical regions of the world (Berg and Corner 2005; Chaudhary et al. 2012). Ficus species produce nutrient rich fruits throughout the year to attract most of frugivores and play a significant role in restoration of tropical ecosystem (Harrison 2006; Cotton-Jones et al. 2016). There are about 115 Ficus species in India. Among all states, Meghalaya is endowed with 43 Ficus species, and therefore, it is considered as a hot spot region for this genus (Chaudhary et al. 2012).
Wood anatomical characteristics are important to solve taxonomical problems for separation of species. They also help to study the relationship between tree growth and environmental factors. The available literature shows that few investigations have made on wood anatomy of Ficus species. Noorman et al. (1984) characterised Ficus species by abundant axial parenchyma in regular apotracheal bands and relatively wide vessels. Adeniyi et al. (2013) reported crystals, silica inclusions, size of rays, vessels and fibres as important features to distinguish seven Ficus species of Nigeria. A little information is available on wood anatomy of Ficus species in India (Gamble 1922; Purkayastha 1996). Raturi et al. (2001) studied the gross features of Ficus species under hand lens and separated in two groups on the presence or absence of ripple marks. Sharma et al. (2014) investigated 12 Ficus species collected from Mizoram. There is no information on anatomical investigations of Ficus species of Meghalaya despite of being a hot spot region for this genus. Therefore, the present study is an attempt to provide comprehensive account of anatomical and physical characteristics of seven Ficus species.
Materials and methods
Seven Ficus species, namely F. drupacea Thunb., F. elmeri Merr., F. hispida L.f., F. microcarpa L.f, F. nervosa B. Heyne ex Roth, F. rumphii Blume and F. virens Aiton were selected from Tura and Rongram of West Garo Hills District, Meghalaya. The geographical coordinates of the sites were taken with the help of a GPS. The height and diameter of the selected trees were measured with Ravi altimeter and measuring tape (Table 1).
Five healthy trees with straight boles, uniform crown and with no visible defects were selected randomly for each species. Wood blocks of 5 cm × 5 cm × 5 cm size were extracted with the help of a hammer and a chisel at breast height. The wood samples were numbered, packed in polythene bags and brought to the laboratory for further investigations. For anatomical investigations, wood samples were cut into small blocks of 2 cm3 size, fixed in FAA for 24–48 h. and were transferred to 50% alcohol for further preservation.
The preserved blocks were sectioned in 3 planes namely Cross Section (C. S.), Tangential Longitudinal Section (T. L. S.) and Radial Longitudinal Section (R. L. S.) with the help of a sliding microtome (Leica SM 2000R). Standard protocols were followed to prepare the permanent slides.
Thin splinters were taken from the radial side of selected wood species and were macerated with Franklin’s solution at 60 °C for 24 h till they turn to soft and white in colour. Temporary slides were prepared with 50% glycerol for the measurement of all anatomical parameters and their dimensions were measured with the help of Scope image 9 software. Quantitative data of fibre, vessel and ray dimensions were based on random 30 measures for each replicate of selected species. While, for vessel frequency, rays per mm. and tissue proportion, 10 fields for each replicate were randomly selected. The percentage of fibre, vessel, ray and parenchyma were determined in the field of 1 mm2 from cross-section. IAWA list of microscopic features for hardwoods identification (Wheeler et al. 1989) was followed for the anatomical descriptions of species. The photomicrographs were taken with the help of image analysis system at different magnifications to study detailed anatomical features of each species.
Water displacement method (Smith 1955) was used to determine the wood density. Moisture content was determined by oven drying method (IS 11215: 1991).
The data were analysed statistically by using SPSS 16 software. One way ANOVA was carried out to see the variation in anatomical characteristics within species. Tukey’s test was performed to compare the means of selected anatomical characteristics among species.
Results
The qualitative anatomical features and tissue percentage of Ficus species were presented in Table 2 and Figs. 1, 2 and 3.
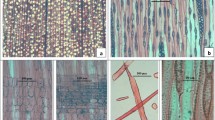
Ficus spp. Cross-section showing wood diffuse-porous, vessels mostly solitary and in radial multiples of 2–3, parenchyma banded and bands more than 3 cells wide, fibres thin walled (a,e,i). Tangential longitudinal section showing rays multiseriate, parenchyma strands 4–6 celled (c,j,k); fusiform parenchyma (c); crystals in parenchyma (f) and sheath cells in rays (j). Radial longitudinal section showing heterocellular rays made up of procumbent body ray cells with marginal over 4 rows of upright and/or squares cells (c–k) and with marginal over 1–2 rows of upright and/or squares cells (g); crystals present in rays (c); vessel ray pits with much reduced border to apparently simple, pits rounded (d–h) and vertical (palisade) (l)
Ficus spp. Cross-section showing wood diffuse-porous, vessels mostly solitary and in radial multiples of 2–3, parenchyma banded and bands more than 3 cells wide (a,d,i,m) fibres thin to walled (a,d,m); fibres thin walled (i). Tangential longitudinal section showing rays mostly multiseriate and parenchyma strands, (b, e, f, j, n and o); rays with long tail and septate fibres present (e). Radial longitudinal section showing homocellular rays of procumbent cells(p); heterocellular rays made up of procumbent body ray cells with marginal 1–2 rows of upright and/or squares cells (c, g,h,k,l,and q); axial laticifers among fibres (l) and horizontal laticifers in rays (p) present
1. Ficus drupacea Thunb. (Fig. 1: a–d)
Vernacular name: Prap (Garo).
Anatomical features—A diffuse-porous wood.
Growth rings—Indistinct.
Vessels—Mostly solitary, in radial multiples of 2–3, oval in outline, barrel shaped with or without tails, 290.59–495.71 µm (Mean 393.65 ± 17 µm) in length, 81.82–250.83 µm (Mean 178.97 ± 30.46 µm) in diameter, vessel frequency 2–7 (Mean 3.82 ± 1.13) per mm2, simple perforation plates, intervessel pits alternate,13–18.2 µm (Mean 15.34 ± 2.05 µm) in size, vessel- ray pits with much reduced border to apparently simple, pits rounded, tyloses present, vessel percentage 24.
Fibres—Thin walled, 1230.72–1931.55 µm (Mean 1544.72 ± 74.15 µm) long, 19.98–37.15 µm (Mean 25.30 ± 3.82 µm) and 13.17–30.08 µm (Mean 18.47 ± 3.90 µm) in diameter and lumen diameter, wall thickness 4.14–10.06 µm (Mean 6.84 ± 1.37 µm), fibres percentage 23.27.
Parenchyma—Fusiform, vasicentric, banded (both apotracheal and paratracheal bands), more than 3 cells wide, bands 4–12 cells wide (Mean 6.40 ± 2.50), 4–6 cells per parenchyma strand, parenchyma percentage 27.64.
Rays—Mostly multiseriate, uniseriate and biseriate rays also present, mean ray height and ray width 404.56–897.67 µm (Mean 564.30 ± 247.60 µm) and 4.14–88.81 µm (Mean 44.32 ± 24.88 µm), rays both homocellular and heterocellular, all homocellular rays of either procumbent cells or upright and/or square cells, heterocellular rays with procumbent, square and/or upright cells mixed throughout the ray. Rays 4–9 (Mean 5.52 ± 1.19) per mm, rays percentage 25.09.
Mineral inclusions—Prismatic crystals present in axial parenchyma and square cells.
Laticifers—Occasionally present among fibres.
2. Ficus elmeri Merr. (Fig. 1: e–h)
Vernacular name: Aminsep (Garo).
Anatomical features—A diffuse-porous wood.
Growth rings—Indistinct.
Vessels—Mostly solitary, in radial multiples of 2–3, oval in outline, barrel and drum shaped, 273.49–589.72 µm (Mean 400.77 ± 22.01) in length, 127.74–239.64 µm (Mean 181.83 ± 23.74 µm) in diameter, vessel frequency 3–12 (Mean 5.94 ± 1.77) per mm2, simple perforation plates, intervessel pits alternate, 10.4–15.6 µm (Mean 12.09 ± 2.56 µm) in size, vessel- ray pits with much reduced border to apparently simple, pits rounded, tyloses present, vessel percentage 25.09.
Fibres—Thin walled, 1239.27–2076.84 µm (Mean 1645.57 ± 57.82 µm) long, 13.84–25.58 µm (Mean 19.14 ± 2.33 µm) and 5.93–19.78 µm (Mean11.56 ± 2.34 µm) in diameter and lumen diameter, wall thickness 4.97–10.23 µm (Mean 7.58 ± 1.25 µm), septate fibres and gelatinous fibres present, fibre percentage 29.45.
Parenchyma—Banded (paratracheal bands), more than 3 cells wide, bands 9–24 cells wide (Mean 13.00 ± 4.90), 4–6 cells per parenchyma strand, parenchyma percentage 24.55.
Rays—Multiseriate, mean ray height and ray width 52.10–468.90 µm (Mean 536.16 ± 101.82 µm) and 10.42–52.10 µm (Mean105.56 ± 11.58 µm), both homocellular and heterocellular rays, all homocellular rays of procumbent cells, either square and/or upright cells, heterocellular rays of procumbent body ray cells with 1–2 rows of upright and/or square ray cells on the margins. Rays 2–6 (Mean 3.76 ± 0.77) per mm, rays percentage 22.91.
Mineral inclusions—Prismatic crystals present in axial parenchyma and square ray cells.
3. Ficus hispida L. f. (Fig. 1: i–l)
Vernacular name: Pantap (Garo).
Anatomical features—A diffuse-porous wood.
Growth rings—Indistinct.
Vessels—Mostly solitary, in radial multiples of 2–3, oval in outline, barrel to oblong shaped with tail like extensions at one or both ends, 299.13–504.25 µm (Mean 413.65 ± 11.60 µm) in length, 106.11–233.38 µm (Mean 139.93 ± 24.57 µm) in diameter, vessel frequency 3–15 (Mean 7.74 ± 2.85) per mm2, simple perforation plates, intervessel pits alternate, 13–18.2 µm (Mean 13.91 ± 2.70 µm) in size, vessel—ray pits with much reduced border to apparently simple, pits vertical (palisade) present in outer 3-4 rows, vessel percentage 21.63.
Fibres—Thin walled, 1162.35–1871.72 µm (Mean1431.22 ± 43.97 µm) long, 13.32–30.01 µm (Mean 21.11 ± 4.31 µm) and 9.03–25.05 µm (Mean 15.86 ± 4.12 µm) in diameter and lumen diameter, wall thickness 2.41–9.83 µm (Mean 5.24 ± 1.62 µm), fibre percentage 26.36.
Parenchyma—Banded (both apotracheal and paratracheal diagonal bands), more than 3 cells wide, bands 6–16 cells wide (Mean 9.30 ± 3.09), 3–6 cells per parenchyma strand, parenchyma percentage 26.91.
Rays—Multiseriate, mean ray height and ray width 361.55–887.40 µm (Mean 579.55 ± 131.10 µm) and 38.50–93.07 µm (Mean 62.78 ± 12.72 µm), rays both homocellular and heterocellular, homocellular rays of either procumbent cells or square cells, heterocellular ray either procumbent body ray cells with marginal 1–2 rows of upright and/or square cells or mixed throughout the ray, body ray cells procumbent. Rays 3–6 (Mean 4.4 ± 0.83) per mm, sheath cells present, ray percentage 25.09.
Laticifers—Occasionally present among fibres.
4. Ficus microcarpa L. f (Fig. 2: a–c)
Vernacular name: Prapsi, tapsi (Garo).
Anatomical features—A diffuse-porous wood.
Growth rings—Both indistinct and distinct and marked by radially flattened thick walled fibres.
Vessels—Mostly solitary, in radial multiples of 2, oval in outline, barrel and drum shaped, 341.87–495.71 µm (Mean 417.932 ± 7.79 µm) in length, 152.18–289.44 µm (Mean 211.30 ± 28.51 µm) in diameter, vessel frequency 4–10 (Mean 6.68 ± 1.73) per mm2, simple perforation plate, intervessel pits alternate in size, 13–20.8 µm (Mean 15.73 ± 2.98 µm) polygonal in outline, vessel -ray pits with much reduced border to apparently simple, pits rounded or tyloses present, vessel percentage 27.64.
Fibres—Thin walled, 1282–2128.12 µm (Mean 1661.98 ± 56.31 µm) long, 11–31.52 µm (Mean 20.34 ± 5.04 µm) and 7.81–24.28 µm (Mean 15.20 ± 4.04 µm) in diameter and lumen diameter, 2.62–7.55 µm (Mean 5.14 ± 1.34 µm) in wall thickness, septate fibres and gelatinous fibres present, fibre percentage 26.
Parenchyma—Banded (both apotracheal and paratracheal wavy bands) more than 3 cells wide, bands 4–8cells wide (Mean 5.70 ± 1.57), 4–7 cells per parenchyma strand, parenchyma percentage 24.91.
Rays—Uniseriate, biseriate and multiseriate, mean ray height and ray width 310.72–537.79 µm (Mean 421.45 ± 57.33 µm) and 30.25–56.06 µm (Mean 42.49 ± 6.49 µm), rays both homocellular and heterocellular, body ray cells procumbent in homocellular rays and in heterocellular rays procumbent body ray cells with marginal rows of upright and/or square cells. Rays 4–8 (Mean 5.8 ± 1.19) per mm, rays percentage 21.45.
Mineral inclusions—Prismatic crystals present in axial parenchyma, fibres, square and procumbent ray cells.
Laticifers—Present among fibres.
5. Ficus nervosa B. Heyne ex Roth (Fig. 2: d–h)
Vernacular name: Bolchap (Garo).
Anatomical features—A diffuse-porous wood.
Growth rings—Indistinct.
Vessels—Mostly solitary, in radial multiples of 2–4, oval in outline, barrel to oblong shaped, 341.87–512.80 µm (Mean 422.37 ± 8.55 µm) in length, 114.09–259.32 µm (Mean 179.75 ± 31.45 µm) in diameter, vessel frequency 2–14 (Mean 5.68 ± 2.77) per mm2, simple perforation plates, intervessel pits alternate, 10.4–18.2 µm (Mean 14.3 ± 2.98 µm) in size, vessel- ray pits with much reduced border to apparently simple, pits rounded, vessels percentage 20.90.
Fibres—Thin to thick walled, 1247.81–2017.01 µm (Mean 1621.47 ± 29.35 µm) long, 27.05–43.80 µm (Mean 34.76 ± 4.42 µm) and 19.59–37.71 µm (Mean 27.65 ± 4.37 µm) in diameter and lumen diameter, wall thickness 4.69–9.41 µm (Mean 7.11 ± 0.92 µm), fibre percentage 29.09.
Parenchyma—Banded (Both apotracheal and paratracheal bands), more than 3 cells wide, bands 6–12 cells wide (Mean 8.70 ± 2.31), 4–9 cells per parenchyma strand, parenchyma percentage 27.09.
Rays—Mostly multiseriate, rarely uniseriate, mean ray height and ray width 517.28–954.61 µm (Mean 684.32 ± 115.46 µm) and 50.13–102.57 µm (Mean73.01 ± 11.9 µm), multiseriate rays with long tail, rays both homocellular and heterocellular, body ray cells of upright and/or square cells in homocellular ray, body ray cells of procumbent with marginal 1–2 rows of upright and/or square cells in heterocellular rays. Rays 3–7 (Mean 4.78 ± 1.03) per mm, rays percentage 22.91.
Mineral inclusions—Prismatic crystals present in axial parenchyma.
6. Ficus rumphii Blume: (Fig. 3: i–l)
Vernacular name: Prap rakseng (Garo).
Anatomical features—A diffuse-porous wood.
Growth rings—Indistinct.
Vessels—Mostly solitary, in radial multiples of 2–3, oval in outline, barrel shaped, 282.04–529.89 µm (Mean 376.73 ± 12 µm) in length, 112.95–231.51 µm (Mean159.55 ± 22.35 µm) in diameter, vessel frequency 3–10 (Mean 5.54 ± 1.66) per mm2, simple perforation plates, intervessel pits alternate in size, 13–18.2 µm (Mean 15.34 ± 1.86 µm) vessel—ray pits with much reduced border to apparently simple, pits vertical (palisade), vessel percentage 22.45.
Fibres—Thin walled, 307.68–1820.44 µm (Mean 1492.07 ± 22.14 µm) long, 14.01–25.95 µm (Mean 20.08 ± 2.58 µm) and 9.51–18.58 µm (Mean 13.54 ± 2.14 µm) in diameter and lumen diameter, wall thickness 3.63–8.73 µm (Mean 6.54 ± 1.08 µm), septate fibres present, fibre percentage 23.50.
Parenchyma—Banded (paratracheal bands), more than 3 cells wide, bands 8–32 cells wide (Mean 16.80 ± 4.32), 5–8 cells per parenchyma strand, parenchyma percentage 30.27.
Rays—Uniseriate, biseriate and multiseriate, mean ray height and ray width 331.22-331.22 µm (Mean 562.83 ± 118.55 µm) and 17.46–26.71 µm (Mean 55.73 ± 17.46 µm), both homocellular and heterocellular rays, homocellular rays of either procumbent cells or of square and/or upright cells, heterocellular rays of procumbent body ray cells with1–2 marginal rows of upright and/or square cells. Rays 3–7 (Mean 4.7 ± 0.86) per mm, rays percentage 23.78.
Mineral inclusions—Prismatic crystals present in axial parenchyma square and/or upright ray cells.
Laticifers—Occasionally present among fibres.
7. Ficus virens Aiton: (Fig. 3: m–q)
Vernacular name: Dieng- sohpoklaw (Khasi).
Anatomical features—A diffuse- porous wood.
Growth rings—Indistinct.
Vessels—Mostly solitary, in radial multiples of 2–4, oval in outline, barrel and drum shaped, 324.77–487.16 µm (Mean 421.52 ± 9.20 µm) in length, 108.55–319.86 µm (Mean 180.49 ± 52.37 µm) in diameter, vessel frequency 3–11 (Mean 5.94 ± 1.77) per mm2, simple perforation plates, intervessel pits alternate, 10.4–15.6 µm (Mean 13.13 ± 1.98 µm) in size, vessel- ray pits with much reduced border to apparently simple, pits scalariform (gash like), vessels percentage 23.45.
Fibres—Thin to thick walled, 1290.55–2179.40 µm (Mean 1637.71 ± 69.16 µm) long,
23.15–41.18 µm (Mean 33.29 ± 4.04 µm) and 15.64–35.72 µm (Mean 26.20 ± 4.47 µm) in diameter and lumen diameter, wall thickness 4.7–11 µm (Mean 7.09 ± 1.45 µm), septate fibres present, fibres percentage 26.
Parenchyma—Banded (both apotracheal and paratracheal bands), more than 3 cells wide, bands 4–15 cells wide (Mean 8.20 ± 3.79), 4–6 cells per parenchyma strand, parenchyma percentage 27.64.
Rays—Mostly multiseriate, rarely biseriate, mean ray height and ray width 612.10–1037.78 µm (Mean 778.90 ± 107.83 µm) and 36.65–82.83 µm (Mean 62.41 ± 8.89 µm), both homocellular and heterocellular rays present, all homocellular rays of either procumbent or upright and/or square cells, heterocellular rays of procumbent body ray cells with marginal 1–3 rows of square and/or upright cells. Rays 3–7 (Mean 5.12 ± 1.15) per mm, rays percentage 22.91.
Mineral inclusions—Prismatic crystals present in square ray cells.
Laticifers—Present in rays.
The results given in Table 3 showed highly significant variations in most of the anatomical characteristics within species. A non-significant variation was observed for ray frequency and intervessel pit size for all Ficus species. The other few anatomical characteristics exhibiting non-significant variation were vessel length (F. microcarpa and F. virens), vessel diameter (F. rumphii) and fibre diameter and fibre lumen diameter (F. elmeri and F. rumphii), fibre wall thickness (F. nervosa and F. rumphii). ray height (F. microcarpa, F. rumphii and F. virens) and ray width ( F. hispida).
Tukey’s HSD test was performed to see the significant differences in anatomical characteristics of selected Ficus species (Table 4). Vessels were significantly longer in F. nervosa and F. virens than other species. Vessel diameter was significantly larger in F. microcarpa and vessel frequency was higher in F. hispida than other species. Fibres were substantially longer in F. microcarpa, F. elmeri, F. virens and F. nervosa whereas fibre diameter and fibre lumen diameter were significantly greater in F. nervosa and F. virens. F. elmeri had maximum wall thickness among selected species. Rays were significantly longer and wider in F. virens and F. nervosa. On the other hand, the number of rays per mm was maximum in F. microcarpa and F. drupacea Parenchyma strands were longer in F. rumphii where as parenchyma bands were significantly wider in F. rumphii and F. elmeri.
Coded descriptions based on IAWA list of microscopic features of Ficus species presented in Table 5 showed similarity in most of the anatomical characteristics.
Maximum wood density and minimum moisture content were recorded in F. elmeri among species. On the other hand, F. rumphii showed minimum wood density with maximum moisture content (Table 6).
Identification key
-
1.
Banded parenchyma, both apotracheal and paratracheal bands with more than 3 cells wide…4
-
1a.
Banded parenchyma, paratracheal bands with more than 3 cells wide …2
-
1a.
-
2.
Fibre length 900–1600 µm, laticifers among fibres present …3
-
2a.
Fibre length ≥ 1600 µm, laticifers among fibres absent …F. elmeri.
-
2a.
-
3.
Vessel frequency 5–20, vessel ray pits vertical, multiseriate rays with sheath cell …F. hispida.
-
3a.
Vessel frequency 5–20, vessel ray pits vertical, multiseriate rays without sheath. Cells …F. rumphii.
-
3a.
-
4.
Ray frequency ≥ 4, fusiform and vascicentric parenchyma present…F. drupacea.
-
4a.
Ray frequency ≤ 4, fusiform and vascicentric parenchyma absent …5.
-
4a.
-
5.
Vessel diameter 100–200 µm, vessel ray pits rounded and laticifers absent in fibres …6.
-
5a.
Vessel diameter ≥ 200 µm, vessel ray pits rounded and laticifers present …F. microcarpa.
-
5a.
-
6.
Prismatic crystals in axial parenchyma, septate fibres absent …F. nervosa.
-
6a.
Prismatic crystals in ray cells, septate fibres present …F. virens.
-
6a.
Discussion
Most of the anatomical characteristics are similar in Ficus species. Diffuse-porous woods with indistinct growth rings, vessels solitary and in radial multiples of 2–3 were present in all species except F. microcarpa in which distinct rings due to presence of radially flattened fibres were observed. The vessels were barrel shaped except drum shaped in F. elmeri and F. microcarpa. The tangential diameters of vessels were in the range of 100–200 µm except F.microcarpa and the range of vessel frequency was 5–20 per square millimetre in all species except F. drupacea. Other common features of vessels were simple perforation plate and intervessel pits alternate. Vessel ray pits were with much reduced border to apparently simple with rounded pits in all species except vertical (palisade) in F. hispida and F. rumphii and scalariform (gash like) in F. virens. Fibres were thin to thick walled in F. nervosa and F. virens. Whereas, these were thin walled in other selected species. Septate fibres were present in F. drupacea and F. hispida The present study on qualitative and quantitative features of fibres and vessels confirms the findings of Noorman et al. (1984) and Adeniyi et al. (2013) who reported similar features of vessel and fibres in Neotropical, African and Nigerian species of Ficus.
Axial parenchyma was abundant, banded and present in both apotracheal and paratracheal bands. On contrary to it, Sharma et al. (2014) reported other forms of parenchyma like lozenge aliform and diffuse in F. hispida and F. variegata, diffuse-in-aggregate in F. racemosa and F. rumphii in addition to banded parenchyma. Raturi et al. (2001) divided Indian Ficus species into two groups on the basis of ripple marks which may be due to storied rays, fibres or parenchyma. In this study, storied (fusiform) parenchyma was observed in F. drupacea only. Rays were mostly multiseriate in all species. Uniseriate and biseriate rays alongwith multiseriate rays were also observed in F. drupacea, F. microcarpa and F. rumphii. The presence of sheath cells in F. elmeri and F. hispida corroborates the findings of Sharma et al. (2014). Rays were homocellular and heterocellular in all species and confirm the findings of other workers (Noorman et al. 1984; Adeniyi et al. 2013; Sharma et al. 2014). Crystals were present in axial parenchyma cells, square and upright ray cells of Ficus species whereas Yaman (2014) reported prismatic crystals in axial parenchyma cells of F. carica subsp. carica.
Laticifers act as a defense system in plants and prevent pest invasion by secretion of latex. Axial/longitudinal laticifers were present in the form of streaks among fibres in F. hispida, F. rumphii, F. microcarpa and occasionally in F. virens also. Horizontal laticifers were observed in rays of F. virens only. The study corroborates the findings of Kaji et al. (2014) who reported the frequent occurrence of both longitudinal and horizontal laticifers in secondary phloem and secondary xylem of F. carica.
Anatomical characteristics are highly variable within a tree, among trees of same or different species (Zobel and Talbert 1984). Pande et al. (2007, 2009) reported non-significant intra-species variation in anatomical features. On contrary to it, the conflicting results have been found in the present study. Most of the anatomical characteristics show highly significant variations while other features are non-significant. Hence, Ficus species can be differentiated on the basis of both qualitative and quantitative anatomical characteristics.
In Ficus species, the percentage of fibres, vessels, rays and parenchyma are more or less uniform. F. elmeri had maximum fibre and vessel percentage. Ray percentage was maximum in F. drupacea and F. hispida whereas, maximum parenchyma percentage was observed in F. rumphii. The banded parenchyma may be the reason of less percentage of fibres in most of species.
The present study reveals maximum wood density and minimum moisture content in F. elmeri. On the other hand, F. rumphii has minimum wood density with maximum moisture content. The higher percentage of fibres with maximum wall thickness and low percentage of parenchyma may attribute to maximum wood density with minimum moisture content in F. elmeri. On the other hand, maximum width of parenchyma bands (16 cells wide) may be the possible reason for maximum moisture content in in F. rumphii.
Conclusions
The wood structure of Ficus species is uniform. Storied (fusiform) parenchyma in F. drupacea, vessel ray pits with much reduced border to apparently simple with vertical pits in F. rumphii, scalariform pits and laticifers in rays of F. virens, both distinct and indistinct growth rings, presence of septate and gelatinous fibres in F. microcarpa and absence of laticifers among fibres and rays in F. elmeri and F. nervosa are the diagnostic features for identification among species. Also, the selected species showed significant variation in most of the anatomical characteristics. Both qualitative and quantitative anatomical characteristics are used for preparation of identification key and are important to differentiate Ficus species.
References
Adeniyi IM, Adejoba OR, Alao OJ, Noah AS, Salaudeen GT (2013) Comparative anatomy of some Ficus species. Res Pl Sci 1(2):15–19
Berg CC (1977) The Castilleae, a tribe of the Moraceae, renamed and redefined due to the exclusion of the type genus Olmedia from the “Olmedieae.” Acta Bot Neerl 26:73–82
Berg CC, Corner EJH (2005) Moraceae-Ficus. Flora Malesiana Series I. 17 (2): 1–70
Berg CC (2001) Moreae, Artocarpeae, and Dorstenia (Moraceae) with introductions to the family and Ficus and with additions and corrections to Flora Neotropica Monograph 7. Flora Neotropica 1–346
Chaudhary LB, Sudhakar JV, Kumar A, Bajpai O, Tiwari R, Murthy GVS (2012) Synopsis of the genus Ficus L (Moraceae) in India. Taiwania 57(2):193–216
Clement WL, Weiblen GD (2009) Morphological evolution in the mulberry family (Moraceae). Syst Bot 34(3):530–552
Cottee-Jones HEW, Bajpai O, Chaudhary LB, Whittaker RJ (2016) The importance of Ficus (Moraceae) trees for tropical forest restoration. Biotrop 48(3):413–419
Datwyler SL, Weiblen GD (2004) On the origin of the fig: phylogenetic relationships of Moraceae from ndhF sequences. Amer J Bot 91:767–777
Gamble JS (1922) A manual of Indian timbers. Bishen Singh and Mahendra Pal Singh publication, Dehradun
Harrison RD (2006) Mortality and recruitment of hemi-epiphytic figs in the canopy of a bornean rain forest. J Trop Eco 22:477–480
Indian Standard 11215 (1991) Moisture content of timber and timber products-Methods for determination. Bureau of Indian Standards New Delhi
Kajii C, Morita T, Kuroda K (2014) Laticifers of Ficus carica and their potential role in plant defence. IAWA J 35(2):109–115
Ludwig F, Middleton W, Gallenmüller F, Rogers P, Speck T (2019) Living bridges using aerial roots of Ficus elastica. Sci Rep 9:12226. https://doi.org/10.1038/s41598-019-48652-w
Noorman JK, Topper SMC, ter Welle BJH (1984) The systematic wood anatomy of the Moraceae (Urticales) III. Tribe Ficeae. IAWA Bull 5(4):330–334
Pande PK, Negi K, Singh M (2007) Wood anatomical variations in species of Shorea of Balau group of Malay Peninsula-A tool for identification. Ind For 133(6):759–777
Pande PK, Negi K, Singh M (2009) Variations in physical and wood anatomical properties of Shorea of Malay Peninsula. Ind For 135(2):209–226
Purkayastha SK (1996) A manual of Indian timbers. Sribhumi Publ, Calcutta
Raturi RD, Chauhan L, Gupta S, Rao RV (2001) Indian woods: Their identification, properties & uses. VI. Euphorbiaceae to Salicaceae. ICFRE, Dehradun
Sharma M, Sharma CL, Lalmalsawma M, Singh MK, Gogoi BR (2014) Wood anatomy of some Ficus species of Mizoram, NE India with reference to their identification. Int J Bot Res 4(2):19–30
Smith DM (1955) A comparison of two methods for determining the specific gravity of small samples of secondary growth, Douglas fir. U S For Prod Lab Rep No 2033: 21
Wheeler EA, Baas P, Gasson PE (1989) IAWA list of microscopic features for hardwood identification. IAWA Bull 10(3):219–332
Yaman B (2014) Anatomical differences between stem and branch wood of F. carica L. subsp. carica. Mod Phytomorph 6:79–83
Zobel B, Talbert T (1984) Applied forest tree improvement. Wiley, New York
Acknowledgements
The authors are thankful to Director, NERIST for providing laboratory facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharma, M., Sharma, C.L., Marak, L.M. et al. Anatomical and physical characteristics of some Ficus species of Meghalaya, NE India. J Indian Acad Wood Sci 19, 67–78 (2022). https://doi.org/10.1007/s13196-022-00300-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13196-022-00300-z