Abstract
The hardwood species Eucalyptus citriodora (Myrtaceae) is used as a raw material in paper industry that has a substantial proportion of heartwood. In addition to libriform fibres and fibre tracheids, heartwood has vessels blocked with tyloses and sapwood has live ray and axial parenchyma cells filled with starch. To understand the negative correlation that exists between extractives content in heartwood and pulp yield, both heartwood and sapwood of different sizes were subjected to kraft cooking separately at 170 °C. The pulps after kraft cooking were chemically characterized and analyzed for various properties using FTIR data, Water retention value and Ash content value. For 150 µm size wood sample, it is found that heartwood pulp has almost fivefold more residual lignin and 2.5 folds less ash content compared to sapwood pulp. For 2000 µm size sample, the water retention value increases by threefold for pulp compared to wood, but within the pulp, sapwood pulp has slightly higher water retention value compared to heartwood pulp. The FTIR also confirms that heartwood pulp contains slightly more lignin than sapwood pulp. As the sample size decreases from 2000 to 150 µm, it results in decrease of residual lignin content. Hence this study will be helpful for optimization of the process parameters during kraft cooking and also to optimize the rotation cycle of trees i.e. least amount of heartwood in wood log as required by the paper industry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wood cell wall consists of a thin primary wall (P), the most external layer, and secondary wall layers called S1 layer, S2 layer, and S3 layer as shown in Fig. 1b. The S2 layer is the thickest among the layers and consists of up to 50 % partly crystalline cellulose micro fibrils wound in a spiral fashion around the stem axis and embedded in a matrix of hemicellulose and lignin (Adusumalli et al. 2010). The portions of the high-polymeric compounds such as cellulose, hemicellulose and lignin building up the cell wall accounts 90 %, whereas low molecular weight substances such as extractives and inorganic substances namely K, Ca, Mg, Si accounts remaining 10 % in tropical woods as a rough approximation. Extractives are organic in nature such as aromatic compounds, terpenes, aliphatic acids and alcohols (Meerts 2002; Fengel and Wegener 1989). Hardwoods with short rotation cycles (shorter fibres of 2 mm length, cell wall thickness of 3–4 µm) are used as a fibre source for paper and board manufacture (Fengel and Wegener 1989; Bamber 1987; Smook 1992).
Hardwoods have libriform fibres, fibre tracheids and conducting vessels. It also has parenchyma cells which are short compact cells with stubby ends. Hardwoods generally consist of 45 % cellulose, 30 % hemicellulose, 20 % lignin and 5 % extractives on a dry weight basis (Smook 1992). During pulping process, lignin is removed from compound middle lamella (CML) and partially from cell walls either by mechanical or chemical methods. Chemical pulping involves treatment of wood chips at an elevated temperature(~170 °C) in a solution of NaOH + Na2S (also called white liquor) until a certain degree of delignification. During this process, a small quantity of cellulose and hemicelluloses also gets degraded. Bleaching is performed on pulp fibres to remove the residual lignin present in the cell wall. Consequent to cooking, bleaching and drying, cell wall deformations such as wrinkles, micro compressions and twists are commonly observed in pulp fibres (Fig. 1b). Due to this the paper offers more flexibility than wood log. The degree of polymerization values (weight average) of cellulose also decreases from 3500 to 600–1500 when wood is transformed into Pulp (Smook 1992). Among several theories proposed to explain the chemistry of pulping reactions, cellulose crystal theory (Young 1994) suggested that the paracrystalline regions in native cellulose fibrils are transformed into amorphous regions during pulping, where the extent of transformation depends on cooking conditions. Fahlén and Salmén (2005) reported the formation of pores in the matrix material and enlargement of the cellulose fibril aggregates as a result of chemical pulping. Besides many changes in wood structure during chemical pulping, the three linkages between the propane side chains and the benzene rings are broken to free the cellulosic fibres (Smook 1992).
The cross section of wood has two concentric regions called heartwood and sapwood (Fig. 1a). Initially the wood cells produced by the cambium become the sapwood. Death of parenchyma cells and other controlled processes gradually transform the sapwood into heartwood, but retaining the sapwood width around 50 mm in case of Eucalyptus citriodora (Myrtaceae) plant. The sapwood is the outer region which constitutes the live parenchyma cells filled with starch grains does the storage and conduction of water and mineral salts through vessels having large lumen diameter and length in few centimetres (Fig. 2). In structural timber applications, sapwood is inferior to heartwood due to its pale colour and susceptibility to fungi/insects, but pulp mills require the wood species having only sapwood (Bamber 1987). Heartwood being the central part of the stem provides the structural support to the tree and consists of polyphenolics (tannins) (Fengel and Wegener 1989; Gominho and Pereira 2000; Gominho et al. 2001; Santiago et al. 2008; Wimmer et al. 2008) and mostly darker in color. During the process of pulping, the amount of heartwood contained is a factor which has a detrimental effect on the quality of pulp. In pulp and paper manufacturing, the presence of heartwood in the wood logs causes problems such as pitch formation, extractive deposition on equipment and pulp, higher chemical consumption and reduced pulp brightness (Kai 1991; Higgins 1984; Del Río et al. 1998). The specific effect of heartwood was studied in a very limited manner (Egas et al. 2002; Irvine et al. 1996) as most of the work was focused on the effect of heartwood in total delignification process rather than a separate investigation of heartwood and sapwood. Although there are a few studies which aim at understanding this correlation (Miranda et al. 2007; Mariani et al. 2005), it is emphasized more on quantifying the end results of the pulping reaction. There is still a lack of comprehensive information regarding the causes for different behavior of heartwood and sapwood under identical conditions of kraft cooking. This study is aimed to gain both quantitative and qualitative understanding of the differences between delignification of heartwood and sapwood immediately after cooking. Apart from a direct empirical evaluation of the lignin content before and after cooking in heartwood and sapwood, certain indirect experimental methods are used (FTIR analysis, water retention value and ash content) to conclude the effects of heartwood in kraft cooking.
Materials and methods
The raw material used in this study was obtained from a first-rotation E. citriodora (Myrtaceae) tree, 6–7 years of age, from a rain-fed eucalyptus clonal plantation grown under the supervision of Telangana Forest Development Corporation in Jawaharnagar, Hyderabad, India. The log selected was 8 m length and 10 cm in diameter. Around 60 % of the inner cross sectional area at 4 m of height from the root side was considered as heartwood (one such cross-section just above the root is shown in Fig. 1a). A 50 cm long section was taken at this height and sapwood and heartwood were separated using a wood turning machine. The sapwood and heartwood were sieved and distributed into 6 sizes ranging from 2000 to 150 microns as shown in Fig. 3. The sieved heartwood and sapwood samples were used instead of chips in order to reduce impregnation difficulties that lead to experimental errors. Since kraft process can tolerate high amount of extractives and for better accuracy in ash content measurement, all samples were used without removing extractives.
Kraft cooking (delignification)
Delignification is carried out in a high pressure reactor (Anton Parr Model 4848). The reaction mixture of white liquor and wood samples were loaded in 1000 ml SS reactor with temperature control and stirrer rotation at 160 rpm. The conditions for pulping of 5 g of oven dry weight wood: liquor-to-wood ratio (mL/g) 4:1; sulfidity 30 % (both Na2S and NaOH expressed as Na2O); active alkali 20 % (as Na2O). Based on the optimal ratio, 18.5 ml of the white liquor i.e. solution of water + NaOH + Na2S was used for delignification. The pulping was carried out under isothermal conditions in two stages at 130 °C for 30 min and at 170 °C for 90 min, with heating time of 10 min for both the temperatures. The delignified samples were separated from the residual liquor using a funnel and a Whatman filter paper. The pulp was dried in a hot air oven at a temperature of 104.5 °C until a constant weight was observed and stored for further analysis. It should be noted that both heart and sapwoods were delignified under same cooking conditions.
Analysis of pulp
Raw heartwood and sapwood were analyzed before delignification and later milled and sieved fractions were analyzed after the kraft pulping process. Ash content for both the raw wood and kraft pulp fractions was determined by incinerating the samples at 525 °C until constant weight was attained in a muffle furnace (TAPPI T211). For incineration experiment, known oven dried weights of the samples were kept in a pre-weighed silica crucible for a period of 2–3 h
The samples were analyzed for their water retention values (WRV) using the centrifuge by placing the pulp slurry in plastic vials. Both the heartwood and sapwood samples of the six sizes were analyzed for their WRV before and after delignification. A refrigerated centrifuge was used for this purpose. The wet samples were centrifuged at 2600 rpm, 23 °C for 15 min, followed by overnight drying in a hot air oven at 104.5 °C and WRV is measured using Eq. 1.
The lignin content of both the raw wood and delignified heartwood and sapwood pulp samples was estimated using the Klason’s lignin protocol (TAPPI T222 and TAPPI UM250). 0.25 g of oven dry sample was taken and ground to a uniform size. The weighed sample was added to 5 ml of 72 % H2SO4 in a 250 ml conical flask and kept at 20 °C for 2 h with intermediate stirring. After 2 h, 192.5 ml water was added to the conical flasks and the flasks were autoclaved at 120 °C for 1 h. After 1 h, filtration was carried out for the autoclaved samples to separate the solids from the liquid. The separated solids were dried overnight and weighed to give the Klason lignin content. The liquid was used to assess the amount of acid soluble lignin using UV Spectrophotometer. The optical density at 203 nm was recorded. Absorptivity coefficient of 110 g l−1 was used to calculate the amount of acid soluble lignin. The total lignin content was recorded as the sum of Klason lignin and acid soluble lignin as shown in Table 1.
All the raw and delignified samples were analyzed using Fourier Transform Infrared (FTIR) spectroscopy in order to detect the various functional groups especially aromatic skeleton vibrations present in them. In order to get the spectral data, samples were dried, ground to fine size and mixed with potassium bromide (KBr) in approx. ratio of 1:100 and compressed to form the pellets. To understand the structural and morphological differences, both sapwood and heart wood were subjected to partial cooking to observe the longitudinal sections using scanning electron microscopy (after gold sputtering).
Results and discussion
Adding NaOH into the wood cells enables the cleavage of the β-O-4 structural linkages of lignin and the cleavage of ether and ester bonds between lignin and hemicellulose. As 10 % of alkali is consumed in initial period of cooking for the neutralization of acids deriving from the polysaccharides (acidic and uronic acids) and 25–30 % of the alkali is consumed for neutralising lignin degradation products. By maintaining the reasonable sulfidity levels (30 %) and varying the size of wood for both heartwood and sapwood, the aim of this study is to understand the process of delignification as it occurs in heartwood and sapwood separately. More specifically, the aim is to observe a possible amplification or a clearer pronouncement of the differences in delignification of heartwood and sapwood of a particular species given that the delignification was carried out at exactly the same reaction conditions for both. In line with the hypothesis, such differences were more clearly observed for a smaller size of raw wood, owing to improved chemical impregnation and availability of a larger surface area for the delignification reaction to occur. This difference was observed in all the results of experimental procedures employed in the study i.e. residual lignin content, water retention value, ash content and FTIR spectrophotometry.
It was found by Klasson’s lignin estimation that the amount of total lignin present in heartwood (25.4 %) and sapwood (26.8 %) is almost equal as shown in Table 1. For the same kraft cooking conditions, the amount of residual lignin decrease with size in both heartwood and sapwood as shown in Table 2. This is an indication of better delignification due to higher surface area leading to better chemical impregnation through the CML (cell interface) and cell wall. When the residual lignin was estimated in the delignified samples, it was clearly seen that the amount of residual lignin was overall higher for heartwood (2.4–3.8 %) when compared with sapwood (0.5–1.5 %) indicating better delignification in sapwood than in heartwood across all sizes. This can be due to the blockage of vessels with tyloses in heartwood, because vessels with large lumen are known to improve the diffusion of the white liquor to cell interfaces. It can also be due to the ray parenchyma cells present in the heartwood which may have much amount of phenolic extractives. Tyloses are ballon like structures formed due to the growth of adjacent parenchyma cells through the pit (Bamber 1987). Sapwood contains more amounts of mono- and disaccharides along with polysaccharides and almost free from tyloses and phenolic extractives. In normal process, residual lignin is removed in series of bleaching stages because lignin is strongly interlinked with cellulose fibrils as a continuous phase in the cell wall resembling unidirectional composite. It is also assumed that bonding is relatively stronger between cellulose fibrils and continuous phase of lignin (matrix) in heartwood compared to sapwood, thereby decreasing the delignification rate of heartwood.
The results obtained from the ash test for heartwood and sapwood before and after delignification are shown in Table 3. The percentage of ash content is much higher in sapwood pulp than in heartwood pulp irrespective of size. This is consistent with earlier studies for similar species (Mariani et al. 2005; Hillis 1987). Since heartwood contains less living matter than sapwood, the ash content i.e. an indication of the amount of inorganic substances is expected to be higher for sapwood than for heartwood. During the conversion of sapwood into heart wood, extensive translocation of chemical compounds occur, while sugars and macronutrients (N, P, K, Ca, Mg) are removed from senescing sapwood rings (Meerts 2002). Surprisingly trend remains same for wood and pulp, indicating that inorganic ash substances are heavily accumulated in cell walls. For cotton stalk the ash content was reported as 1.5 % (Meng et al. 2012) which is almost threefold higher than the value reported for sapwood in this study. This could be due to the pith, negligible heartwood content and large amount of NPK fertilizers applied during the growth of the cotton.
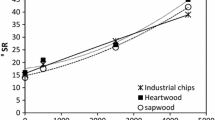
Lignin is hydrophobic in nature. The WRV of the samples was measured to know the approximate amount of the lignin present in the pulp. If the removal of lignin is more, WRV should be higher. Since the amount of lignin is similar in heartwood and sapwood, the WRV of the raw heartwood and sapwood are same across all sizes as shown in Table 4. However, it observed that though the WRV increases for both heartwood and sapwood pulps due to the removal of lignin, the increase in the values for sapwood is higher than heartwood due to the differences in their residual lignin content of the pulp as shown in Table 2. In comparison to raw wood, the increase in WRV is much higher for sapwood (0.84–3.06) than heartwood (0.85–2.33) in the case of 150 µm size particles. It indicates that the differences observed in Tables 2, 3 and 4, could be a direct or indirect reason for the relative difficulty in the delignification of heartwood portion of the log. Similar results were reported in literature (Lourenço et al. 2008, 2010).
In Figs. 4, 5, FTIR spectra of delignified pulp and in Fig. 6, spectra of raw wood are illustrated. The absorption peak at ~3400 cm−1 due to the OH stretching and peak at ~2920 cm−1 due to the C–H stretching in methyl and methylene group of lignin and extractives were observed in all FTIR spectra’s (not shown). The absorption peak at ~2360 cm−1 corresponds to the lignin is observed in 2000 µm pulp and raw wood samples, but no such peaks are observed in 425 µm pulp samples indicating that size reduction has improved the delignification process. In the fingerprint region of 900–1800 cm−1 many absorption bands related to various functional groups of lignin are observed. The band around 1593 and 1420 cm−1 representative of aromatic skeletal vibrations is present in all FTIR spectra’s. The bands for aromatic skeleton vibrations assigned at 1508 cm−1 are present in all spectra’s except for sapwood of size 425 µm which have the 0.9 % residual lignin.
FTIR Spectrum of the lignin in raw wood samples of size 425 μm. Peaks in the region 900–1800 cm−1 are characteristic of lignin and 1507 cm−1 indicates the occurrence of aromatic structures in the lignin and peak at 1735 cm−1 characteristic of carbonyl stretching of unconjugated ketone and carboxyl groups
The band around 1460 cm−1 representative of C–H deformations (asymmetric) is absent again in sample size of 425 µm (for both heartwood and sapwood). The band around 1339 and 1234 cm−1 representative of syringyl ring breathing with CO stretching is also absent in sample size of 425 µm (for both heartwood and sapwood pulp). This confirms the fact that the size reduction improves the delignifaction capacity of the wood, because lignin is removed not only from CML but also from the cell wall. The band around 1160, 1114 and 1055 cm−1 representative aromatic C–H in-plane deformation is present in all speactra’s shown in Figs. 4, 5 and 6. The band around 890 cm−1 representative of aromatic C–H out-of-plane deformation is present in all FTIR spectra’s, but intensity is low for sapwood pulp indicating the easier removal of lignin in smaller size (425 µm) sapwood samples. The presence of the peak at 1735 cm−1 in the FTIR spectra of raw wood (Fig. 6) could be due to the carbonyl (C=O) stretching vibration of the carboxyl groups of hemicellulose and lignin. Such peak is not observed in FTIR spectra of pulp samples shown in Figs. 4 and 5 except as a low intense peak for 2000 µm heartwood pulp. This confirms the fact that wood has more residual lignin than pulp, but cooking after size reduction can decrease the residual lignin content as shown in Table 2.
Conclusion
Most of the earlier research works were focused on the negative effect of heartwood on delignification of the total wood but not on heartwood in particular. From the experimental analysis, it can be concluded that though the amount of lignin is almost the same in heartwood and sapwood for the given species, it is difficult to remove lignin from heartwood than from sapwood. This could be due the blockage of vessels and accumulation of secondary compounds in heartwood. Hence pulp mills reject the wood logs having heartwood even in smaller quantities. This study concludes the relative difficulty of delignification of heartwood not only by using established experimental procedures of wood analysis but also using other non-standard experiments such as the water retention value test and ash content assessment. These results are in line with the results of the residual lignin estimation. To accept the wood logs with less quantity of heartwood, one strategic approach could be directed at nullifying or mitigating the negative effects caused by heartwood content after the reaction. This in effect is what is currently practiced in the industry and could be improved upon further.
References
Adusumalli RB, Raghavan R, Ghisleni R, Zimmermann T, Michler J (2010) Deformation and failure mechanism of secondary cell wall in Spruce late wood. Appl Phys A Mater Sci Process 100(2):447–452
Bamber RK (1987) Sapwood and heartwood, vol 2. Forestry Commission of New South Wales, Wood Technology and Forest Research Division, Beecrolt, pp 1–7. ISBN: 0724020675
Del Río JC, Gutiérrez A, González-Vila F, Martín F, Romero J (1998) Characterization of organic deposits produced in the Kraft pulping of Eucalyptus globulus wood. J Chromatogr A 823:457–465
Egas APV, Simão JPF, Costa IMM, Francisco SCP, Castro JAAM (2002) Experimental methodology for heterogeneous studies in pulping of wood. Ind Engin Chem Res 41:2529–2534
Fahlén J, Salmén L (2005) Pore and matrix distribution in the fibre wall revealed by atomic force microscopy and image analysis. Biomacromolecules 6:433–438
Fengel D, Wegener G (1989) Wood: chemistry, ultrastucture, reactions. Walter de Gruyter & Co, Berlin
Gominho J, Pereira H (2000) Variability of heartwood content in plantation-grown Eucalyptus Globulus Labill. Wood Fiber Sci 32(2):189–195
Gominho J, Figueira J, Rodrigues JC, Pereira H (2001) Within-tree variation of heartwood extractives and wood density in the Eucalyptus hybrid Urograndis (Eucalyptus Grandis x E. Urophylla). Wood Fiber Sci 33(1):3–8
Higgins HG (1984) Pulp and paper. In: Hills WE, Brown AG (eds) Eucalyptus for wood production. CSIRO/Academic Press, Australia, pp 289–312
Hillis WE (1987) Heartwood and tree exudates. Springer-Verlag, Berlin
Irvine GM, Clark NB, Recuperos C (1996) Extended delignification of mature and plantation Eucalypt wood. Part I: the principles of extended delignification. Appita J 49(4):251–257
Kai Y (1991) In: Hon DNS, Shiraishi N (eds) Chemistry of extractives in wood and cellulosic chemistry. Marcel Dekker Inc., New York, pp 215–251
Lourenço A, Baptista I, Gominho J, Pereira H (2008) The influence of heartwood on the pulping properties of Acacia melanoxylon wood. J Wood Sci 54:464–469
Lourenço A, Gominho J, Pereira H (2010) Pulping and delignification of sapwood and heartwood from Eucalyptus globulus. J Pulp Paper Sci 36(3–4):85–90
Mariani S, Torres M, Fernandez A, Morales E (2005) Effects of Eucalyptus nitens heartwood in Kraft pulping. Tappi J 4(2):8–10
Meerts P (2002) Mineral nutrient concentrations in sapwood and heartwood: a literature review. Ann For Sci 59:713–722
Meng L, Kang S, Zhang X, Wu Y, Sun R (2012) Comparative characterization of lignins extracted from cotton stalk based on complete dissolution in différent systems. Ind Eng Chem Res 51:9858–9866
Miranda I, Gominho J, Lourenço A, Pereira H (2007) Heartwood, extractives and pulp yield of three Eucalyptus globulus clones grown in two sites. Appita J 60(6):485–488
Santiago AS, Neto CP, Vilela C (2008) Impact of effective alkali and sulfide profiling on Eucalyptus globulus kraft pulping. Selectivity of the impregnation phase and its effect on final pulping results. J Chem Technol Biotech 83:242–251
Smook GA (1992) Handbook for pulp & paper technologists. Angus Wilde publications Inc., Vancouver
Wimmer R, Downes G, Evans R, French J (2008) Effects of site on fibre, kraft pulp and handsheet properties of Eucalyptus globulus. Ann For Sci 65(6):602
Young RA (1994) Comparison of the properties of chemical cellulose pulps. Cellulose 1:107–130
Acknowledgments
We are thankful to Dr.Karthik Chetan, Mr. Appalanaidu, Mr. Raju, Mr. Appalareddy, Mr. Bipin Chakravarthy and Mr. Uday Kumar for their valuable suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Puntambekar, R., Pydimalla, M., Dinda, S. et al. Characterization of Eucalyptus heartwood and sapwood pulp after kraft cooking. J Indian Acad Wood Sci 13, 8–15 (2016). https://doi.org/10.1007/s13196-016-0159-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13196-016-0159-5