Abstract
Management of periacetabular metastatic bone disease (MBD) is challenging, specifically if associated with bone loss or fracture. The aim of this study was to evaluate the complications and outcomes after undergoing peri-acetabular reconstruction using an ‘ice-cream cone’ pedestal cup endoprostheses for the most severe cases of (impending) pathological acetabular fractures. Fifty cases with severe periacetabular disease were identified. Acetabular defects were classified using the Metastatic Acetabular Classification (MAC). Pre- and post-operative mobility was assessed using the Eastern Cooperative Oncology Group (ECOG) Performance Status. Pain levels were assessed using a verbal rating scale. Surgical complications and patient survival were analysed; the Prognostic Immune Nutritional Index (PINI) was applied retrospectively to survival. There were 32 females and 18 males with a median age of 65 (41–88). Median post-operative follow-up was 16 months (IQR 5.5–28.5 months). Thirty-nine had complete, and 11, impending pathological fractures. The observed five-year survival was 19%, with a median survival of 16 months (IQR 5.8–42.5 months). Significantly worse survival was observed with PINI scores < 3.0 (p = 0.003). Excluding three perioperative deaths, 13 complications occurred in 12 patients: Implant failure in six patients (four aseptic loosening, one dislocation and one infection). At the final follow-up, mobility and pain levels were improved in 85% and 100%, respectively. Reconstruction of significant pelvic MBD with the ‘ice-cream cone’ reduces pain and improves mobility. Whilst the mortality rate is high, it remains a reasonable option for bed-bound, immobile patients. We advocate the use of an ‘ice-cream cone’ prosthesis for selected patients balancing the reported risks with the observed benefits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone is the third most common site of metastasis (after lung and liver) for solid organ tumours [1,2,3]. Peri-acetabular tumours are common and the third most prevalent non-spinal site for metastatic bone disease (MBD) after the femur and humerus [4]. Peri-acetabular disease can cause severe pain, inability to bear weight and can progress to pathological fracture. Non-operative management options include analgesia, walking aids, bone-targeted therapies (bisphosphonates or denosumab), external beam radiation therapy and depending on the tumour histiotype, immuno-, chemo- or hormonal therapy [5]. However, in patients with treatment-resistant or large-volume disease (with impending or established acetabular fractures), surgery is indicated to preserve mobility and is often challenging. An individualised therapeutic strategy is necessary, taking into account the patient’s systemic disease, life expectancy, severity of symptoms and extent of the acetabular defect [5, 6].
Contemporary literature advocates surgery when life expectancy is estimated to exceed 3 to 6 months [6]. Predicting survival of those with advanced cancer is difficult and involves multiple variables needing assessment [7]; consequently, a number of prognostic scores, within the orthopaedic literature, have been developed with this intent (e.g. Baumber et al. & Stevenson et al.) [8, 9]. This is also common for other cancer types with Jung et al. developing the Prognostic Immune Nutritional Index (PINI) score in predicting survival in patients with colorectal carcinoma [10]. Kayikcioglu et al. further validated this score in those with metastatic disease [11].
Reconstructions of metastatic acetabular lesions and pathological fractures are complex, high-risk procedures, specifically in patients with terminal disease [12]. Surgical reconstruction of the acetabulum aims to reduce pain and restore immediate weight-bearing to preserve mobility, utilising a technique that will outlast the patient’s life expectancy [5]. Non-biological reconstructive techniques are preferred in patients with MBD; depending on the degree of bone destruction, surgical options include cementoplasty, cemented total hip arthroplasty, Harrington’s procedure [13,14,15], acetabular cage fixation [16] and acetabular endoprostheses [17,18,19,20]. Acetabular endoprosthetic replacement with a pedestal cup prosthesis has been described following primary bone tumour resections of the peri-acetabulum [18, 21,22,23] and also recently as an option in the treatment of traumatic osteoporotic acetabular fractures [24].
The aim of this study was to determine whether acetabular reconstruction using an ‘ice-cream cone’ pedestal cup endoprosthesis is an appropriate and effective surgical option in patients with severely destructive peri-acetabular MBD. Specifically, we wish to assess a predictive mortality score, complications and patient-specific outcomes (function and pain).
Patients and Methods
The medical records from a prospectively maintained departmental database were reviewed retrospectively after institution approval. The database was interrogated to identify all patients who had undergone surgery to insert a pedestal cup pelvic endoprosthesis. Patient records were included for analysis where the indication for surgery was metastatic bone disease and a coned hemi-pelvic prosthesis (Stanmore METS, Elstree, England) had been used for reconstruction. Patients with a primary bone tumour, a non-oncological diagnosis, previous ipsilateral hip surgery, other types of coned hemi-pelvic implant used, follow-up of less than 6 months (for patients still alive) and whose prosthesis was inserted prior to the introduction of electronic radiology were excluded from the analysis.
Clinical and radiological records of all patients meeting inclusion criteria were reviewed. Surgical complications were identified. Implant failure was defined as any patient requiring removal of the ice cream cone or femoral prosthesis or patients in whom revision surgery was felt to be warranted but who were medically unsuitable or declined revision surgery; dislocation was also counted as a failure. Pre- and post-operative mobility was assessed using the Eastern Cooperative Oncology Group (ECOG) Performance Status (Table 1) [25, 26].
Pre-operative axial imaging for each patient was reviewed by the authors (JM, CB, JS), and the acetabular defect was classified as per the Metastatic Acetabular Classification (MAC) (Table 2) [27]. The MAC involves evaluating four anatomical sections: the dome, the medial wall, the anterior column and the posterior column and deeming each as either sufficient or insufficient based on the presence of fracture, segmental defect or cavitary defect.
Pain was assessed using a verbal rating scale as described by the British Pain Society [28].
We retrospectively utilised the PINI developed by Jung et al. to assess survival. The score was calculated as follows: (albumin [g/L] × 0.09) − (monocytes [cells/µL] × 0.0007). The score was calculated for each patient using immediate pre-operative blood results; note, the required indices were routinely included in pre-operative blood samples. According to Jung et al., patients with a PINI ≥ 3.0 had better overall survival than those with a PINI of < 3 [10, 11].
Statistical analysis was conducted using R Studio (Boston, MA, USA). Patient survival was calculated from the date of surgery to the most recent follow-up or death. Implant survival was calculated from the date of surgery to the date of implant failure (in our cohort, implant failure was defined as implant loosening/migration, dislocation and infection indicating revision whether the patient underwent revision surgery or not). Kaplan–Meier method was applied for patient and implant survival and the log rank test compared survival variables.
Surgical Procedure
All patients were treated at a single tertiary orthopaedic oncology centre in the UK. All data was routinely recorded prospectively on the departmental database. Following receipt of a referral, patients were discussed the following working day at a diagnostic multidisciplinary team meeting of orthopaedic oncologists and musculoskeletal radiologists; oncological staging investigations (CT chest, abdomen and pelvis and whole-body scintigraphy) were reviewed. Only patients who were estimated, by the referring team, to have a prognosis greater than 3 months were deemed suitable for surgical management and underwent CT and MRI of the pelvis for surgical planning. The indication for pedestal cup reconstruction in all cases was MBD with severe periacetabular defects (MAC type 3 or 4) that were felt not suitable, for the other aforementioned medical or surgical management options, by the treating surgical team. Pre-operative embolization was performed to lesions traditionally deemed to be highly vascular, such as renal metastases. It should be noted that during the study period, approximately 2 Harrington rod reconstructions were performed annually for MAC 2/3 disease, but only pedestal cup reconstructions were used for MAC 4 disease apart from two hemipelvic reconstructions after en-bloc resection of solitary renal disease.
Surgery utilised the Kocher-Langenbeck approach in all patients [29]. Releasing the gluteus maximus tendon allowed anterior translation of the femur to optimise stem placement of the pedestal cup in all cases. Computer navigation-assisted surgery was used in 15 patients (Stryker Orthomap 3D Navigation System II; Stryker, Kalamazoo, MI). The acetabular prosthesis used in all 50 cases was a coned hemi-pelvic prosthesis (Stanmore METS, Elstree, England).
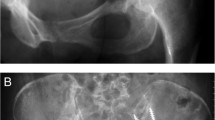
Intralesional placement of the prosthesis after curettage of the tumour was performed, and all tissue was submitted for histological analysis. After curettage or resection of the tumour and hand reaming towards the posterior superior iliac spine, the stem of the prosthesis was positioned in the posterior ilium avoiding the sacroiliac joint. Once the uncemented stem has been impacted, the lesional cavity was filled surrounding the prosthesis with antibiotic-laden bone cement, (Palacos-G, Biomet, Swindon, UK) with added vancomycin (1 g per mix), to reduce rates of infection, as described by Fisher et al. [21]. The femur was then prepared in the standard fashion using a variety of femoral components. In 43 patients, a polished, tapered cemented stem was used (Exeter, Stryker, UK) (Fig. 1). One patient received an uncemented femoral prostheses (Corail, Depuy Synthes), and six patients had the procedure combined with a proximal femoral replacement (Stanmore METS, Elstree, England) due to concurrent femoral metastatic disease. A cemented dual-mobility acetabular articulation was used in all patients.
The post-operative physiotherapy protocol was the same for all patients, and comprised of bed rest until the following day, partial weightbearing for 6 weeks and routine hip replacement precautions regarding the avoidance of deep flexion. All patients received antibiotic prophylaxis as for a primary total hip replacement in line with local antimicrobial policies and mechanical and chemical venous thromboembolism prophylaxis in keeping with local and national policy. Patients were routinely reviewed at 6 weeks, 3, 6 and 12 months post-operatively if well enough to attend clinic.
Results
Fifty eligible cases were retrospectively identified from a prospectively collated institutional database with greater than 56,000 patients registered since 1979. We identified 32 females and 18 males with a mean age of 65 years (range 41–88 years), who met the inclusion criteria. Patient demographics are presented in Table 3. Median follow-up was 16 months (IQR 5.5–28.5 months). The most common primary histotypes were breast (n = 15) and renal cell (n = 10) carcinoma as described in Table 3; 23 patients had multiple osseous metastases at the time of referral. All patients had a MAC of 3 or 4 with 39 patients having a confirmed fracture with 11 impending pathological fractures.
Survival
Three patients (6%) died on the day of surgery, and at final follow-up, nine patients (18%) remain alive. Patient survival is demonstrated in Fig. 2a (and Table 1s available in the supplementary material). Median survival was 16 months (IQR 5.8 to 42.5 months). Estimated survival after 2 years was 37.4% (95% CI, 26.1 to 53.5%) and after 5 years was 19.0% (95% CI, 9.9 to 36.1%).
a Overall survival with 95% confidence intervals. Estimated survival after 2 years was 37.4% (95% CI, 26.1 to 53.5% and after 5 years was 19.0% (95% CI, 9.9 to 36.1%). b Kaplan–Meier survival analysis comparing patients with a PINI > 3 or < 3; showing significantly worse survival was observed with PINI scores < 3.0 (p = 0.003)
When retrospectively applying the PINI score using a cutoff of < 3 or ≥ 3 (as previously described by Jung et al.) [10], we found there was a statiscally significant difference in overall survival (p = 0.0034). This is comparable to the published literature of Jung et al. and Kayikcioglu et al. [10, 11]. This shows that the PINI score may be able to predict survival outcomes in patients with osseous metastatic disease. Although more research on this subject would be required (see Fig. 2b).
Complications
Three patients suffered likely intraoperative cardiac events leading to perioperative mortality on the day of surgery. A further 13 complications were documented in 11 patients. Five (10%) patients returned to theatre (one patient on two occasions). A summary of complications and return to theatre can be found in Table 4.
At the time of writing, we determined that 7 (14%) patients had implant failure (four aseptic loosening/implant migration, two dislocations, one infection). One patient had implant migration and dislocation, and another patient had revision for aseptic loosening and subsequently developed a deep infection 2 years after revision surgery (the first date and mode of implant failure were used in statistical analysis for each). It should be noted that three patients classed as having implant failure did not require any surgical intervention (all three patients had asymptomatic implant migration and post-operative radiotherapy). Implant survival after 2 years is 85.4% (95% CI, 74.1 to 98.4%), and survival at 5 years is 57% (95% CI, 34.7 to 93.3%) (see Fig. 3).
One patient, who was noted to have implant migration after 6 months which was asymptomatic, also suffered a dislocation at 12 months post-operatively. The patient was comfortable, continued to mobilise with crutches and a shoe raise and was therefore managed conservatively. One further patient was noted to have a dislocation 2 months post-operatively, and this was managed with open reduction. According to our records, no further dislocation was documented until death approximately 8 years later.
Three patients were noted to have radiographic evidence of implant migration at 3, 4 and 9 months post-operatively, and all patients were asymptomatic and managed conservatively.
Two patients required revision surgery: the first for prosthetic joint infection diagnosed 3 years post-surgery secondary to Pseudomonas spp. (confirmed on deep samples). This patient was treated with a 1st stage revision with a view to second-stage reimplantation after eradication of infection. However, due to concurrent progression of metastatic disease, the patient remains on long-term antibiotic suppression. The second patient was revised for aseptic loosening 4 years post-operatively, however subsequently developed a deep infection and converted to a cement spacer with no further surgery planned.
Two further patients required return to theatre without revision surgery. One for wash out of a superficial haematoma 48 h post operatively. The other was for re-attachment of the greater trochanter (GT), which had avulsed from the proximal femoral replacement, 8 weeks post-operatively.
Functional Outcome and Pain Scores
ECOG scores were assessed according to the scoring system demonstrated in Table 1. Pre- and post-operative data was available for 41 patients; an improvement was noted in 35 (85%) patients with the remaining patients showing no change. Of note 30 (73%), patients were at best, wheelchair-bound pre-operatively. No patients had a lower ECOG score post-operatively (Fig. 4a). Pain was assessed as severe, moderate or mild [28]. Pre- and post-operative data was available for 36 patients. Pre-operatively, all patients had either severe or moderate pain. All patient’s pain improved post-operatively, with no patient suffering severe pain after surgery (Fig. 4b).
Discussion
Patients with acetabular metastatic bone disease present a significant therapeutic challenge. Surgical treatment is technically challenging and a significant undertaking, particularly in the context of disseminated disease, compromised physiological reserve, limited prognosis and often on the background of previous irradiation of the surgical site. Risks must be balanced with the potential benefits of pain reduction, improved independence and increased quality of life. Advances in supportive care and systemic therapies (such as hormone therapy, denosumab and chemotherapy) have achieved an increased life expectancy for patients with MBD, and in the majority of cases, therapy aligns with chronic disease management principles [30].
There are several surgical techniques available for the treatment and reconstruction of acetabular tumours; biological techniques or allograft-prosthesis composites are infrequently used in the context of metastatic disease due to the time to union, extended length of rehabilitation and risk of failure with adjuvant radiotherapy. Non-biological options are therefore preferred, with options ranging from cementoplasty to hemipelvis endoprostheses, depending on the extent of the disease. Non-biological fixation allows patients to achieve function and pain relief quicker in view of their often-guarded prognosis.
The commonest cause of implant failure in our cohort was asymptomatic implant subsidence, this occurred in three patients, all in the context of post-operative adjuvant radiotherapy. More than half of our patients (31/50) received radiotherapy either pre- or post-operatively or both. Whether radiotherapy has an influence on implant survival in patients with acetabular MBD is unclear from this study but worthy of further research.
The use of pelvic endoprostheses in MBD was explored by Hipfl et al. who reported results from 21 similar patients having used a titanium pedestal cup and reported an overall complication rate of 19%. The revision rate in their cohort was 14% [31]. Their cohort had a similar mean patient age (63 years) and tumour histiotypes; however, a direct comparison is difficult as the pelvic defect severity and use of adjuvant treatments were unclassified and unreported.
Stihsen et al. cautioned against the use of pedestal cups, particularly in patients with pelvic discontinuity, recognizing high rates of revision for aseptic loosening (17%) and infection (11%) in a cohort of 35 patients undergoing revision surgery for failed arthroplasty [32]. Consequently, the authors recommended the use of anti-protrusion cages combined with posterior column plating. However, elective revision arthroplasty represents a physiologically fitter, functionally more demanding, and disparate population than patients with MBD. The durability of an acetabular cage reconstruction in patients with metastatic disease was explored by Rowell et al., who reported good functional results in 50 patients with an overall major complication rate of 18% and a re-operation rate of 16% after 4 years [16]. The authors conceded, however, that the cup-cage technique may not be adequate in cases with extensive proximal ilium bone loss or where the ischium is deficient, as it is in the cases of severe bone loss (MAC grade 3 or 4) for which pedestal cup reconstruction is indicated in our institution.
To our knowledge, this is the largest cohort of patients treated with an ‘ice-cream cone’ pedestal cup endoprosthesis for acetabular metastatic bone disease. Acknowledging the limitations of the single-centre retrospective study design, our results show that surgical treatment of peri-acetabular metastases achieves pain control and improved mobility, the principal goals in this palliative group [5]. Our overall length of follow-up has been limited due to the prognosis of patients with MBD. Our survival rate is comparable with patients with severe MBD [33]. There is a significant risk of surgical complication; however, our observed overall complication rate is comparable to previous case series of surgery in the context of acetabular malignancy. Reported overall complication rates amongst patients treated with the Harrington procedure [14, 15, 34] and by pedestal cup reconstruction [31].
Predicting the survival of those with advanced cancer is difficult with a number of factors needing to be taken into account, including medical assessment, symptoms, performance status and lab values [7]. Jung et al. reviewed and assessed the accuracy of a number of different predictive scoring parameters and developed a novel prognostic index for patients with metastatic colon cancer [10]; the PINI was found to have a better predictive performance than the other assessed scores [10]. The PINI score has further been assessed by Kayikcioglu et al. and was again discovered to be an independent prognostic factor in metastatic colorectal cancer [11].
A predictive score to aid clinical judgement when making management decisions is invaluable when making shared decisions about major pelvic surgery in this context. As a team, we wanted this to be based on easily accessible routine data. After a review of published scoring systems, we chose to assess the PINI score as this involved two serological parameters which were routinely included in our pre-operative blood samples. PINI was calculated as follows: (albumin [g/L] × 0.09) − (monocytes [cells/µL] × 0.0007). According to Jung et al., patients with a PINI ≥ 3.0 had better overall survival than those with a PINI of < 3 [10, 11]. We retrospectively assessed the PINI score for all patients in our cohort, regardless of cancer subtype, to assess if this novel index could aid prediction of survival. For our patient population, the result was found to be significant.
Although this score was created for patients with metastatic colorectal cancer, the authors felt using an albumin-based nutritional score for the included patient population, who often present as frail and co-morbid, was appropriate. As the score is easily calculated from routine results, it will potentially aid future decision making.
Conclusion
Treating patients with severe acetabular defects secondary to metastatic disease requires an individualised approach. Reconstruction of impending or confirmed pathological acetabular fractures due to MBD with ‘ice-cream cone’ endoprostheses reduces pain and improves mobility in patients with significant destruction when other reconstruction options are not appropriate. While the mortality rate is high, it remains reasonable when compared to those with significant MBD [33]. Therefore, we would advocate the use of the ‘ice-cream cone’ for selected patients balancing the risk of mortality and complications with the observed benefits.
Data Availability
Anonymised data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Macedo F, Ladeira K, Pinho F, Saraiva N, Bonito N, Pinto L et al (2017) Bone metastases: an overview. Oncol Rev 11(1):321
D’Oronzo S, Coleman R, Brown J, Silvestris F (2018) Metastatic bone disease: pathogenesis and therapeutic options: up-date on bone metastasis management. J Bone Oncol 15(10):004
Coleman RE (2001) Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev 27(3):165–176
Ratasvuori M, Wedin R, Keller J, Nottrott M, Zaikova O, Bergh P et al (2013) Insight opinion to surgically treated metastatic bone disease: Scandinavian Sarcoma Group Skeletal Metastasis Registry report of 1195 operated skeletal metastasis. Surg Oncol 22(2):132–138
British Orthopaedic Oncology Society, British Orthopaedic Association (2015) Metastatic bone disease: a guide to good practice. Revision 1–59. (Last accessed on 08/03/2024) Available at https://baso.org.uk/media/61543/boos_mbd_2016_boa.pdf
Angelini A, Trovarelli G, Ruggieri P (2019) Metastases to the Pelvis: Algorithm of Treatment. In: Denaro V, Di Martino A, Piccioli A (eds) Management of Bone Metastases. Springer, Cham. https://doi.org/10.1007/978-3-319-73485-9_10
Krishnan M, Temel JS, Wright AA, Bernacki R, Selvaggi K, Balboni T (2013) Predicting life expectancy in patients with advanced incurable cancer: a review. J Support Oncol 11:68–74
Baumber R, Gerrand C, Cooper M, Aston W (2021) Development of a scoring system for survival following surgery for metastatic bone disease. Bone Joint J 103-B(11):1725–1730
Stevenson JD, McNair M, Cribb GL, Cool WP (2016) Prognostic factors for patients with skeletal metastases from carcinoma of the breast. Bone Joint J 98-B(2):266–270
Jung SH, Hao J, Shivakumar M et al (2022) Development and validation of a novel strong prognostic index for colon cancer through a robust combination of laboratory features for systemic inflammation: a prognostic immune nutritional index. Br J Cancer 126:1539–1547
Kayikcioglu E, Iscan G (2023) A novel prognostic index for metastatic colon cancer: the prognostic immune nutritional index. Cureus 15(1):e33808
Issack PS, Kotwal SY, Lane JM (2013) Management of metastatic bone disease of the acetabulum. J Am Acad Orthop Surg 21(11):685–695
Harrington KD (1981) The management of acetabular insufficiency secondary to metastatic malignant disease. J Bone Joint Surg Am 63(4):653–664
Tillman R, Tsuda Y, Puthiya Veettil M, Sree D, Fujiwara T, Abudu A et al (2019) The long-term outcomes of modified Harrington procedure using antegrade pins for periacetabular metastasis and haematological diseases. Bone Joint J 101–B(12):1557–62
Ho L, Ahlmann ER, Menendez LR (2010) Modified Harrington reconstruction for advanced periacetabular metastatic disease. J Surg Oncol 101(2):170–174
Rowell P, Lowe M, Sommerville S, Dickinson I (2019) Is an acetabular cage and cement fixation sufficiently durable for the treatment of destructive acetabular metastases? Clin Orthop Relat Res 477(6):1459–1465
Issa SP, Biau D, Babinet A, Dumaine V, Le Hanneur M, Anract P (2018) Pelvic reconstructions following peri-acetabular bone tumour resections using a cementless ice-cream cone prosthesis with dual mobility cup. Int Orthop 42(8):1987–1997
Aboulafia AJ, Buch R, Mathews J, Li W, Malawer MM (1995) Reconstruction using the saddle prosthesis following excision of primary and metastatic periacetabular tumors. Clin Orthop Relat Res 314:203–213
Kitagawa Y, Ek ET, Choong PF (2006) Pelvic reconstruction using saddle prosthesis following limb salvage operation for periacetabular tumour. J Orthop Surg 14(2):155–162
Menendez LR, Ahlmann ER, Falkinstein Y, Allison DC (2009) Periacetabular reconstruction with a new endoprosthesis. Clin Orthop Relat Res 467(11):2831–2837
Fisher NE, Patton JT, Grimer RJ, Porter D, Jeys L, Tillman RM et al (2011) Ice-cream cone reconstruction of the pelvis: a new type of pelvic replacement. J Bone Joint Surg Br 93–B(5):684–8
Bus MPA, Szafranski A, Sellevold S, Goryn T, Jutte PC, Bramer JAM et al (2017) LUMiC ® endoprosthetic reconstruction after periacetabular tumor resection: short-term results. Clin Orthop Relat Res 475(3):686–695
Jaiswal PK, Aston WJS, Grimer RJ, Abudu A, Carter S, Blunn G et al (2008) Peri-acetabular resection and endoprosthetic reconstruction for tumours of the acetabulum. J Bone Jt Surg Br 90(9):1222–1227
McMahon SE, Diamond OJ, Cusick LA (2020) Coned hemipelvis reconstruction for osteoporotic acetabular fractures in frail elderly patients. Bone Joint J 102–B(2):155–61
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5(6):649–655
Azam F, Latif MF, Farooq A, Tirmazy SH, AlShahrani S, Bashir S, Bukhari N (2019) Performance status assessment by using ECOG (Eastern Cooperative Oncology Group) Score for cancer patients by oncology healthcare professionals. Case Rep Oncol 12(3):728–736
Healey JH, Lenard HB (2015) Pathological pelvic fractures and acetabular reconstruction in metastatic disease. In: Tile M, Helfet D, Kellam J, Vrahas M (eds) Fractures of the pelvis and acetabulum - principles and methods of management, 4th edn. Thieme, pp 835–848
Outcome measures. British Pain Society and The Faculty of Pain Medicine. (last accessed on 07/03/2024) Available at: https://www.britishpainsociety.org/media/resources/files/outcome_measures_FINAL.PDF
Hoppenfeld S, DeBoer P, Buckley R (2016) Surgical exposures in orthopaedics: the anatomic approach 5th edn. Wolters Kluwer. Lippincott, Williams & Wilkins, USA
Steinauer K, Huang DJ, Eppenberger-Castori S, Amann E, Güth U (2014) Bone metastases in breast cancer: frequency, metastatic pattern and non-systemic locoregional therapy. J Bone Oncol 3(2):54–60
Hipfl C, Stihsen C, Puchner SE, Kaider A, Dominkus M, Funovics PT et al (2017) Pelvic reconstruction following resection of malignant bone tumours using a stemmed acetabular pedestal cup. Bone Joint J 99B(6):841–848
Stihsen C, Hipfl C, Kubista B, Funovics PT, Dominkus M, Giurea A et al (2016) Review of the outcomes of complex acetabular reconstructions using a stemmed acetabular pedestal component. Bone Joint J 98–B(6):772–9
Zacharia B, Joy J, Subramaniam D et al (2021) Factors affecting life expectancy after bone metastasis in adults — results of a 5-year prospective study. Indian J Surg Oncol 12:759–769
Kask G, Nieminen J, van Iterson V, Naboistsikov M, Pakarinen T-K, Laitinen MK (n.d.) Modified Harrington’s procedure for periacetabular metastases in 89 cases: a reliable method for cancer patients with good functional outcome, especially with long
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
Ethical approval was not required.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Macdonald, J., Baird, C., Jeys, L. et al. Outcomes Following Pedestal Cup Reconstruction of (Impending) Pathological Fractures of the Acetabulum due to Metastatic Bone Disease. Indian J Surg Oncol 15, 428–436 (2024). https://doi.org/10.1007/s13193-024-01917-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13193-024-01917-x