Abstract
Low-grade serous carcinoma (LGSC) is a rare histologic subtype of ovarian cancer. We present detailed management of 15 cases of advanced LGSC from a tertiary cancer center of India. Fifteen cases of advanced LGSC who underwent cytoreductive surgery (CRS) were analyzed from a prospectively maintained database. Baseline demographic characteristics, surgical details, and chemotherapy details were recorded. Descriptive statistics were summarized, and progression-free survival (PFS) and overall survival (OS) were estimated. The median age was 37 years. Nine patients had received NACT. All cases were FIGO stage III. Mean PCI was 15. Eleven patients had a completeness of cytoreduction score of 0–1. The median surgical time was 7.5 h; nine patients required multiple gastrointestinal resections. Median blood loss was 2500 ml. Median postoperative ventilation, ICU stay, and hospital stays were 1, 2, and 16 days, respectively. One patient had a grade III complication. Four patients received adjuvant chemotherapy. There was no postoperative mortality at the end of 90 days of surgery. All the patients except one were offered hormonal maintenance therapy. At a median follow-up of 43 months, 4 patients were disease-free, 9 had a recurrence, one died of disease progression, and one was lost to follow-up. Most recurrences were locoregional in the peritoneal cavity or pelvis. Four-year OS and PFS were 71.8% and 29.7%, respectively. Advanced LGSCs occur mostly in young premenopausal women with favorable oncologic outcomes. Optimal CRS is the mainstay of treatment. Relative chemo-resistance and hormone receptor positivity provide an excellent therapeutic opportunity for endocrine therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Low-grade serous carcinoma (LGSC) of the ovary comprises less than 15% of all serous carcinomas of the ovary. LGSC has distinct features such as younger age at presentation, good performance status, and overall better prognosis compared to high-grade serous carcinoma (HGSC) stage versus stage. Activation of the mitogen-activated protein kinase pathway (MAPK) plays a prominent role in the pathogenesis of LGSC, in contrast to a predominant P53-driven pathway in HGSC. LGSC does not seem to be a part of hereditary breast and ovarian cancer (HBOC) syndrome [1]. LGSC and HGSC have distinct biology and clinical outcomes which led to the binary grading system of serous carcinomas and have replaced the FIGO 3-tier grading system [2, 3].
Evidence is not robust on natural history of the disease with or without available treatment options. Even though surgery is the mainstay of treatment, the impact of disease burden and residual disease on oncological outcomes are derived from HGSC. There is heterogeneity of data with regard to response to chemotherapy and hormone therapy. Due to lower incidence of the disease, generating evidence from randomized controlled trials is not possible in the near future; hence, case series are important.
We present detailed management of 15 cases of advanced LGSC from a national tertiary cancer center with a literature review.
Methods
Fifteen cases of advanced LGSC (July 2016–March 2019) were analyzed from a prospectively maintained database. All patients underwent surgical debulking at the Centre. Histopathologic diagnosis of LGCS was based on morphology along with immunohistochemistry, and all cases were reported by specialty onco-pathologists. Baseline demographic characteristics such as age, performance status, along with clinical and radiology findings, tumor markers, and FIGO stage were recorded.
Surgical details (Table 2) included intraoperative findings and peritoneal carcinomatosis index (PCI) (5), type and duration of surgery, blood loss and transfusion of blood products, residual tumor, and completeness of cytoreduction (CC) score. The 30- and 90-day post-operative complications as per Clavien-Dindo classification and duration of hospital stay were recorded.
Details related to chemotherapy included the timing in relation to the surgery, chemotherapeutic agents offered along with the dose and duration, adverse reactions, and grade of toxicity were recorded. A standard regimen of paclitaxel and carboplatin was administered intravenously 3-weekly, and the dose and intervals were altered depending on the general condition and toxicity profile. All patients were advised for endocrine therapy after the completion of primary treatment. All the patients were followed up with 3 to 6 monthly intervals, as per the institutional protocol. Recurrences were identified based on clinico-radiological evaluation. When in doubt, tissue diagnosis was obtained. Depending on site, time to recurrence, and prior therapy, the recurrences were salvaged with surgery, chemotherapy, change of endocrine therapy, or a combination.
Statistical Analysis
Descriptive statistics were summarized using frequencies, percentages, medians, and ranges. Continuous data were presented as mean (SD) and medians with IQR. Progression-free survival (PFS) was defined as the duration between date of completion of treatment to date of first documented clinical or radiological or serological progression or death due to any cause whichever was earlier or date of last follow. Overall survival (OS) was calculated from completion of first line treatment till date of death due to any cause or date of last follow up. Kaplan–Meier method was used for the estimation of the probability of PFS, OS. All analyses were performed using R version 3.4.2, from the Comprehensive R Archive Network (R Core team, 2020).
Results
Baseline Characteristics
The baseline demographic characteristics are outlined in Table 1. The median age was 37 years (22–51). All patients had a good performance status (ECOG 0 or 1) with median preoperative albumin of 3.8 gm/dl (3.2–4.7). Eleven patients did not have any comorbidities (ASA 1), whereas four patients categorized as ASA 2 had comorbidities such as diabetes, hypertension, or hypothyroidism. Nine out of 15 patients had received neoadjuvant chemotherapy, with no or partial response before undergoing surgery. Six patients underwent upfront surgery. All cases were FIGO stage III. The median preoperative CA125 was 442 U/ml (15–7609). The surgical characteristics are depicted in Table 2. Mean PCI was 15 (3–25). Eleven patients had CC score 0–1, three had CC-2, and one had CC-3. The median surgical time was 7.5 h. Twelve patients underwent near-total peritonectomy, nine patients required multiple gastrointestinal (GI) resections, and three patients underwent stoma formation. A single patient underwent distal pancreatico-splenectomy. Two patients underwent fertility-preserving surgery with conservation of uterus at their request and after extensive counseling. Median blood loss was 2500 ml (200–6000). Median postoperative ventilation and median ICU stay were 1 and 2 days, respectively, and median hospital stay was 16 days. Fourteen patients had minor postoperative complications. A single patient developed Clavien-Dindo grade III complication (wound dehiscence). Four patients with prior partial response to chemotherapy received adjuvant chemotherapy. There was no postoperative mortality 30 days and 90 days of surgery. Immunohistochemically, estrogen receptor (ER) staining was positive in all 15 patients, ranging from 10 to 95%. Progesterone receptor (PR) immunostaining was positive in five out of thirteen patients in varying percentages. All the patients were offered hormonal maintenance therapy, except one, who was planning for conception immediately following surgery. Thirteen patients received tamoxifen and a single patient received letrozole.
Outcome
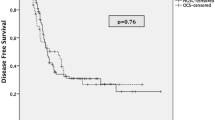
At a median follow-up of 43 months (12–61), 4 patients were disease free, 9 had recurrence, one died of disease progression, and one was lost to follow-up (Table 3). Most recurrences were locoregional in the peritoneal cavity or in pelvis as implants. The median PFS in our cohort of patients was 28 months (95% CI; lower bound not reached to upper bound 57 months), and median OS was not reached for calculation; all patients had more than 50% survival rate. At a median follow-up of 43 months, its robust to report 4-year overall survival rate was 71.8% (95% CI; 41.1% to 88.4%), whereas 4-year progression-free survival was 29.7% (95% CI; 7.89 to 56.0%).
Treatment at Relapse
The recurrent lesions were treated with surgical resection, wherever feasible or with chemotherapy and/or change of endocrine therapy. Three patients underwent secondary cytoreductive surgery; one patient underwent palliative surgery (stoma revision). CC-0 was achieved in 2 cases. Two patients are clinically controlled with the salvage treatment, four are alive-with-disease, and four patients died as a result of disease (Table 4) (Figs. 1 and 2).
Discussion
In current study, the median age at presentation was 37 years with a median albumin of 3.8 gm/dl and is similar to published studies [4]. Despite similar tumor burden in the abdominal cavity, advanced HGSC tend to have weight loss, cachexia, and lower performance status compared to advanced LGCS emphasizing the effects of tumor biology on cancer cachexia as a probable separate mechanism. Volume of ascites and median CA125 has been noted to be lower in LGCS compared to HGCS [4]. Median CA125 was 442 in our series. Calcified psammoma bodies are more common and numerous in advanced LGSC [5]. In the published randomized studies, a combination of ascitic fluid cytology and raised CA125 is sufficient to confirm a diagnosis of advanced serous ovarian cancers, prior to administering neoadjuvant chemotherapy [6]. However, interpretation of grade of the tumor is difficult with cytology alone [7] and has implications in the management of LGCS with neoadjuvant chemotherapy, given these tumors are relatively chemo-unresponsive. Histopathology, either a tissue biopsy or ascitic cell block remains the gold standard for accurate diagnosis as radiologically both present in advanced stage with similar tumor burden causing diagnostic dilemma [8]. Most serous carcinomas can be categorized as either low-grade or high-grade, based on morphological features. However, immunohistochemistry is helpful in cases where morphological distinction is challenging. LGSC shows a focal or patchy staining for P53, p16INK4A, variable immunostaining for WT 1; low Ki-67/MIB-1 proliferation index, in contrast to HGSC which shows a diffuse, intense staining, or complete loss of staining (null) for P53; and diffuse staining for p16INK4A, a relatively more diffuse WT 1 staining score, and a high Ki-67/MIB-1 proliferation index. A systematic review and meta-analysis showed higher ER/PR expression in LGSC versus HGSC ( 80.7%/54.4% vs 61.5%/30.7%, respectively) [9]. In current series, a MIB-1 index of < 20% was seen similar to reported in the literature (< 50%) [10], and also an expression of ER/PR was consistent with the available literature (100%/42.8%) [9].
Traditionally, principles of management of advanced LGSC have been similar to those of advanced HGSC. Only in recent years, specific features related to advanced LGCS such as diagnostic dilemmas, chemo-resistance, hormone responsiveness, and targeted therapies are being addressed. Standard guidelines include surgery, chemotherapy, and endocrine therapy in the management of LGCS. However, as a result of relative chemo resistance, surgery is the cornerstone of treatment even in advanced diseases with an excess tumor burden. Similar to HGSC, optimal debulking has been associated with improved survival outcomes. However, stage versus stage, with similar CC and surgical residual disease scores, outcomes are largely different for HGCS and LGCS [11, 12]. Patients with LGCS tend to have better DFS, PFS and OS, compared to HGSC with similar CC and residual disease scores and more in keeping with low-grade tumors occurring at other sites such as the GI tract. The extent of intraperitoneal disease depicted by PCI and CC scores has been studied in colorectal and gastric cancers and has been shown to be reliable tools to assess the disease. In the current series, we used the same tools to assess the intraabdominal disease (Table 2). During surgery, we also noticed that LGSC tend to be more fibrotic and densely adherent to the surrounding tissues, similar to surgery for endometriosis and in contrast to friable tumors observed in cases of HGSC. More than half of our patients underwent bowel resections as the tumor nodules were seen densely infiltrating the bowel serosa. We also observed that advanced LGCS was more confined to the peritoneal cavity (stage IIIC) and none had pleural disease despite extensive disease on diaphragmatic peritoneum and on Glisson’s capsule. Despite a higher disease burden requiring major bowel resections and long operating time, patients performed well during the postoperative period, compared to patients undergoing primary debulking surgery with advanced HGSC [6, 13].
The role of conservative surgery to preserve reproductive function or prescription of hormone replacement therapy following radical debulking in advanced LGSC is not clear. At present, empirical advice to patients is solely based on the knowledge that LGSC tend to have estrogen receptors, hence circulating endogenous or exogenous estrogens might have adverse prognosis. However, this aspect needs to be studied in future given the younger age at presentation of LGSC. In our series, we performed conservative surgery on one patient at patient request following extensive counseling. She is clinically controlled of disease with uterus in situ.
Prospective trials on NACT in advanced LGSC are lacking. In a retrospective analysis of an institutional database of 25 patients over more than 25 years, more than 50% of patients had a serological response, 4% had a radiological complete response (CR), 88% had stable disease (SD), and 8% had radiological disease progression(PD) [14]. In a further expansion of the prior work by the MD Anderson group analyzing 36 patients receiving NACT, 6 (11%) had a partial response (PR), 30 (83%) had SD, and 2/36 (6%) had PD. Although the gynecologic cancer intergroup (GCIG) consensus review does not opine NACT as a recommended approach in advanced LGSC, the authors in the MD Anderson group explained that the candidates who are not suitable for upfront surgery due to extensive tumor burden or medical comorbidities were feasibly treated with NACT. However, the OS was not dissimilar to the corresponding HGSC group and could be attributed to poor disease biology. In contradiction, however, in the review of literature, the LGSC patients successfully undergoing upfront CRS had better survival outcomes. Adjuvant postoperative chemotherapy is recommended in advanced LGSC (stages II–IV). Which of these tumors would benefit from chemotherapy is difficult to interpret, although it seems logical to administer adjuvant chemotherapy based on neoadjuvant chemotherapy response. Further, the physician choice of chemotherapy arm showed higher than predicted chemotherapy response in the MILO/ENGOT-ov11 trial comparing head-on with binimetinib. NCCN version 2.2020 recommends adjuvant chemotherapy after primary surgery with maintenance hormonal therapy in LGSC stages IC and above, although the recommended guidelines also suggest that hormone therapy can be used as an adjuvant. In IC tumors, no adjuvant chemotherapy is an option only for patients with complete surgical staging [15]. The European guidelines have similar recommendations for advanced LGSC (stages II–IV). Nine out of 15 patients in current series received NACT (prior to referral to our Centre or due to high tumor burden). Seven patients had stable disease, 1 had a partial response, and 1 had progressive disease (RECIST1.1). Four patients out of 15 received postoperative adjuvant chemotherapy. The remaining did not receive postoperative adjuvant chemotherapy, either due to prior suboptimal chemo-response, toxicity profile, or patient and physician choice following consultation. Table 5 depicts a summary of Clinical trials in LGSC.
Maintenance hormonal therapy (HT) is beneficial in stage II to IV LGSC. In a large single center retrospective and prospective study from the MD Anderson group, analyzing the outcomes of hormonal therapy, compared with routine observation after primary CRS and adjuvant platinum-based CT, in 203 patients, over 30 years, the median progression-free survival (PFS) of the HT group was significantly higher vs the observation (64.9 months vs 24.3 months), although OS was not statistically different. Further, in the subgroup analysis of patients who were disease-free or had persistent disease at the end of chemotherapy, the median PFS was significantly superior in the HT group (81.1 vs 30.0 months and 38.1 vs 15.2 months, respectively). Moreover, the HT group had a significantly lower risk of disease progression compared to the observation group. However, the patients who had received NACT were excluded [16]. Thus, it can be concluded that adjuvant hormonal maintenance should be considered after completion of primary CRS and adjuvant CT. A phase III randomized trial (NRG-GY-019) is currently comparing the role of adjuvant letrozole maintenance after upfront CRS alone vs letrozole maintenance after upfront CRS and 6 cycles adjuvant platinum-based doublet chemotherapy in stage II–IV advanced LGSC. In the above-quoted study, on analyzing the receptor immunoexpression, there were no significant differences in the median PFS and OS, while comparing ER positive/PR positive tumors with ER positive/PR negative tumors. However, considered isolated, the median PFS of ER positive or PR positive tumors undergoing maintenance HT was significantly higher than the observation group, although median OS was not statistically different. In current series, all patients except one (planning for immediate conception) were treated with adjuvant HT (Table 4). The choice and the magnitude of benefit of tamoxifen and letrozole when used as maintenance therapy on DFS is not clear. However, tamoxifen has been shown to reduce menopausal adverse side effects and improves quality of l life in young women. Based on this understanding, in the current series, we prescribed tamoxifen in majority of women and letrozole in recurrent setting.
Recurrent LGSC also appears to be relatively chemo-resistant. In a retrospective study of 58 patients receiving 108 separate chemotherapy regimens over a period of 17 years, overall response rate was only 3.7%, 4.9% for the platinum sensitive cohort, and 2.1% for the platinum-resistant cohort, the difference being non-significant [17]. A retrospective analysis of 41 patients by the MD Anderson group showed a significant PFS benefit in the no gross residual disease group versus those having the gross residual disease after secondary CRS (60.3 months vs 10.7 months, respectively) with 61% having complications but no death in the study. In the current series, 2 patients received chemotherapy at recurrence.
Endocrine therapies have moderate anti-tumor activity in patients with recurrent LGSC. There is a growing body of evidence of using endocrine therapy as maintenance, adjuvant, or recurrent setting [18]. A retrospective analysis by the same investigator revealed a clinical benefit in 71% of cases (9% overall response and 62% SD) in 64 patients receiving 89 separate patient hormone therapy regimens; the benefit approached but did not reach clinical significance between ER + /PR + tumors versus ER + /PR- tumors ( 8.8 months vs 6.2 months, respectively; p = 0.053 [19]. In current series, patients who recurred were prescribed letrozole, megestrol, anastrazole, and fulvestrant (see Table 4).
Secondary cytoreduction is beneficial in the management of recurrent tumors of ovary, In LGCS, the selection criteria for secondary cytoreduction is based on a combination of disease characteristics such as chemoresistance and hormone sensitivity. The dilemma whether the treatment needs to be started at radiological/serological evidence of recurrence versus symptomatic recurrence is not clear in LGSC. It has been observed that the disease tends to be stable without symptoms for longer period of time without intervention. In the current series, despite presence of disease in the abdomen, patients remained largely symptom free with good quality of life. In our series, 3 patients underwent secondary cytoreduction with CC-0 achieved in 2 cases (see Table 4).
Hyperthermic intraperitoneal chemotherapy (HIPEC) is an increasingly used modality in the treatment of peritoneal metastasis in gastrointestinal and ovarian malignancy, gastrointestinal tumors showing mixed results with regard to oncological outcomes and toxicity [20]. OVHIPEC trial [21] evaluated the addition of HIPEC in interval debulking setting in stage III ovarian cancer with significant PFS and OS benefit (31). However, the number of LGSC was underrepresented for meaningful conclusions in both CRS (2/123) and CRS + HIPEC arms (4/122). Encouraging results are obtained with low-grade appendiceal neoplasms, combing CRS with HIPEC, though results are debated [22]. We did not use HIPEC or IP chemotherapy in current series.
MEK inhibitors, cyclin-dependent kinase (CDK) 4/6 inhibitors, are currently under evaluation as molecular targeted therapies in recurrent or progressive settings. Bevacizumab alone or in combination has also been tried in recurrent settings. In a retrospective analysis of 40 patients receiving 45 separate bevacizumab containing patient regimens, clinical benefit (CR + PR + SD = 7.5% + 40% + 30%) was seen in 77.5% with median PFS of 10.2 months and median OS of 34.6 months; 15 patients discontinued bevacizumab due to toxicity [23].
Details of primary treatment and treatment on recurrence or progression of the disease are given in Table 4.
In the recent years, MEK inhibitors are the prime targeted therapy under investigation. The analysis of mutational profiles demonstrates predominant KRAS (17–40%) and BRAF (28–45%) mutations in LGSC which are upstream regulators of MAPK pathway. GOG239, a phase II trial investigating a MEK inhibitor selumetinib, revealed improved response rates and stable disease rates over chemotherapy or hormonal therapy in recurrent settings. Subsequently, a randomized phase III study (MILO/ENGOT-ov11 trial) evaluating binimetinib versus physician choice chemotherapy in recurrent settings did not meet its primary endpoint since the chemotherapy responses were higher than predicted [24]. However, another phase II–III trial (GOG 0281) evaluating trametinib versus physician’s choice standard of care chemotherapy showed significant PFS and objective response rates over chemotherapy [25]. Interestingly, a Japanese study showed PIK3CA/AKT is the main signaling pathway (60%) in contrast to the MAPK pathway in the European population; thus, there might be ethnic differences in biomarker expression. The PI3K inhibitor voxtalisib was evaluated in a phase II trial with MEK inhibitor pimasertib for recurrent LGSC without added benefit (EMR 20006–012). Metformin alone or in combination with MEK inhibitors might be beneficial by its antitumoral effects through AMP-activated protein kinase activation and PI3K-mTOR inhibition [26]. Cyclin-dependent kinase (CDK) inhibitor ribociclib (in conjunction with letrozole) is currently under investigation in a phase II trial for recurrent LGSC (NCT03673124). Abemaciclib, another CDK inhibitor plus fulvestrant, is under a pilot phase II study for patients with stage III–IV LGSC evaluating clinical benefit rate (NCT03531645). Table 6 depicts a summary of targeted therapies in LGSC. Analysis for mutational profiles for KRAS, NRAS, or BRAF was not performed in our series as it is not a part of routine biomarker analysis at our center and also MEK inhibitors are not available for routine clinical use outside trials. Germline BRCA mutations have rarely been identified in LGCS or borderline ovarian tumors.
In conclusion, advanced LGSC occurs mostly in premenopausal women and has better oncological outcomes, compared to advanced HGSCs. Optimal debulking surgery is the mainstay of treatment as LGCS is relatively chemo-resistant. ER/PR positivity provides an excellent therapeutic opportunity for endocrine therapy. However, the magnitude of the benefit of various chemotherapeutic agents and hormone therapy in LGSC needs to be studied further. MEK and CDK inhibitors are investigational in recurrent settings and biomarker analysis holds promise in guiding the therapy. Following our initial experience, we have started a phase II clinical trial of advanced LGCS treated with surgery and maintenance with letrozole without postoperative chemotherapy at our institution.
References
Vineyard MA et al (2011) Is low-grade serous ovarian cancer part of the tumor spectrum of hereditary breast and ovarian cancer? Gynecol Oncol 120(2):229–232. https://doi.org/10.1016/j.ygyno.2010.10.033
Malpica A et al (2004) Grading ovarian serous carcinoma using a two-tier system. Am J Surg Pathol 28(4):496–504. https://doi.org/10.1097/00000478-200404000-00009
McGee J et al (2017) Fifth Ovarian Cancer Consensus Conference: individualized therapy and patient factors. Ann Oncol 28(4):702–710. https://doi.org/10.1093/ANNONC/MDX010
Kaldawy A, Segev Y, Lavie O, Auslender R, Sopik V, Narod SA (2016) Low-grade serous ovarian cancer: a review. Gynecol Oncol 143(2):433–438. https://doi.org/10.1016/j.ygyno.2016.08.320
Amante S, Santos F, Cunha TM (2021) Low-grade serous epithelial ovarian cancer: a comprehensive review and update for radiologists. Insights Imaging 12(1):60. https://doi.org/10.1186/s13244-021-01004-7
Vergote I et al (2018) Neoadjuvant chemotherapy versus debulking surgery in advanced tubo-ovarian cancers: pooled analysis of individual patient data from the EORTC 55971 and CHORUS trials. Lancet Oncol 19(12):1680–1687. https://doi.org/10.1016/S1470-2045(18)30566-7
Sneige N, Thomison JB, Malpica A, Gong Y, Ensor J, Silva EG (2012) Peritoneal washing cytologic analysis of ovarian serous tumors of low malignant potential to detect peritoneal implants and predict clinical outcome. Cancer Cytopathol 120(4):238–244. https://doi.org/10.1002/CNCY.21219
Rekhi B et al (2020) Evaluation of cell blocks from effusion specimens in gynecologic oncopathology: an experience of 220 cases, diagnosed at a tertiary cancer referral center. Indian J Pathol Microbiol 63(3):427. https://doi.org/10.4103/IJPM.IJPM_858_19
Voutsadakis IA (2021) A systematic review and meta-analysis of hormone receptor expression in low-grade serous ovarian carcinoma. Eur J Obs Gynecol Reprod Biol 256:172–178
Sundov D et al (2013) P53, MAPK, topoisomerase II alpha and Ki67 immunohistochemical expression and KRAS/BRAF mutation in ovarian serous carcinomas. Diagn Pathol 8(1):1. https://doi.org/10.1186/1746-1596-8-21
Grabowski JP et al (2016) Operability and chemotherapy responsiveness in advanced low-grade serous ovarian cancer. An analysis of the AGO Study Group metadatabase. Gynecol Oncol 140(3):457–462. https://doi.org/10.1016/J.YGYNO.2016.01.022
Gourley C et al (2014) Gynecologic Cancer InterGroup (GCIG) consensus review for ovarian and primary peritoneal low-grade serous carcinomas. Int J Gynecol Cancer 24(9 Suppl 3):S9–S13. https://doi.org/10.1097/IGC.0000000000000257
Fagotti A et al (2020) Randomized trial of primary debulking surgery versus neoadjuvant chemotherapy for advanced epithelial ovarian cancer (SCORPION-NCT01461850). Int J Gynecol Cancer 30(11):1657–1664. https://doi.org/10.1136/IJGC-2020-001640
Schmeler KM et al (2008) Neoadjuvant chemotherapy for low-grade serous carcinoma of the ovary or peritoneum. Gynecol Oncol 108(3):510–514. https://doi.org/10.1016/J.YGYNO.2007.11.013
Colombo N et al (2019) ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann Oncol 30(5):672–705. https://doi.org/10.1093/annonc/mdz062
Gershenson DM (2016) Low-grade serous carcinoma of the ovary or peritoneum. Ann Oncol 27(Supplement 1):i45–i49. https://doi.org/10.1093/annonc/mdw085
Gershenson DM et al (2009) Recurrent low-grade serous ovarian carcinoma is relatively chemoresistant. Gynecol Oncol 114(1):48–52. https://doi.org/10.1016/J.YGYNO.2009.03.001
Gershenson DM, Bodurka DC, Coleman RL, Lu KH, Malpica A, Sun CC (2017) Hormonal maintenance therapy for women with low-grade serous cancer of the ovary or peritoneum. J Clin Oncol 35(10):1103–1111. https://doi.org/10.1200/JCO.2016.71.0632
Gershenson DM et al (2012) Hormonal therapy for recurrent low-grade serous carcinoma of the ovary or peritoneum. Gynecol Oncol 125(3):661–666. https://doi.org/10.1016/J.YGYNO.2012.02.037
Ceelen W, Demuytere J, de Hingh I (2021) Hyperthermic intraperitoneal chemotherapy: a critical review. Cancers 2021 13(13):3114. https://doi.org/10.3390/CANCERS13133114
van Driel WJ et al (2018) Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med 378(3):230–240. https://doi.org/10.1056/nejmoa1708618
Sugarbaker PH (2006) New standard of care for appendiceal epithelial neoplasms and pseudomyxoma peritonei syndrome? Lancet Oncol 7(1):69–76. https://doi.org/10.1016/S1470-2045(05)70539-8
Dalton HJ, Fleming ND, Sun CC, Bhosale P, Schmeler KM, Gershenson DM (2017) Activity of bevacizumab-containing regimens in recurrent low-grade serous ovarian or peritoneal cancer: a single institution experience. Gynecol Oncol 145(1):37–40. https://doi.org/10.1016/J.YGYNO.2017.01.027
Monk BJ et al (2020) MILO/ENGOT-ov11: Binimetinib versus physician’s choice chemotherapy in recurrent or persistent low-grade serous carcinomas of the ovary, fallopian tube, or primary peritoneum. J Clin Oncol 38(32):3753–3762. https://doi.org/10.1200/JCO.20.01164
Gershenson DM, Gourley C, Paul J (2020) ‘MEK inhibitors for the treatment of low-grade serous ovarian cancer: expanding therapeutic options for a rare ovarian cancer subtype’. 38(32):3731–3734. https://doi.org/10.1200/JCO.20.02190
Mert I et al (2017) Synergistic effect of MEK inhibitor and metformin combination in low grade serous ovarian cancer. Gynecol Oncol 146(2):319–326. https://doi.org/10.1016/J.YGYNO.2017.05.019
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dash, B., Shylasree, T.S., Rekhi, B. et al. Clinical Observations and Outcomes in Advanced Low-Grade Serous Carcinoma of the Ovary: Case Series from a Tertiary Cancer Center. Indian J Surg Oncol 14, 784–792 (2023). https://doi.org/10.1007/s13193-023-01775-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13193-023-01775-z