Abstract
We are reporting a rare case of primary gastric synovial sarcoma in a young male. Synovial sarcoma of the stomach is a very rare tumor. The common involved sites of occurrence of synovial sarcomas are upper and lower extremities. In the English literature, only 47 cases of primary synovial sarcoma of stomach have been reported. Spindle-shaped tumor cells are the basic content of synovial sarcomas with varying degrees of epithelial differentiation. The basic classification of synovial sarcoma depends on the histological pattern and the degree of differentiation and it is classified as monophasic, biphasic, and poorly differentiated. Synovial sarcoma presents with classical chromosomal translocation where they form fusion genes of SS18-SSX1, SS18-SSX2, and SS18-SSX4. Fluorescence in situ hybridization (FISH) and reverse transcription polymerase chain reaction (RT-PCR) are the molecular analysis techniques to detect these fusion genes. As the available literature support is limited, the role of adjuvant chemotherapy, radiation therapy, and intra-operative lymphadenectomy is still unclear. However, surgical resection with clear margin is the gold standard treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of synovial sarcoma among all soft tissue sarcomas is around 8–10% and it is considered a rare malignant tumor with origin from mesenchymal tissue [1]. The common sites of occurrence of synovial sarcoma are upper and lower extremities with rare occurrence in mediastinum, heart, liver, head, neck and gastrointestinal tract [2, 3]. Synovial sarcoma of the stomach is a very rare scenario and the commonest mistaken diagnosis is gastrointestinal stromal tumor (GIST). In the English literature, only 47 cases have been reported of primary synovial sarcoma of stomach [4,5,6,7]. Spindle-shaped tumor cells are the basic content of synovial sarcomas with varying degrees of epithelial differentiation. The basic classification of synovial sarcoma depends on the histological pattern and the degree of differentiation and it is classified as monophasic, biphasic, and poorly differentiated. Monophasic type contains a uniform proliferation of spindle cells without epithelial component, a biphasic type contains a mixture of distinct epithelial cells and spindle-shaped cells and a poorly differentiated type contains anaplastic spindle and/or round cells. The cell of origin of synovial sarcoma is still unclear; however, they have a unique chromosomal translocation t(X;18) (p11; q11). This translocation forms fusion genes of SS18-SSX1, SS18-SSX2, and SS18-SSX4. These fusion genes are detected with molecular analysis technique like fluorescence in situ hybridization (FISH) and reverse transcription polymerase chain reaction (RT-PCR). As gastric synovial sarcoma is a rare variety, there is a paucity of literature describing role of D2 lymphadenectomy and adjuvant chemotherapy. We are reporting a case of primary synovial sarcoma of stomach in a young male.
Case Report
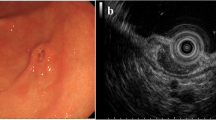
A 36-year-old gentleman with Eastern Cooperative Oncology Group Performance Status 1 (ECOG PS 1) with no comorbidity presented with two episodes of hematemesis and one episode of melena within a period of 1 month. We had advised him to undergo upper endoscopy. Upper gastrointestinal endoscopy defined an ulcero-proliferative growth involving distal part of body and antrum of stomach with complete circumferential involvement of the stomach. Scope was negotiated till duodenum 2nd part and biopsy of the lesion was sent for histopathological examination (HPE). HPE was suggestive of spindle cell tumor of stomach. For metastatic staging, we had advised him to undergo CECT (contrast enhanced computed tomography) of thorax, abdomen, and pelvis. CECT was suggestive of a growth limited to the distal part of body and antrum of stomach with no regional lymph node and distant metastasis. Case was discussed in our institutional multidisciplinary tumor board and board decided to plan for surgery. Patient was posted for surgery and intraoperative, a new finding was discovered where a tumor was found infiltrating into the mesentery of the transverse colon (Fig. 1). Thus, patient underwent radical subtotal gastrectomy with D2 lymphadenectomy with right hemicolectomy (Fig. 2). Oral liquid diet started after 72 h of surgery. Postoperative course was uneventful and he was discharged on 6th postoperative day.
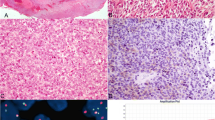
Final histopathological examination was crucial in this case as features of sarcomatoid differentiation were found in the tumor measuring 9 × 7.5 cm. The differential diagnosis was malignant GIST (gastrointestinal stromal tumor), leiomyosarcoma, and synovial sarcoma. Immunohistochemical (IHC) analysis was carried out to rule out all possibilities. Tumor cells were strongly and diffusely positive for vimentin, CD 99 and focally positive for EMA. Tumor cells were negative for caldesmon, CD 34, CD117, C KIT, DESMIN, and DOG 1. The diagnosis of synovial sarcoma was confirmed after molecular analysis. The translocation t(X;18) was detected on fluorescence in situ hybridization technique (FISH) (Fig. 3). Around 90% of synovial sarcoma possess a fusion between the SS18 gene on chromosome 18 and an SSX gene found on the X chromosome. Around 42 lymph nodes were retrieved and all were free from the disease. Hence, final histopathological examination was suggestive of primary synovial sarcoma of stomach with AJCC 8th edition staging—pT4a pN0 pM0. Patient had been advised adjuvant chemotherapy of doxorubicin monotherapy. However, patient and his relatives were not willing for adjuvant chemotherapy and they opted negative consent for the same. Patient had been advised regular institutional periodic follow-up protocol (3 monthly). PET CT scan had been advised at 6 months and 1 year of completion of treatment and there was no recurrent or residual disease. After 1 year of completion of treatment, he is alive and disease free. He had been advised long-term follow-up.
Discussion
The first case of primary gastric synovial sarcoma was reported in 2000 and then near about 47 cases reported in the English literature [8]. The typical endoscopic view of gastric synovial sarcoma is presentation of white submucosal plaque with central depression. GIST presents with similar features. But, in the index case, there was an ulcero-proliferative growth measuring 9 × 7.5 cm with location in the body and antrum. Histopathological examination demonstrates similar features of monophasic and biphasic spindle cell in GIST tumors as like in synovial sarcoma. However, GIST tumors shows C-Kit positivity on immunohistochemistry which is absent in gastric synovial sarcoma. The diagnostic test for gastric synovial sarcoma is chromosomal translocation with formation of the fusion products of the SS18 gene combined to either SSX1, SSX2, or SSX4 gene [9]. In the index case, fusion products with translocation genes were diagnosed on fluorescence in situ hybridization (FISH) technique. Out of all the reported cases, monophasic type accounts around 89% and biphasic type accounts around 8% and poorly differentiated type has been reported in only one case [10, 11].
As it is a rare disease, literature support for the treatment of gastric synovial sarcoma is lacking. Complete margin negative surgical resection of the tumor with lymphadenectomy is the standard treatment based on NCCN and ESMO guidelines [12, 13]. We did subtotal gastrectomy with D2 lymphadenectomy as intraoperative, enlarged perigastric lymph nodes were visible. Billings et al. was the first person who reported first two cases of primary gastric synovial sarcoma in 2000 [14] followed by Makhlouf et al. [15] who reported a series of 10 cases of gastric synovial sarcoma in 2008. The most common presentation of gastric synovial sarcoma is epigastric pain and anemia [16]. Our index case presented with upper abdominal pain and anorexia. IHC examination is the landmark test for diagnosis of gastric synovial sarcoma. In most of the cases of synovial sarcoma, TLE-1 is positive. However, it is not considered specific for it as it is positive in other tumors also like epithelioid sarcoma, solitary fibrous tumor, and endometrial stromal sarcoma. The confirmatory diagnostic test for gastric synovial sarcoma is detection of fusion product by fluorescence in situ hybridization (FISH) or polymerase chain reaction (PCR) [17, 18].
These tumors are commonly located in the fundus and body of stomach with median size around 5.46 cm. In the index case, tumor was located in the distal part of body of stomach and antrum with size around 9 × 7.5 cm. The reported 5-year survival of gastric synovial sarcoma is around 75% and 10-year survival rate is 34% [19]. The poor prognostic factor of synovial sarcoma is tumor diameter ≥ 5 cm, microscopically positive margins, 10 thread divisions or more at 10 high-power fields [20]. Ten-year survival rate is 100% if the size of the tumor < 5 cm [21]. The index case had a very large tumor. Krupinska et al. reported that patients with synovial tumors larger than 72 mm had a significantly lower probability of survival. The rate of distant metastasis of synovial sarcoma is around 50–70% as reported by Kering et al. Hence, long-term follow for >10 years is the recommendation [22]. The drug of choice for unresectable soft tissue sarcomas is doxorubicin monotherapy and it has been found that it has useful role as an adjuvant chemotherapy for resectable localized soft tissue sarcomas [23, 24]. The role of radiation therapy has been explained in adjuvant settings for local control of the tumor [24]. However, it is not yet a recommended option.
Major cases of gastric synovial sarcoma show monophasic type. The index case had also a monophasic pattern. As the standard treatment of gastric synovial sarcoma is surgical resection, we did complete oncological resection with clear margins. The literature is lacking in demonstrating the role of adjuvant chemotherapy as the total number of reported cases are very less. We had advised him adjuvant doxorubicin monotherapy as it was infiltrating into the mesentery of the transverse colon. However, the role of adjuvant chemotherapy is still not clear and our patient also refused the adjuvant treatment. Further research and studies over primary gastric synovial sarcoma are necessary to form a consensus treatment protocol.
Conclusion
Primary gastric synovial sarcoma is a very rare disease and the only diagnostic modality to confirm its diagnosis is immunohistochemical and molecular analysis with detection of chromosomal translocation. Surgical resection with clear margin stands as the primary therapeutic modality for gastric synovial sarcoma. Role of lymphadenectomy and neo-adjuvant / adjuvant chemotherapyis still under research.
References
Jo VY, Fletcher CD (2014) WHO classification of soft tissue tumors: an update based on the 2013 (4th) edition. Pathology. 46(2):95–104
Hazelbag HM, Szuhai K, Tanke HJ et al (2004) Primary synovial sarcoma of the heart: a cytogenetic and molecular genetic analysis combining RT-PCR and COBRA-FISH of a case with a complex karyotype. Mod Pathol 17(11):1434–1439
Srivastava A, Nielsen PG, Dal Cin P et al (2005) Monophasic synovial sarcoma of the liver. Arch Pathol Lab Med 129(8):1047–1049
Olsen G, Beal E, Pfeil S, Dillhoff M (2018) Primary gastric synovial sarcoma mimicking a gastrointestinal stromal tumor (GIST): gastric synovial sarcoma. J Gastrointest Surg 22:1450–1451
Ogino S, Konishi H, Ichikawa D, Hamada J, Shoda K, Arita T et al (2018) Detection of fusion gene in cell-free DNA a gastric synovial sarcoma. World J Gastroenterol 24:949–956
Fuente I, Bruballa R, Corradetti S, Cavadas D, Beskow A, Wright F (2019) Gastric synovial sarcoma. J Gastrointest Surg 23:1515–1517
Bialick S, Schwartz S, Khiew YC, Budina A, Harter L (2019) 1925 GIST kidding: primary gastric synovial sarcoma presenting as hematemesis. Am J Gastroenterol 114:S1076–S1077
Marchand Crety C, Bellefqih S, Amroun K et al (2020) Primary gastric synovial sarcoma: a case report and literature review. Int J Surg Case Rep 78:270–273
Romeo S, Rossi S, Acosta Marín M et al (2015) Primary synovial sarcoma (SS) of the digestives: a molecular and clinicopathological study of fifteen cases. Clin Sarcoma Res 12(2):5–7
Wong H, Law S, Collins R (2020) Gastric synovial sarcoma: a case report and literature review. Hong Kong Med J 26:142–145
Folpe AL, Schmidt RA, Chapman D et al (1998) Poorly differentiated synovial sarcoma: immunohistochemical distinction from primitive neuroectodermal tumors and high-grade malignant peripheral nerve sheath tumors. Am J Surg Pathol 22(6):673–682
National Comprehensive Cancer Network. (2018). Soft tissue sarcoma (version 2.2018). Retrieved from https://jnccn.org/view/journals/jnccn/16/5/article-p536.xml
Casali PG, Abecassis N, Aro HT et al (2018) Soft tissue and visceral sarcomas: ESMO-EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 29(Suppl 4):iv268–iv269
Billings SD, Meisner LF, Cummings OW, Tejada E (2000) Synovial sarcoma of the upper digestive tract: a report of two cases with demonstration of the X;18 translocation by fluorescence in situ hybridization. Mod Pathol 13:68–76
Makhlouf HR, Ahrens W, Agarwal B et al (2008) Synovial sarcoma of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 10 cases. Am J Surg Pathol 32:275–281
Crew AJ, Clark J, Fisher C et al (1995) Fusion of SYT to two genes, SSX1 and SSX2, encoding proteins with homology to the Kruppel-associated box in human synovial sarcoma. EMBO J 14:2333–2340
Tsuji S, Hisaoka M, Morimitsu Y et al (1998) Detection of SYT-SSX fusion transcripts in synovial sarcoma by reverse transcription-polymerase chain reaction using archival paraffin-embedded tissues. Am J Pathol 153:1807–1812
Terry J, Barry TS, Horsman DE et al (2005) Fluorescence in situ hybridization for the detection of t(X;18) (p11.2;q11.2) in a synovial sarcoma tissue microarray using a break apart style probe. Diagn Mol Pathol 14:77–82
Lewis JJ, Antonescu CR, Leung DH, Blumberg D, Healey JH, Woodruff JM et al (2000) Synovial sarcoma: a multivariate analysis of prognostic factors in 112 patients with primary localized tumors of the extremity. J Clin Oncol 18(10):2087–2094
Singer S, Baldini EH, Demetri GD, Fletcher JA, Corson JM (1996) Synovial sarcoma: prognostic significance of tumor size, margin of resection, and mitotic activity for survival. J Clin Oncol 14(4):1201–1208
Krieg AH, Hefti F, Speth BM, Jundt G, Guillou L, Exner UG et al (2011) Synovial sarcomas usually metastasize after >5 years: a multicenter retrospective analysis with minimum follow-up of 10 years for survivors. Ann Oncol 22(2):458–467
Bramwell VH, Anderson D, Charette ML, Group SDS (2003) Doxorubicin-based chemotherapy for the palliative treatment of adult patients with locally advanced or metastatic soft tissue sarcoma. Cochrane Database Syst Rev 3:CD003293
Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M (2008) A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer. 113(3):573–581
Rosenberg SA, Tepper J, Glatstein E, Costa J, Baker A, Brennan M et al (1982) The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg 196(3):305–315
Data availability
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Informed consent was obtained from the patient for being included in the study.
Consent for Publication
An informed consent to publish this case was obtained from the patient.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kadam, S.S., Kadam, T. Primary Gastric Synovial Sarcoma in a Young Male: a Rare Case Report and Review of Literature. Indian J Surg Oncol 14, 690–693 (2023). https://doi.org/10.1007/s13193-023-01738-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13193-023-01738-4