Abstract
The use of open cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) has shown improved oncological survival in terms of treating peritoneal surface malignancies (PSM). However, this procedure often comes with associated morbidity. The move towards use of laparoscopic surgery in this field is postulated to lead to a reduction in morbidity and earlier return to function, but literature on its use for CRS and HIPEC has been scarce. We performed a retrospective review of 6 patients with PSM who underwent laparoscopic CRS and HIPEC in our institution and analysed the patient characteristics, oncological history, perioperative and postoperative outcomes. Median peritoneal cancer index (PCI) score was 0 (IQR 0–1.25). All 6 patients had appendiceal primaries. Median operative time was 285 min (IQR 228.8–300); median length of stay was 7.5 days (IQR 5–8.8). All patients achieved complete cytoreduction, and there was no conversion to open surgery. One patient developed port site infection and another 2 patients subsequently developed adhesions. Median follow-up time was 35 (IQR 17.5–41) months. No patients had developed recurrence at the time of data collection. We conclude that in patients with limited PCI sore (< 2), laparoscopic CRS and HIPEC are safe and feasible. With increasing experience, a select group of patients with limited PSM may be treated via minimally invasive surgery, minimising the morbidity of a traditional laparotomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The development of cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) has resulted in a shift of the role of surgery of peritoneal surface malignancies (PSM) from palliative to curative [1]. With surgical resection of macroscopic disease and heated chemotherapy to microscopic disease, a 5-year overall survival of 30–80% may be achieved for PSM [2]. In patients with a less aggressive form of PSM such as pseudomyxoma peritoneii (PMP) and localized malignant mesothelioma (MM), 5-year overall survival of up to 80–95% can be achieved [3]. As such, it has become the mainstay curative treatment for PMP and MM. CRS and HIPEC are also performed in several centres worldwide for treatment of PSM of colorectal and ovarian origin with curative intent [1].
CRS and HIPEC traditionally involve a long midline laparotomy to evaluate the extent of peritoneal disease and for adequate cytoreduction. This has been associated with significant morbidity, such as poor wound healing and infection, resulting in prolonged hospital stay and delay in the initiation of adjuvant chemotherapy [1, 4].
In the recent years, the use of laparoscopic surgery for abdominal conditions has become more widely accepted and has been regarded to confer similar oncological outcomes, with reduced morbidity and faster recovery [5, 6]. Early reports on the use of laparoscopic surgery in CRS and HIPEC for PSM have been promising. Several studies have demonstrated that laparoscopic CRS and HIPEC for a variety of conditions including PSM, PMP and MM have demonstrated the ability to achieve complete cytoreduction with minimal to no conversion to open surgery and minimal complications [7,8,9]. One small study from Mercier et al. assessing long-term oncological outcomes of laparoscopic CRS and HIPEC for PMP and MM found only 2 cases of peritoneal recurrence out of 32 patients who underwent laparoscopic CRS and HIPEC, with a median follow-up of 31.6 months [10]. Studies that compared outcomes of laparoscopic CRS and HIPEC with open in case–control conditions have reported equivalent operative time, morbidity and rate of locoregional recurrence (LRR) compared to its open counterpart, while touting shorter length of stay and surgical morbidity [11,12,13].
At present, there has been no study from Asia, looking at outcomes of laparoscopic CRS and HIPEC in patients with peritoneal carcinomatosis. We aim to present our institutions’ experience with performing laparoscopic CRS and HIPEC for the treatment of PSM in a predominantly Asian population and review the perioperative and oncological outcomes.
Materials and Methods
We performed a retrospective review of our institution prospective database of all consecutive patients who underwent laparoscopic CRS and HIPEC for PSM between January 2015 and September 2020. Patients who did not have extensive peritoneal disease, as confirmed on review of pre-operative imaging and intra-operatively on diagnostic laparoscopy were included. Various information were collected including PCI score, underlying tumour characteristics, perioperative procedure and postoperative complications. This study was approved by the local institution review board.
We found a total of 6 patients who underwent laparoscopic CRS and HIPEC who met the criteria for analysis.
Data Collection
All clinical data were retrieved from a prospectively maintained computerised clinical database (Sunrise Clinical Manager, Eclipsys Corporation, Atlanta, GA, USA) and patient’s clinical charts. Operative data, on the other hand, were retrieved from a separate prospective computerised database (OTM 10, IBM, Armonk, NY, USA).
Basic demographic and clinicopathologic data were collected for each patient. Oncological and perioperative data, including the primary tumour, PCI score, the HIPEC agent used and number of organs resected were collected. Postoperative complications and postoperative recovery data were also collected. These include operative time, complications, time to commencement of diet, length of stay, need for readmission, completeness of cytoreduction and conversion to open surgery. Additional data including time to adjuvant chemotherapy, overall survival (OS), disease-free interval (DFI) and time to locoregional relapse are included as well.
Operative Technique
Patients who have been identified for laparoscopic CRS and HIPEC are first counselled by both the surgical team and medical oncologist. Patients are admitted the day before the procedure for bowel preparation and venous thromboembolism prophylaxis in the form of subcutaneous enoxaparin 20 mg at 8 p.m. on the night before the procedure.
The procedure was performed under general anaesthesia in the Llyod Davis position. Infraumbilical open cutdown technique was used for initial placement of 10-mm port in all the cases. After entry, diagnostic laparoscopy is first performed to assess the extent of disease and to determine the PCI score. Laparoscopic CRS and HIPEC were performed in patients who were confirmed not to have extensive peritoneal disease.
All patients underwent greater omentectomy with preservation of the gastroepiploic arcade, even in the absence of macroscopic omental disease. Selective peritoneal stripping was performed as needed to achieve a complete cytoreduction. Bowel resection, where needed, was performed with Endo GIA 100 (Medtronic, Dublin, Ireland). The infra-umbilical incisions would then be extended for retrieval of specimen and extracorporeal anastomosis.
Total of four drains are placed, one in each quadrant of the abdomen. These drains are then connected — two inflow and two outflow, to the perfusion catheters. HIPEC was then administered using either the Belmont (Belmont Instruments, Billerica, MA) or Transmedic (Andover, Massachusetts, United States) perfusion systems with varying doses (12–24) mg of Mitomycin C at 42°Celsius for 60 min, depending on the patient’s body surface area (BSA). At the end of the procedure, bowel anastomoses, where applicable, were performed with Endo GIA 100 extra-corporeally in a functional end to end fashion, and the midline wound is then closed in the usual fashion.
Definitions
Diagnosis of PSM is made based on imaging findings in correlation with tissue diagnosis. All cases of PSM were discussed at a multidisciplinary tumour board comprising surgical oncologists, medical oncologists, radiation oncologists, pathologists and radiologists. Decision for CRS and HIPEC was made collectively by the board. All patients were followed up at intervals of 3 to 6 months after CRS and HIPEC with cross-sectional imaging using computerised tomography.
Postoperative complications were categorized according to the Clavien-Dindo classification and recorded for up to 30 days, or during the same hospitalisation for surgery [14]. Major morbidity was defined as any complication more than grade 2. Overall survival (OS) was defined as time from surgery to death (all causes) or date of last follow-up. DFI was calculated from time of surgery to disease recurrence or death.
Statistical Analysis
All statistical analyses were conducted using the computer program Statistical Package for Social Sciences for Windows, version 23.0 (SPSS Inc., Chicago, IL, USA). All continuous data were expressed as the mean with standard deviation (SD) for normally distributed variables and median with interquartile range (IQR) for non-normally distributed variables. All proportions were expressed as a fraction and percentage.
Results
Clinicopathological Characteristics
Between January 2015 and September 2020, 6 patients underwent laparoscopic CRS and HIPEC (Table 1). All 6/6 (100%) were of appendiceal primary; 5/6 (83.3%) were of low-grade mucinous appendiceal neoplasm (LAMN) origin; and 1/6 (16.7%) had goblet cell carcinoma. Median PCI score performed during CRS and HIPEC was 0 (IQR 0–1.25). All 6/6 (100%) patients had undergone prior laparoscopic appendectomies, with 1/6 (16.7%) of whom required a conversion to open appendectomy. All 6/6 (100%) patients had PSM diagnosed on histology postappendectomies; 1 patient had proximal margin involved with LAMN; 1 patient had mesenteric margin involved with LAMN; 1 patient had upfront perforation with mucinous deposits seen along peritoneal surface at index operation; 2 patients had extracellular mucin pools on histology; and 1 patient received CRS and HIPEC after having localised perforated appendicitis and was diagnosed to be pT3 goblet cell carcinoma. Median number of organs resected in the laparoscopic CRS and HIPEC group was 1.5 (IQR 1–3.25), with 6/6 (100%) having undergone omentectomies, 3/6 (100%) having limited peritonectomies, 2/6 (33%) having right hemicolectomies and 1/6 (16.7%) having bilateral salpingoophrectomy. All patients received mitomycin C for their HIPEC. Subsequent histologies were negative for gross malignancy for all 6 patients. No patient received neoadjuvant chemotherapy.
Perioperative Outcomes
Perioperative outcomes of laparoscopic CRS and HIPEC are summarised in Table 2. Median operative time was 285 (IQR 228.75–300) min. Median blood loss was 150 (IQR 62.5–275) ml in the laparoscopic group with 1/6 (16.7%) patient needing intraoperative transfusion. Median time to diet was 5 (IQR 4–6.25) days, and median length of stay was 7.5 (IQR 5–8.75) days.
About 1/6 (16.7%) patient who underwent laparoscopic CRS and HIPEC experienced a Clavien-Dindo I morbidity in the form of port site infection, and 2/6 (33.3%) patients subsequently developed adhesions. Of which, one patient required readmission, laparotomy and adhesiolysis for bowel obstruction 14 months post initial surgery. All patients had complete cytoreduction, and none of the patients who underwent laparoscopic CRS and HIPEC required conversion to open surgery.
Oncological Outcome
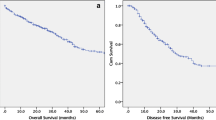
Oncological outcomes are summarised in Table 2. Median follow-up time was 35 (IQR 17.5– 41) months with no patients lost to follow-up. Median DFI and OS were the same at 35 (17.5–41) months. There were no recurrences detected in all 6 patients who underwent laparoscopic CRS/HIPEC and none requiring a repeat CRS/HIPEC at the time of manuscript writing. No patients required adjuvant chemotherapy.
Discussion
Development of CRS and HIPEC has revolutionised the management of patients with PSM and can be considered as a form of curative treatment [1]. However, this may come with significant morbidity associated with traditional laparotomies, such as intraabdominal collections, adhesions and poor wound healing [4]. In patients where survival outcomes are contingent on receiving timely systemic therapy, such morbidity may impede the initiation of adjuvant chemotherapy and result in suboptimal treatment. It is with the aim of minimising the operative morbidity that laparoscopic CRS and HIPEC, which has been shown to be equally effective in the distribution of chemotherapeutic agents, are used [2].
While we have set out to investigate the outcomes of laparoscopic CRS and HIPEC for PSM of all aetiologies in our institution with PCI < 10, we found that all patients who underwent laparoscopic CRS and HIPEC had appendiceal primaries, with PCI scores ranging from 0 to 2. Majority of these patients had previously undergone appendectomies, where histology revealed the presence of LAMN. LAMN is known to be an entity of low risk. In patients for whom repeat diagnostic laparoscopy did not demonstrate further macroscopic disease, prophylactic HIPEC, while not an established standard of care, was nevertheless offered in the context of our clinical trial. This reflects the present treatment philosophy in our institution to carefully select patients for laparoscopic CRS and HIPEC in patients with low risk, minimal peritoneal disease and limited need for multiorgan resection, to avoid the morbidities of a laparotomy.
Our early findings are encouraging. Patients with limited PCI scores were able to receive laparoscopic CRS and HIPEC with complete cytoreduction and reasonable operative time, blood loss, length of stay, morbidity and time to diet. Morbidity was minimal with 1 patient developing port-side infection and 2 patients developing subsequent adhesions, and there was no mortality encountered. In addition, one of our patients had previously undergone laparotomy for an unrelated condition, suggesting that past open surgery does not preclude the use of future laparoscopy for CRS and HIPEC. This also shows that laparoscopic CRS and HIPEC may be safely performed in patients with low tumour burden. All of our patients who underwent laparoscopic CRS and HIPEC had complete cytoreduction and had no recurrence on median follow-up of 35 (IQR 17.5–41) months, suggesting that the use of laparoscopic surgery does not compromise on oncological safety.
Existing literature suggests that the use of laparoscopic CRS and HIPEC may be employed safely for PCI scores of up to 10, in multiple different tumour aetiologies. At present, the largest study on the use of laparoscopic CRS/HIPEC to date is a multicentre study by Arjona-Sanchez et al. [8]. In their study, 90 patients from 7 centres underwent laparoscopic CRS and HIPEC. Appendiceal cancers were the most common (64.9%), followed by colorectal cancers (16.5%), MM (11%) and ovarian cancers (4.4%). One patient had cholangiocarcinoma and one had Goblet cell carcinoid tumour. Their mean PCI score was 4.1, with 41% of patients requiring bowel resection. In their series, all patients had complete cytoreduction. Only 3 and 6.5% of patients had grade 3 or 4 morbidity, respectively, and their mean hospital stay was 7.4 days. They concluded that laparoscopic CRS and HIPEC were safe and feasible procedures for selected patients with PCI < 10 with PSM. Other smaller studies, with median PCI score ranging between 2 and 4.1 [7, 9, 10], for aetiology including MM, PMP, colorectal and ovarian have supported this conclusion.
There are also studies comparing outcomes between laparoscopic and open CRS and HIPEC. These studies have demonstrated that patients who undergo laparoscopic surgery have shorter length of stay [11, 13], reduced time to initiation of adjuvant chemotherapy [11] and shorter operative time [12, 13]. However, the limitation of many existing studies [11, 13] is that the open CRS and HIPEC comparison group were not matched for PCI scores and tumour aetiology. This might have confounded the results where the better outcomes of laparoscopic CRS and HIPEC were due to less aggressive disease. In addition, Rodriguez et al. [11], the only study which looked at oncological outcomes between both the laparoscopic and open group only presented data up to 2 years post operation. Our current group of patients are likely deemed to be very low risk. As we conquer the learning curve, it would be interesting to note for future patients who undergo laparoscopic CRS and HIPEC, if the oncological and survival outcomes can be comparable to its open counterpart.
Some studies have suggested other minimally invasive approach, either as an end in itself or as a transition to laparoscopic CRS and HIPEC. Lotti et al. [15] proposed a hybrid technique where open CRS is performed, followed up with abdominal wall closure and establishment of pneumoperitoneum and port insertion for HIPEC to be performed. Salti and Naffouje [16] proposed the use of hand-assisted laparoscopic CRS and HIPEC to allow for the benefits of open surgery, such as its tactile feedback, retraction and ease of hemostasis, while minimising the length of wound and complications, touting its benefit over a pure laparoscopic approach for complicated surgeries. Gabriel et al. [17] described a case where robotic-assisted CRS and HIPEC were performed, which allowed for a higher quality imaging, improved ergonomics and increase ease of performing complex anastomosis. These may be useful considerations in developing the expertise in performing a full laparoscopic CRS and HIPEC.
There are several limitations to our study, the first of which is its retrospective nature. As this was a retrospective case series, cases that underwent laparoscopic CRS and HIPEC would have naturally been deemed to be safe and favourable compared to cases of similar PCI score that have been performed as open surgery. Secondly, our group of patients are highly selected. Hence, complications and impact on oncological outcomes may not be apparent. Also, while we initially set out to determine if laparoscopic CRS and HIPEC are safe and feasible in PSM of all aetiologies with PCI score < 10, our data only pertained to appendiceal tumours with PCI < 2. Further studies of larger numbers, involving the different aetiologies of PSM and of randomised nature would be useful in expanding the consensus on the extent of PSM that is safe for laparoscopic resection. Such studies could also look into whether the location of PSM, number of organs resected and number of anastomosis needed affect the outcome and safety of minimally invasive CRS and HIPEC.
Conclusion
We conclude that in patients with PSM with limited PCI score (< 2), laparoscopic CRS and HIPEC are safe and feasible with good surgical and oncologic outcomes. With increasing experience, more patients with limited PSM may be treated via minimally invasive surgery, minimising the morbidity of a traditional laparotomy.
References
Chua TC, Yan TD, Saxena A, Morris DL (2009) Should the treatment of peritoneal carcinomatosis by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy still be regarded as a highly morbid procedure?: A systematic review of morbidity and mortality. Ann Surg 249(6):900–907
Padilla-Valverde D, Villarejo P, Redondo J et al (2019) Laparoscopic cytoreductive surgery and HIPEC is effective regarding peritoneum tissue paclitaxel distribution. Clin Transl Oncol 21(9):1260–1269
Delhorme J-B, Severac F, Averous G et al (2018) Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for pseudomyxoma peritonei of appendicular and extra-appendicular origin. Br J Surg 105(6):668–676
Mahner S, Eulenburg C, Staehle A et al (2013) Prognostic impact of the time interval between surgery and chemotherapy in advanced ovarian cancer: analysis of prospective randomised phase III trials. Eur J Cancer 49(1):142–149
Martínez-Cecilia D, Cipriani F, Vishal S et al (2017) Laparoscopic versus open liver resection for colorectal metastases in elderly and octogenarian patients: a multicenter propensity score based analysis of short- and long-term outcomes. Ann Surg 265(6):1192–1200
Song X-J, Liu Z-L, Zeng R, Ye W, Liu C-W (2019) A meta-analysis of laparoscopic surgery versus conventional open surgery in the treatment of colorectal cancer. Medicine 98(17):e15347
Dumont F, Duchalais E, Aumont A, Thibaudeau E (2020) Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy by laparoscopy via a single-port approach for low-grade peritoneal malignancy. Surg Endosc 34(6):2789–2795
Arjona-Sanchez A, Esquivel J, Glehen O et al (2019) A minimally invasive approach for peritonectomy procedures and hyperthermic intraperitoneal chemotherapy (HIPEC) in limited peritoneal carcinomatosis: the American Society of Peritoneal Surface Malignancies (ASPSM) multi-institution analysis. Surg Endosc 33(3):854–860
Esquivel J, Averbach A (2012) Laparoscopic cytoreductive surgery and HIPEC in patients with limited pseudomyxoma peritonei of appendiceal origin. Gastroenterol Res Practice 2012:1–5
Mercier F, Jeremie G, Alyami M et al (2020) Long-term results of laparoscopic cytoreductive surgery and HIPEC for the curative treatment of low-grade pseudomyxoma peritonei and multicystic mesothelioma. Surg Endosc 34(11):4916–4923
Rodríguez-Ortiz L, Arjona-Sánchez A, Ibañez-Rubio M et al (2021) Laparoscopic cytoreductive surgery and HIPEC: a comparative matched analysis. Surg Endosc 35(4):1778–1785
Abudeeb H, Selvasekar CR, O’Dwyer ST et al (2020) Laparoscopic cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for perforated low-grade appendiceal mucinous neoplasms. Surg Endosc 34(12):5516–5521
Passot G, Bakrin N, Isaac S et al (2014) Postoperative outcomes of laparoscopic vs open cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy for treatment of peritoneal surface malignancies. European J Surg Oncol (EJSO) 40(8):957–962
Dindo D, Demartines N, Clavien P-A (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213
Lotti M, Capponi M, Piazzalunga D et al (2016) Laparoscopic HIPEC: a bridge between open and closed-techniques. J Min Access Surg 12(1):86
Salti GI, Naffouje SA (2019) Feasibility of hand-assisted laparoscopic cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal surface malignancy. Surg Endosc 33(1):52–57
Gabriel E, Elli E, Bagaria S et al (2019) Robotic-assisted cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (CRS-HIPEC). J Robotic Surg 13(1):175–179
Funding
This study is funded by the NCCS Cancer Fund. CAJO is funded by the National Medical Research Council Transition Award (NMRC/TA/0061/2017).
Author information
Authors and Affiliations
Contributions
Conceptualization: YLL, JSMW, CSC; data curation: YLL, SCJ; formal analysis: YLL; funding acquisition: CAJO, CSC; investigation: YLL, SCJ; methodology: SCJ, JSMW, CSC; project administration: SCJ, JSMW, CSC; Resources: CAJO, CSC; Supervision: CAJO, CSC; validation: YLL, SCJ, JSMW; visualization: YLL; writing — original draft: YLL, JSMW; writing — review and editing: YLL, SCJ, JSMW, CAJO, CSC.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Le Linn, Y., Seo, C.J., Wong, J.S.M. et al. An Asian Tertiary Centre’s Early Experience with Laparoscopic Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Peritoneal Carcinomatosis. Indian J Surg Oncol 14 (Suppl 1), 175–180 (2023). https://doi.org/10.1007/s13193-022-01632-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13193-022-01632-5