Abstract
Recurrent laryngeal nerve (RLN) palsy is one of the feared complications following thyroid surgery. Intraoperative neuromonitoring (IONM) has been used as an adjunct to reduce this complication. In the present study, we attempted to evaluate the IONM parameters such as latency, current requirement, and baseline amplitude that could predict temporary RLN palsy along with factors that could influence these parameters during thyroid surgery. This was a retrospective study of patients who underwent hemi, total, or completion thyroidectomy for cancer at our institute between June 1, 2017 to May 31, 2019 in whom IONM was used during surgery. The study consisted of 84 consecutive patients with 138 nerves at risk. The RLN palsy rate in our study was 5% (n = 7). Patients with low baseline amplitude and/or requiring higher current to maintain normal baseline amplitude were often associated with temporary RLN palsy. In the multivariate analysis, age > 40 years (p = 0.001, OR = 4.14) influenced the baseline EMG amplitude the most. The intraoperative current management was influenced by advanced pT stage (p = 0.001, OR = 2.87), and structural nerve injury (p = 0.001, OR = 3.15). Patients with low baseline amplitude and/or requiring higher current to maintain normal baseline amplitude were often associated with temporary RLN palsy. Factors such as age, pT stage, and structural nerve injury influenced the IONM stimulation and recording parameters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recurrent laryngeal nerve palsy (RLNP) is one of the common and devastating complications following thyroid surgery which significantly influences the patient’s quality of life. The purpose of IONM was to reduce this complication by assisting the surgeon to identify the recurrent laryngeal nerve (RLN) and assure its functional integrity. [1] The benefits of the application of IONM in thyroid surgery are not just in the identification of RLN but also in the external branch of the superior laryngeal nerve (EBSLN) with a significantly improved identification rate and a reduced risk of injury. [2, 3] IONM can also help clinicians predict vocal cord dysfunction and assist in intraoperative decision-making and planning subsequent treatment in the event of nerve palsy being identified or anticipated intraoperatively. [1, 4] Studies have been conducted to investigate the role of IONM in reducing early clinical RLNP. [5] In general, IONM can indicate non-dissociative damage such as transaction, clamping, traction, electrocautery injury, ligature entrapment, or ischemia, which cannot be recognized and judged visually. [6,7,8] The negative predictive value for identifying the postoperative nerve function is between 92 and 100% and a low positive predictive value between 10 and 90%. [9] Previous studies have suggested certain factors such as malignant histology, central compartment dissection, and previous thyroid surgery as strong predictors for RLNP among others. [10, 11] However, the usefulness of IONM parameters such as current requirement, latency, and amplitude changes in predicting temporary RLNP is not known. We did this retrospective study with the aim to evaluate the IONM parameters predicting temporary postoperative RLNP and factors influencing these IONM parameters in patients who underwent surgery for thyroid malignancy.
Methodology
This retrospective study was done on prospectively collected data of patients who underwent thyroid surgery between June 1, 2017 and May 31, 2019 for malignancy with IONM in a single unit of a tertiary head and neck cancer center. All clinical, radiological, and histopathology details were retrieved from the electronic medical records and the departmental database. As per our institute policy, all patients underwent an examination of the vocal cord status with 90° Hopkins rigid endoscope and/or fiber optic laryngoscopy examination preoperatively and postoperatively (before discharge). This procedure was performed by one of the members of the operating team. After discharge, the vocal cord status is recorded at every follow-up visit. The temporary RLNP was defined as vocal cord palsy when present in the postoperative period, i.e., before discharge and subsequently during follow-up until 6 months from surgery. [12, 13].
Intraoperative neuromonitoring (IONM) was done using standard reinforced electromyography (EMG) endotracheal tubes with a size of 7.0, 7.5, or 8.0 mm (Medtronic Xomed, Jacksonville, FL, USA) for intubation. The tube was placed (3 cm of the surface electrodes) in good contact with the vocal cords (vocalis muscle) under direct video laryngoscopy. [14] A prass monopolar stimulator probe (Medtronic Xomed) was used with a current delivery of 0.5–2.5 mA (supramaximal stimulation) at the rate of 4 Hz/s with 100 µs pulse width to monitor the integrity of the nerve. The EMG activity was recorded on a NIM-response 3.0 monitor (Medtronic Xomed). The monitoring system-generated stimuli with a time window of 25 ms and an amplitude scale were set to 500 µV/division. The event threshold on the NIM was set to 100 µV, and impedance differences were recorded to be less than 1.0 mΩ on each channel (left and right RLN). No muscle relaxants were used till the end of IONM use. The neuromonitoring device was used in various phases during surgery. Specifically, before draping the patient, mechanical stimulation was applied by gently tapping the trachea at a level corresponding to vocalis muscles to ensure that the monitoring system was working; later, stimulation was then applied to the structure believed to be the RLN (Fig. 1a, d). After thyroid removal and complete hemostasis within the surgical field, stimulation was reapplied to the vagus nerve (indirect stimulation) and the RLN (direct stimulation) for predicting the postoperative outcome. [15] It is well known that IONM EMG amplitudes may vary significantly within a patient and among patients and is yet to be systematically standardized. In general, a relatively higher (> 1500 µV) or lower (< 400 µV) RLN baseline amplitude would be involved in the prediction of laryngeal mobility outcome. [16] In our clinical setup, we have considered ≥ 300 µV to be the normal baseline amplitude and have used this cutoff for postoperative voice outcome analysis. Also, baseline EMG (vocalis) amplitude reduction by > 50% was considered abnormal. The latency in the range between 1.2 and 1.5 ms was considered normal in the study and an increase by > 10% was considered abnormal responses. Also, the requirement of current > 1.0 mA to maintain baseline amplitude (≈300 µV) was considered abnormal [17]. All these parameters were documented and analyzed to understand whether they could predict temporary RLN palsy.
Statistical Analysis
The statistical analysis was performed using SPSS version 24 (IBM Corp, Armonk, USA). The occurrence of palsy in the immediate postoperative period (days 1 to 7) was defined as the event. Based on the available evidence in the literature and our experience, multiple factors like age, gender, pathological T stage (early vs. advance), tracheoesophageal groove (TEG) involvement, central compartment lymph nodes (CCLN) positivity, intraoperative events, and injury were considered for analysis among others. Univariate analysis was done using the Chi-square test to check for the associations of these factors on the current requirement and amplitude. Multivariate analysis was done subsequently using binomial logistic regression (forward stepwise selection). A p-value of < 0.05 was considered significant. The risk preference for each factor was generated. The sensitive risk factors were measured with the odds ratio.
Results
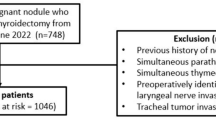
A total of 255 patients underwent thyroid surgeries during the abovementioned time period. All cases in which the final histopathology was benign (n = 27), and in those cases where the IONM was not used (n = 144) were excluded. A total of 84 patients satisfied the eligibility criteria and were included in the study. The majority of them were differentiated thyroid carcinoma, papillary thyroid carcinoma being the most common variety within (n = 74, 88%), followed by poorly differentiated thyroid carcinoma (n = 5, 5.9%), medullary thyroid carcinoma (n = 4, 4.7%), and anaplastic thyroid carcinoma (n = 1.1.4%). The details of the surgical procedures are given in Table 1. Based on the procedure performed on each patient, two RLN were at risk with total thyroidectomy, while a unilateral procedure put one nerve at risk (NAR). Hence, 138 nerves at risk of developing RLN palsy were studied. The decision to use IONM was taken on a case to case basis by the operating surgeon based on one or more of the following factors: revision surgery (completion thyroidectomy), ETE on imaging, TEG involvement on imaging, advanced T stage, and when CC lymph nodes were expected to be positive or as seen in imaging (CECT). The overall postoperative (temporary) RLN palsy rate was 5% (7 out of 138 NAR) in our series. None of the patients developed bilateral vocal cord palsies. In our analysis, there were no issues pertaining to vocalis muscle latency.
Out of the 138 NAR, 84 NAR displayed normal baseline amplitude (≥ 300 µV) (Fig. 2). One NAR out of this 84 developed temporary RLNP subsequently. Fifty-four NAR displayed lower baseline amplitude (< 300 µV), out of which 3 NAR developed temporary RLNP subsequently. All these three NAR were in patients who underwent completion thyroidectomy. Eighty-three NAR required normal current (≤ 1.0 mA) to elicit a response, out of which 2 NAR developed temporary RLNP. Fifty-five NAR required a higher current (> 1.0 mA) to elicit a response, out of which one NAR developed a temporary RLNP. Interestingly, this patient also underwent a completion thyroidectomy (Fig. 2).
Factors Influencing (RLN) Baseline EMG Amplitudes (Table 2, Fig. 1b)
The median age of the patients was 40 years in our study (range 12–80 years). The effect of different preoperative and intraoperative factors on EMG amplitudes and current is summarized in Table 2. In our study, age was the only preoperative factor that had an influence on the baseline EMG amplitudes on both univariate and multivariate analyses. We got lesser baseline EMG amplitudes (mean 212 ± 72 µV) in patients aged > 40 years (p < 0.01, OR = 4.14) as compared to patients ≤ 40 years of age (mean 812 ± 39 µV). Certain factors like intraoperative blood loss and when central compartment dissection was performed were found to influence the baseline EMG amplitude only in univariate analysis. Intraoperative blood loss of > 500 ml (mean 325 ± 48 µV) was associated with lower baseline EMG amplitude (p < 0.05) as compared to patients with blood loss ≤ 500 ml (mean 651 ± 27 µV). Also, when bilateral central compartment node dissection was done, it was associated with reduced baseline EMG amplitudes (p < 0.05), and this was more frequent on the right side (p < 0.05) than on the left side.
Factors Influencing the Current Requirement (Table 2, Fig. 1c)
The pT stages (T3–T4) and intraoperative structural injury to the nerve influenced the current required to elicit a response on both univariate and multivariate analyses. Patients with early pT-staged tumors required normal current to elicit a baseline EMG amplitude. In contrast, a higher current (mean 2.4 ± 0.3 mA) was required to get a normal baseline amplitude in patients with advanced T stage, and on certain occasions, the amplitude remained lower despite increasing the current. This was significant on both univariate (p = 0.047) and multivariate analysis (p = 0.001; OR = 2.87). Similarly, patients with involvement of the TEG (n = 8) required higher current (mean 1.8 ± 0.6 mA) (p < 0.05) to elicit stable baseline EMG amplitudes as compared to those patients where the TEG was uninvolved (mean 0.8 ± 0.3 mA). Intraoperative nerve structural injury was associated with a higher current required to maintain the baseline EMG amplitude. The intraoperative structural injury tends to significantly increase the current requirement (mean 2.2 ± 0.3 mA from 0.7 ± 0.3 mA) (p < 0.01, OR = 3.15) as compared to patients with no injury (mean 1.0 ± 0.1 mA from 0.8 ± 0.3 mA) to obtain baseline EMG amplitude.
Discussion
Our study suggests that patients with low baseline amplitude and/or those requiring higher current to maintain normal baseline amplitude were often associated with temporary RLN palsy in the postoperative period. Patients aged > 40 years had a negative influence on the baseline EMG amplitude, and those patients with advanced pT stage and intraoperative nerve injury necessitated higher current to obtain a baseline EMG amplitude.
There are many preoperative and intraoperative risk factors for RLN injury including older age, pathological T stage, TEG involvement, previous thyroidectomy, intraoperative bleeding, side of resection among others. [18,19,20,21] IONM has rarely been shown to be significantly superior to visual identification in terms of RLN palsy rate following thyroid surgery by many studies. [3, 9, 22] However, it is useful for assessing nerve integrity, as a visually intact nerve is not necessarily functional and is of prognostic value when palsy occurs. [9] The American Academy of Head and Neck Surgery recommends IONM to be included for the identification of recurrent laryngeal nerves to reduce transient RLN palsy, and also to remove the risk of bilateral RLN palsy. [18, 23]
Factors Influencing IONM Parameters
Baseline EMG Amplitude
Lorenz et al. have suggested that baseline EMG amplitude was better in patients with < 40 years than in the higher age group; however, no difference in latency and duration was reported. [24] Similarly, in our study, too, age influenced the baseline EMG amplitude the most, and latency did not give any information. It is well known that aging results in a decrease in nerve tissue mass, neuronal density, and concentration of neurotransmitters [25] along with pathology that may cause less excitation input to the corresponding muscles with electrical stimulation. This could be one of the reasons that we obtained lower baseline EMG amplitudes from the vocalis muscle with > 40 years of age than the younger patients in our study. On the other hand, aging with preoperative comorbidities like diabetes would have an influence on the baseline RLN latencies and amplitudes in thyroidectomy patients. [26] However, we did not find any significant (p = 0.3) influence of this factor on RLN latency and amplitudes in our study. Also, there were no differences in anesthetic drug maintenance between the two age groups in this study. Other factors that showed to have some influence on the baseline EMG were intraoperative blood loss of > 500 ml and central compartment dissection. Patients with > 500 ml blood loss were mostly associated with advanced disease necessitating extensive surgery. Another surgery-related risk factor for RLN injury is central compartment dissection. [20, 27, 28] A study by Ling et al. suggested that IONM is highly recommended for patients with a high positive rate of lymph node metastasis requiring central and lateral cervical lymph node dissection. [29] A higher ratio of positive central lymph nodes was associated with a greater risk of RLN injury due to direct invasion of the nerves by the nodes and/or due to the extensive central compartment dissection, and this was frequent on the right side. [29, 30] These findings were also seen in our study and bilateral central compartment node positivity was likely to reduce the EMG amplitude. This probably suggests that IONM might be pre-emptively used to reduce the risk of RLN injury in patients with central lymph node metastasis requiring extensive nodal dissection bilaterally.
Current Requirement for Stimulation
Advanced stage (T3–T4) thyroid malignancy is often associated with the risk of RLN injury as compared to the early stage (T1–T2). [29, 30] In our study, the advanced malignancy influenced determining the baseline current requirement to elicit stable EMG amplitudes by more than 2 times as compared to earlier stage malignancy. This could be due to pathology-induced deranged neuronal cells excitation, necessitating higher current to elicit stable EMG responses from the vocalis muscles. Generally, RLN is a physiologically very sensitive structure and can easily be injured by different intraoperative actions such as clamping, stretching, compressing, and heating and retraction of the thyroid gland. [31] These intraoperative actions can lead to absent or reduced EMG amplitude followed by direct vagus or RLN stimulation. The neurobiological explanation for reduced EMG amplitude is due to a lower number of axons transmitting the electrical signals after the injury, resulting in lesser depolarization of the monitored muscle. [32] This was reflected in our study that structural nerve injury (stretching/traction, compression, thermal injury) necessitated 3 times more current to maintain stable EMG responses intraoperatively. Another factor that showed some influence on the current requirement was the involvement of the TEG by the disease. During thyroid surgery, TEG is a valuable landmark for identifying the RLN. However, TEG involvement is one of the high-risk factors for RLN injury due to its handling during surgery. [20] In our study, patients with TEG involvement required higher current to elicit EMG responses as compared to those in whom TEG was not involved. This could be due to the compression of the nerve and/or its handling.
Some of the limitations of our study are its small sample size and its retrospective nature and hence it bears all its inherent weaknesses. The follow-up for a significant number of patients (n = 16.19%) in this study was less than 1 year, thus permanent RLN palsy rates could not be reported. In the future, we would like to validate these findings in a larger cohort of patients and also find additional risk factors, if any, influencing the IONM parameters to predict postoperative RLN palsy in thyroid surgery for malignancy. However, this is one of the few studies that have looked into the influence of the IONM parameters in predicting temporary RLN palsy and factors influencing these parameters.
Conclusion
In our study, temporary RLN palsy was associated with low baseline amplitude and/or requirement of higher current to maintain normal baseline amplitude during IONM for thyroid malignancy surgery. These could be utilized as surrogate indicators to predict temporary RLN palsy. Age, advanced pT stages (T3–T4), and intraoperative nerve structural injury significantly influenced the IONM parameters. Keeping in mind the limitations of the study, our study will potentially help us identify patients who may develop temporary RLNP following thyroid surgery.
Data Availability
On request.
References
Dionigi G, Barczynski M, Chiang FY et al (2010) Why monitor the recurrent laryngeal nerve in thyroid surgery? J Endocrinol Invest 33(11):819–822
Barczyński M, Konturek A, Stopa M, Honowska A, Nowak W (2012) Randomized controlled trial of visualization versus neuromonitoring of the external branch of the superior laryngeal nerve during thyroidectomy. World J Surg 36:1340–1347
Dralle H, Sekulla C, Lorenz K, Brauckhoff M, Machens A (2008) Intraoperative monitoring of the recurrent laryngeal nerve in thyroid surgery. World J Surg 32:1358–1366
Calò PG, Medas F, Conzo G et al (2017) Intraoperative neuromonitoring in thyroid surgery: is the two-staged thyroidectomy justified? Int J Surg 41 Suppl 1:S13–S20
Yang S, Zhou L, Lu Z, Ma B, Ji Q, Wang Y (2017) Systematic review with meta-analysis of intraoperative neuromonitoring during thyroidectomy. Int J Surg 39:104–113
Chiang FY, Lu IC, Kuo WR, Lee KW, Chang NC, Wu CW (2008) The mechanism of recurrent laryngeal nerve injury during thyroid surgery the application of intraoperative neuromonitoring. Surgery 143:743–749
Snyder SK, Lairmore TC, Hendricks JC, Roberts JW (2008) Elucidating mechanisms of recurrent laryngeal nerve injury during thyroidectomy and parathyroidectomy. J Am Coll Surg 206:123e30
Dionigi G, Alesina PF, Barczynski M, Boni L, Chiang FY, Kim HY et al (2012) Recurrent laryngeal nerve injury in video-assisted thyroidectomy: lessons learned from neuromonitoring. Surg Endosc 26:2601–2608
Pisanu A, Porceddu G, Podda M, Cois A, Uccheddu A (2014) Systematic review with meta-analysis of studies comparing intraoperative neuromonitoring of recurrent laryngeal nerves versus visualization alone during thyroidectomy. J Surg Res 188(1):152–161
Godballe C, Madsen AR, Sørensen CH, Schytte S, Trolle W, Helweg-Larsen J, Barfoed L, Kristiansen L, Sørensen VZ, Samuelsen G, Pedersen HB (2014) Risk factors for recurrent nerve palsy after thyroid surgery: a national study of patients treated at Danish departments of ENT Head and Neck Surgery. Eur Arch Otorhinolaryngol 271(8):2267–2276
Nayyar SS, Thiagarajan S, Malik A, Chakraborthy A, Velayutham P, Chaukar D (2020) Risk factors predisposing for recurrent laryngeal nerve palsy following thyroid malignancy surgery: experience from a tertiary oncology centre. Eur Arch Otorhinolaryngol 277(4):1199–1204
Chiang F, Lee KW, Huang YF, Wang LF, Ku WR (2004) Risk of vocal palsy after thyroidectomy with identification of the recurrent laryngeal nerve. Kaohsiung J Med Sci 20(9):431–6
Jiang Y, Gao B, Zhang X, Zhao J, Chen J, Zhang S, Luo D (2014) Prevention and treatment of recurrent laryngeal nerve injury in thyroid surgery. Int J Clin Exp Med 7(1):101–107
Eid I, Miller FR, Rowan S, Otto RA (2013) The role of nerve monitoring to predict postoperative recurrent laryngeal nerve function in thyroid and parathyroid surgery. Laryngoscope 123(10):2583–2586
Liddy W, Barber SR, Cinquepalmi M, Lin BM, Patricio S, Kyriazidis N, Bellotti C, Kamani D, Mahamad S, Dralle H, Schneider R, Dionigi G, Barczynski M, Wu CW, Chiang FY, Randolph G (2017) The electrophysiology of thyroid surgery: electrophysiologic and muscular responses with stimulation of the vagus nerve, recurrent laryngeal nerve, and external branch of the superior laryngeal nerve. Laryngoscope 127(3):764–771
Wu CW, Wang MH, Chen CC et al (2015) Loss of signal in recurrent nerve neuromonitoring: causes and management. Gland Surg 4(1):19–26
Schneider R, Randolph GW, Sekulla C, Phelan E, Thanh PN, Bucher M, Machens A, Dralle H, Lorenz K (2013) Continuous intraoperative vagus nerve stimulation for identification of imminent recurrent laryngeal nerve injury. Head Neck 35(11):1591–1598
Shindo MKE, McCaffrey J, Portefield J et al (2013) Management of locally invasive well-differentiated thyroid cancer: an evidence-based American head and neck society consensus statement. Head Neck 36(10):1379–1390
Bergenfelz A, Jansson S, Kristoffersson A et al (2008) Complications to thyroid surgery: results as reported in a database from a multicenter audit comprising 3,660 patients. Langenbecks Arch Surg 393:667–673
Ready AR, Barnes AD (1994) Complications of thyroidectomy. Br J Surg 81(11):1555–1556
Hermann M, Keminger K, Kober F, Nekahm D (1991) Risk factors in recurrent nerve paralysis: a statistical analysis of 7566 cases of struma surgery. Chirurg 62(3):182–7; discussion 188
Barczynski M, Konturek A, Cichonl A (2009) Randomized clinical trial of visualization versus neuromonitoring of recurrent laryngeal nerves during thyroidectomy. Br J Surg 96(3):240–246
Julien N, Ferrary E, Sokoloff A, Lamas G, Sterkers O, Bernardeschi D (2017) Vagal and recurrent laryngeal nerves neuromonitoring during thyroidectomy and parathyroidectomy: a prospective study. Eur Ann Otorhinolaryngol Head Neck Dis 134(2):77–82
Lorenz K, Sekulla C, Schelle J, Schmeiss B, Brauckhoff M, Dralle H, German Neuromonitoring Study Group (2010) What are normal quantitative parameters of intraoperative neuromonitoring (IONM) in thyroid surgery? Langenbecks Arch Surg 395(7):901–9
Kanonidou Z, Karystianou G (2007) Anesthesia for the elderly. Hippokratia 11(4):175–177
Ozemir IA, Ozyalvac F, Yildiz G, Eren T, Aydin-Ozemir Z, Alimoglu O (2016) Importance of latency and amplitude values of recurrent laryngeal nerve during thyroidectomy in diabetic patients. Int J Surg 35:172–178
Steurer M, Passler C, Denk DM, Schneider B, Niederle B, Bigenzahn W (2002) Advantages of recurrent laryngeal nerve identification in thyroidectomy and parathyroidectomy and the importance of preoperative and postoperative laryngoscopic examination in more than 1000 nerves at risk. Laryngoscope 112(1):124–133
Moritani S (2015) Impact of lymph node metastases with recurrent laryngeal nerve invasion on patients with papillary thyroid carcinoma. Thyroid 25:107–111
Ling Y, Zhao J, Zhao Y, Li K, Wang Y, Kang H (2020) Role of intraoperative neuromonitoring of recurrent laryngeal nerve in thyroid and parathyroid surgery. J Int Med Res 48(9):1–11
Rice DH, Cone-Wesson B (1991) Intraoperative recurrent laryngeal nerve monitoring. Otolaryngol Head Neck Surg 105(3):372–375
Heikkinen M, Mäkinen K, Penttilä E, Qvarnström M, Kemppainen T, Löppönen H, Kärkkäinen JM (2019) Incidence, risk factors, and natural outcome of vocal fold paresis in 920 thyroid operations with routine pre- and postoperative laryngoscopic evaluation. World J Surg 43:2228–2234
Dralle H, Sekulla C, Haerting J et al (2004) Risk factors of paralysis and functional outcome after recurrent laryngeal nerve monitoring in thyroid surgery. Surgery 136:1310–1322
Author information
Authors and Affiliations
Contributions
Study concepts: Shivakumar Thiagarajan, Parthiban Velayutham, and Devendra Chaukar; study design: Shivakumar Thiagarajan and Parthiban Velayutham; data acquisition: Christina Daniel, Manali Shaikh, Adhara Chakraborthy, Nithyanand Chidambaranathan, and Shikar Sawhney; quality control of data and algorithms: Shivakumar Thiagarajan and Parthiban Velayutham Devendra Chaukar; statistical analysis: Shivakumar Thiagarajan and Parthiban Velayutham; manuscript preparation: all authors; manuscript editing: all authors; and manuscript reviewing: all authors.
Corresponding author
Ethics declarations
Ethics Approval
The study was conducted in adherence to the existing ethical standards. The authors confirm strict adherence to the ethical standards. All received the standard of care for their condition and was as per the ethical standards.
Consent to Participate
No identifying information about participants is available in the article. All patients have given consent for the treatment they have received.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Velayutham, P., Thiagarajan, S., Daniel, C. et al. Importance of Intraoperative Neuromonitoring Parameters in Predicting Temporary Recurrent Laryngeal Nerve Palsy Following Thyroid Surgery for Malignancy. Indian J Surg Oncol 13, 218–224 (2022). https://doi.org/10.1007/s13193-021-01490-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13193-021-01490-7