Abstract
Urothelial carcinoma has a varied and wide histological spectrum posing a diagnostic challenge in H&E examination alone. Immunohistochemical markers like GATA-3 along with other appropriate panel of IHC can be used. However, the percentage positivity and its intensity may vary in different variants and grades of primary and metastatic urothelial carcinoma. To observe the GATA-3 expression patterns in all the grades and different variants of primary and metastatic urothelial carcinomas. It is a prospective and retrospective observational study. All the clinically suspected urothelial carcinoma (UC) during January 2016 to December 2017 were included in the study. Depending on the differential diagnosis considered, immunohistochemistry (IHC) markers including CK7, CK20, p63, AMACR, CDX2, and p16 were done to differentiate UC from other primary carcinomas. The tumors confirmed as UC were analyzed further for GATA-3 expression by Chi-square test. The number of UC in the present study was 126 including 122 (bladder in 107, ureter in 7, renal pelvis in 5, and urethra in 3) primary and 4 metastatic UC (3 in lung and 1 in liver). Age of the patients ranged from 29 to 80 (mean 61.28) years with male/female ratio 4:1. GATA-3 showed positivity in 97 (79.5%) primary UC. GATA-3 was positive in all normal urothelium and non-invasive UC (100%), while it was positive in 69/94 (73.4%) invasive UC including variants. GATA-3 was positive in 35/39 LP invasive (89.74%) and 34/55 (61.81%) MP invasive UC. GATA-3 was positive in 39/40 papillary cases (97.5%) and 45/59 (76.27%) cases of non-papillary UC. GATA-3 showed strong expression in all metastatic UC (100%). GATA-3 expression was seen in 101/126 (80.15%) of UC including primary and metastatic carcinomas and hence was a useful marker in diagnosing UC. The GATA-3 positivity decreased from normal urothelium to UC; low-grade UC to high-grade UC; non-invasive to invasive UC; lamina propria invasive to muscle invasive UC; papillary to non-papillary UC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Majority of invasive urothelial carcinomas (UC) are high grade. Invasive UC has a wide histological spectrum and has a pronounced ability for divergent differentiation, which poses diagnostic challenge [1, 2]. However, characterization of the tumors has diagnostic, therapeutic, or prognostic implications, significantly impacting the management [2]. Invasive high-grade UC can be difficult to differentiate from other high-grade carcinomas because morphology alone is not always specific. In such situations, immunohistochemistry (IHC) plays a vital role in the diagnosis and differential diagnosis of UC [3, 4]. A large number of IHC markers may be expressed by these tumors but there is no ideal marker or established panel to confirm urothelial differentiation [5].
GATA-3 is a zinc finger transcription factor which is important in the differentiation of urothelium, breast epithelium, and subsets of T-lymphocytes [6]. It was discovered as a UC marker by CDNA expression microarray [7]. It is down-regulated in invasive bladder cancer, and hence useful in the distinction between urothelial and prostatic adenocarcinoma.GATA-3 is expressed in 67% to 90% of UC [6, 8]. However, GATA-3 is multi-specific; hence, it has to be interpreted in the context of clinical and morphological features. There are very few studies on the expression of GATA-3 in the different grades of UC [6, 9,10,11,12,13,14,15].
In this study, we aim to study GATA-3 expression patterns in all the grades and variants of UC to determine its efficacy for the diagnosis.
Subjects and Methods
This was both a prospective and retrospective observational study done during the period of January 2016 to December 2017. All the patients with clinically suspected UC and whose samples were obtained from either primary (by transurethral resection of bladder tumor (TURBT), cystectomy, cysto-prostatectomy, cystoprosto-ureterectomy, radical nephrectomy with ureterectomy), or metastatic sites were included in the study. Samples with inadequate tissue/no available paraffin blocks were excluded. The demographic details and clinical presentation were collected from medical records. Imaging details and cystoscopy findings were obtained and the location of the tumor was noted. The samples were fixed in 10% buffered formalin and processed for paraffin-embedded sections and stained with hematoxylin and eosin (H&E). Normal urothelium was included along with tumor wherever possible. The tumors were diagnosed as UC or its variants and were graded according to WHO 2016 criteria [8]. Depending on the differential diagnosis considered, other IHC markers including CK7, CK20, p63, AMACR, CDX2, and p16 were done to differentiate from other primary carcinomas from adjacent structures like prostate, cervix, vagina, rectum, and others. The tumors confirmed as UC based on imageology (for the predominant site of the tumor), morphology, and IHC were analyzed further for GATA-3 expression.
IHC was done either on Leica Bond III or Ventana. Sections were prepared on poly-L-Lysine-coated slide after de-paraffinization followed by antigen retrieval. IHC was performed with GATA-3 antibody which was mouse monoclonal antibody (PathinSitu, US; test clone L50-823; catalog no: CM199; 0.1 ml concentrated; dilution: 1:75). The reactivity of GATA-3 was considered as positive when there was nuclear staining. This expression was based on the proportion of positive cells and the intensity of the color.
Scoring:
The intensity of nuclear positivity was scored from 0 to 3 as follows: score 0: not-stained; score 1: weak; score 2: moderate; score 3: strong. The percentage of positively stained cells for each staining-intensity was estimated in the respective lesions and recorded as: negative: (0) (< 5% of tumor cells stained); 1+ (5–25%); 2+ (26–50%); 3+ (51–75%), 4+ (> 75%).
Statistical Methods
The Student’s Chi square test was calculated to know whether GATA-3 expression correlates with morphological pattern, histological grade, and muscle invasion. p value of < 0.01 was considered statistically significant.
Results
The number of UC in the present study was 126 including 122 primary and 4 metastatic UC. Age of the patients ranged from 29 to 80 (mean 61.28) years with majority (38.88%) in the age group of 61–70 years. There was male predominance (80.8%) with male/female ratio was 4:1.
The site of primary UC was bladder in 107, ureter in 7, renal pelvis in 5, and urethra in 3. In the bladder, the most common site was lateral wall (22.4%). Metastatic UC was present in the lung in 3 and in the liver in one.
The primary UC was classified histologically as non-invasive/invasive; lamina propria (LP) invasive/muscularis propria (MP) invasive; papillary/non-papillary/UC variants; high grade/low grade UC.
Primary Urothelial Carcinoma (n = 122)
The primary UC included 28 non-invasive and 94 invasive carcinoma.
Non-invasive UC (n = 28)
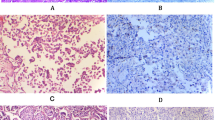
It included 22 (5 low grade and 17 high grade) papillary and 6 (1 low grade and 5 high grade) non papillary carcinoma (Fig. 1).
Invasive UC (n = 94)
In primary invasive UC, 39 were LP invasive and 55 were MP invasive.
There were only 6 low grade UC, while 116 cases were high grade. All the low-grade UC were non-invasive UC (Table 1; Fig. 2).
Invasive UC. a Photomicrograph: invasive papillary UC, high grade, LP invasive, H&E, 4×. b Photomicrograph: invasive urothelial carcinoma, micropapillary variant, H&E, 4×. c Photomicrograph: invasive UC with neuroendocrine differentiation, H&E, 10×. d Photomicrograph: invasive UC with squamous differentiation, H&E, 10×
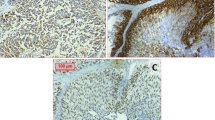
GATA-3 Immunohistochemistry in UC (Table 2; Fig. 3)
GATA-3 Expression in Primary UC (n = 122)
The primary UC on IHC with GATA-3 showed positivity in 97(79.5%).
Comparison of GATA-3 Expression in Normal Urothelium, Non-invasive UC, LP Invasive UC, and MP Invasive UC
GATA-3 was positive in all normal urothelium and non-invasive UC (100%), while it was positive in 69/94 (73.4%) invasive UC including variants. GATA-3 was positive in 35/39 LP invasive (89.74%) and 34/55 (61.81%) MP invasive UC.
Comparison of GATA3 Expression in Non-invasive UC and Invasive UC Including Variants
GATA-3 was positive in all 28 cases of non-invasive UC (100%), whereas it was positive in 69/94 (73.4%) of invasive UC including variants.
Comparison of GATA-3 Expression in Invasive UC and Invasive UC Variants
GATA-3 was positive in 56/71 (78.87%) of invasive UC whereas it was positive in 13/23 (56.52%) variants. The UC variants with GATA-3 positivity included 2 UC with neuroendocrine differentiation and 11 UC with squamous differentiation.
Comparison of GATA-3 Expression in Papillary and Non-papillary UC Excluding Variants
GATA-3 was positive in 39/40 papillary cases (97.5%), whereas it was positive in 45/59 (76.27%) cases of non-papillary UC.
Comparison of GATA-3 Expression in Low-Grade and High-Grade UC
In 122 primary UC cases, 6 cases were low grade and 116 were high grade UC. GATA-3 was strong positive in all low-grade UC (100%), while it was positive in 91 (78.44%) high–grade UC. The expression was strong to moderate in 83 (91.2%) and weak in 8 (8.79%).
GATA-3 Expression in Metastatic UC (n = 04)
GATA-3 showed strong nuclear expression in all metastatic UC (100%). GATA-3 expression between the TURBT and the subsequent cystectomy specimens in recurrent cases was not studied.
GATA-3 expression correlation with the survival outcomes was not done.
Statistical Analysis
GATA-3 was done in all 126 cases. The primary UC (n = 122) on IHC with GATA-3 showed positivity in 97 (79.5%). All metastatic UC (n = 4) were GATA-3 positive (100%).
GATA-3 positivity was higher in normal urothelium (100%), non-invasive UC (100%), and in low grade UC (100%) than invasive UC including variants (73.4%) and high-grade UC (78.44%), also it was higher in invasive UC (78.87%) than in variants (56.52%). The p value was 0.03499 which was not significant at p < 0 .01.
GATA-3 positivity was higher in papillary lesions (97.5%) compared to non-papillary (76.27%). The p value was 0 .003843. This result was significant at p < 0.01.
GATA-3 positivity was higher in LP invasive UC compared to muscle invasive UC. By using Chi-square test, the p value was 0 .002535. This result was significant at p < 0.01.
Discussion
In the present study, the clinicopathological features and GATA-3 expression in the different morphological subtypes of UC were studied. Urothelial carcinomas constitute 90% of urinary bladder tumors and majority occur in men over 50 years and present with hematuria [16, 17]. There was male predominance with the mean age of 61.28 years, and the most common presentation was hematuria in the present study. Urothelial carcinomas can occur from renal pelvis to distal urethra with most common site being urinary bladder, and the lateral walls of bladder being the commonest site (37%) [18, 19]. In the present study, urinary bladder was the most common site for primary UC in 107(87.7%), followed by ureter in 7 (5.73%), renal pelvis in 5 (4.09%), and urethra in 3 (2.45%). In urinary bladder, most common site was lateral wall (22.4%). Imaging was helpful in identifying the primary or predominant site of involvement as well as metastatic or contiguous site involvement in the present study. Metastatic UC was detected in four patients in the present study, three in lung and one in liver similar to earlier reports [1, 17]. In the present study, no significant association of the GATA 3 expression with symptoms (hematuria) or any other clinical parameter was found.
Primary UC (n = 122)
In the present study, primary UC included 28 (22.95%) non-invasive and 94 (77.04%) invasive UC. In invasive UC (n = 94), 55 cases (58.51%) were muscle invasive and 39 (41.48%) were lamina propria invasive tumors. Non-invasive tumors were predominantly papillary (78.57%) and high grade (78.57%). The invasive tumors (including variants) were predominantly muscularis propria invasive (58.5%), non-papillary (56.38%), and high grade (100%). These observations were in agreement with other studies [7, 20]. Higgins et al. reported 208 invasive tumors versus 113 non-invasive tumors [7]. Hasan et al. reported 17.85% of lamina propria invasive and 64.28% of muscle invasive UC [20].
GATA-3 Expression in Urothelial Carcinoma
In the present study, GATA-3 was positive in 101/126 UC (80.15%) including97/122 (79.5%) primary, 4 (100%) metastatic UC. These results were comparable to those from other studies [6, 15, 21].
GATA-3 Expression in Normal, Low–Grade, and High-Grade UC
Mienittien et al. reported strong GATA-3 positivity in the urothelium of renal pelvis, ureter, and urinary bladder. They observed strong positivity of GATA-3 in 49/54 (90.74%) cases of UC and negative in high grade, poorly differentiated, and UC with squamous differentiation [6]. Miyamato et al. showed that GATA3 was positive in 98% of non-neoplastic urothelial tissues [10]. In the present study, all the cases, where normal urothelium was included in the sample (n = 08), were positive for GATA-3. Miyamato et al. showed GATA3 positivity in 86% of urothelial neoplasms including 98% of low-grade and/or non-muscle-invasive tumors versus 72–80% of high-grade and/or muscle-invasive tumors [10].
In the present study of 122 primary UC including 6 low grade and 116 high grade, 97 cases(79.5%) were positive for GATA-3 with moderate to strong nuclear positivity in 89 (91.75%) and weak nuclear positivity in 8 (8.24%). GATA-3 was positive in all low-grade UC (100%). In high-grade UC, GATA-3 was positive in 91 (78.44%) including 83 with strong to moderate expression (91.2%) and 8 (8.79%) with weak expression. The results of the present study were comparable with the study by Clark et al., who showed GATA-3 positivity in 62/72 (86%) high-grade UC. Diffuse and strong nuclear staining was noted in 65% of UC [22]. Hence, a decreasing positivity pattern was seen between the GATA 3 expression and the histopathological high-grade tumors which include parameters of nuclear pleomorphism, mitosis, and necrosis. Tumor-infiltrating lymphocytes correlation with GATA-3 expression was not done.
Chang et al. reported that 80% of primary high-grade UC which showed GATA-3 positivity. All GATA-3 staining was non focal and it was moderate to strong staining in 25 (89%) and weak in 3 (11%) cases [21].
Agarwal et al. reported 77% GATA-3 positivity in UC with moderate to strong GATA-3 expression in low-grade and non-invasive tumors while weak or no expression in high-grade and invasive tumors [15].
GATA-3 Expression Invasive and Non-invasive UC
In the present study of122primary UC, 94 were invasive and 28 were non-invasive. GATA-3 was strong to moderate positive in all 28 (100%) non-invasive UC, whereas GATA-3 expression was seen in 69/94 (73.4%) invasive UC with intensity of expression varying from strong to moderate in 61 (88.4%) and weak in 8 (11.59%) cases.
GATA-3 positivity was 61.81% in muscle invasive versus 89.74% lamina propria invasive UC. GATA-3 was positive in 13/23 (56.52%) variants UC. These results were comparable with the results by Liu et al. who showed GATA-3 positivity in 62 of 72 UC (86%). Seventy-two cases were high-grade with muscularis propria invasion. Diffuse (3+ or 4+) and strong nuclear staining was noted in 65% of cases [11].
Higgins et al. observed GATA-3 positivity in 67% of 321 UC of the bladder including 208 invasive and 113 non-invasive tumors [7].
Expression of GATA-3 in Metastatic UC
There were six cases in our study which had suspicion of metastatic UC. After IHC, four cases were resolved as metastatic UC (3 lung, 1 liver) and two cases were metastatic non-UC. In all the four cases, GATA-3 was strong positive (100%). The most common problem is differentiating spread of UC to the lung versus a primary pulmonary SCC. Chang et al. performed GATA-3 IHC in on 15 pulmonary UC metastases and 25 SCCs of the lung and 5 pulmonary non small cell cancer with squamous features. Twelve (80%) of the metastatic UC to the lung were positive for GATA3 with 11 cases showed diffuse moderate or strong staining and one case showed focal moderate staining. None of the pulmonary SCC or non small cell cancers with squamous features were GATA3 positive [21].
GATA-3 expression between the TURBT and the subsequent cystectomy specimens in recurrent cases was not studied.
GATA-3 expression correlation with the survival outcomes was not done.
Conclusion
GATA-3 expression was seen in 101/126 (80.15%) of UC including primary and metastatic carcinomas and hence was a useful marker in diagnosing UC. The GATA-3 positivity as well as intensity decreased from normal urothelium to UC; low grade UC to high grade UC; non-invasive to invasive UC and invasive UC to variants of UC (statistically not significant). The expression decreased from lamina propria invasive to muscle invasive UC and papillary to non-papillary UC (statistically significant). GATA-3 expression could help in the diagnosis of UC in metastatic sites in lung and liver.
Abbreviations
- UC:
-
Urothelial carcinoma
- LP:
-
Lamina propria invasive
- MP:
-
Muscle invasive
References
Moch H, Humphrey PA, Ulbright TM, Reuter VE (eds) (2016) World Health Organization Classification of Tumours of the Urinary system and Male Genital organs, 4th edn. IARC Press, Lyon, pp 78–80
Amin MB (2009) Histological variants of urothelial carcinoma: diagnostic, therapeutic and prognostic implications. Mod Pathol 22(Suppl 2):96–118
Coleman JF, Hansel DE (2009) Utility of diagnostic and prognostic markers in urothelial carcinoma of the bladder. Adv Anat Pathol 16:67–78
Hodges KB, Lopez-Beltran A, Emerson RE, Montironi R, Cheng L (2010) Clinical utility of immunohistochemistry in the diagnoses of urinary bladder neoplasia. Appl Immunohistochem Mol Morphol 18:401–410
Amin MB, Trpkov K, Lopez-Beltran A, Grignon D (2014) Best practices recommendations in the application of immunohistochemistry in the bladder lesions: report from the International Society of Urologic Pathology consensus conference. Am J Surg Pathol 38(8):e20–e34
Miettinen M, McCue PA, Sarlomo RM, Janusz R, Czapiewski P, Krzysztof W et al (2014) GATA3: a multispecific but potentially useful marker in surgical pathology: a systematic analysis of 2500 epithelial and nonepithelialtumors. Am J Surg Pathol 38:13–22
Higgins JP, Kaygusuz G, Wang L, Montgomery K, Mason V, Zhu SX et al (2007) Placental S100 (S100P) and GATA3: markers for transitional epithelium and urothelial carcinoma discovered by complementary DNA microarray. Am J Surg Pathol 31:673–680
Gonzalez R, Wang J, Kraus T, Sullivan H, Adams AL, Cohen C (2013) GATA-3 expression in male and female breast cancer comparison of clinicopathologic parameters and prognostic relevance. Hum Pathol 44:1065–1070
Chuang AY, DeMarzo AM, Veltri RW, Sharma RB, Bieberich CJ, Epstein JI et al (2007) Immunohistochemical differentiation of high grade prostate carcinoma from urothelial carcinoma. Am J Surg Pathol 31:1246–1255
Miyamoto H, Izumi K, Yao JL, Li Y, Yang Q, McMahon LA et al (2012) GATA binding protein 3 is down-regulated in bladder cancer yet strong expression is an independent predictor of poor prognosis in invasive tumor. Hum Pathol 43:2033–2040
Liu H, Shi J, Wilkerson ML (2012) Immunohistochemical evaluation of GATA3 expression in tumors and normal tissues: a useful immunomarker for breast and urothelial carcinomas. Am J Clin Pathol 138:57–64
Ellis CL, Chang AG, Cimino MA, Argani P, Youssef RF, Kapur P et al (2013) GATA-3 immunohistochemistry in the differential diagnosis of adenocarcinoma of the urinary bladder. Am J Surg Pathol 37(11):1756–1760
Siddiqui MT, Seydafkan S, Cohen C (2014) GATA3 expression in metastatic urothelial carcinoma in fine needle aspiration cell blocks: a review of 25 cases. Diagn Cytopathol 42(9):809–815
Bezerra SM, Lotan TL, Faraj SF, Karram S, Sharma R, Schoenberg M et al (2014) GATA3 expression in small cell carcinoma of bladder and prostate and its potential role in determining primary tumor origin. Hum Pathol 45(8):1682–1687
Agarwal H, Babu S, Rana C, Kumar M, Singhai A, Shankhwar SN, Singh V, Sinha RJ (2019) Diagnostic utility of GATA-3 immunohistochemical expression in urothelial carcinoma. Indian J Pathol Microbiol 62:244–250
Kumar V, Abbas AK, Aster JC (2015) lower urinary tract and male genital system. Robbins & Cotran Pathologic Basis of Disease, 9th edn. Saunders/Elsevier, Philadelphia, pp 961–969
Rosai J (2012) In: 10th (ed) Urinary tract. Rosaiand Ackerman’s surgical pathology. Elesevier, Mosby, pp 1317–1337
Stephenson WT, Holmes FF, Noble MJ, Gerald KB (1990) Analysis of bladder carcinoma by subsite.Cystoscopic location may have prognostic value. Cancer. 66(7):1630–1635
Naeem A, Naseem N, Anwar S, Butt S, Nagi AH (2015) Clinico-pathological pattern, classification and staging of urinary bladder carcinomas--a five years experience at a tertiary care hospital in Central Punjab. J Ayub Med Coll 27(1):131–134
Hasan MS, Imtiaz F, Hasan MS (2007) Frequency of transitional cell carcinoma in local suburban population of Karachi. J Liquat Uni Med Health Sci 2:83–85
Chang A, Amin A, Gabrielson E, Illei P, Roden RB, Sharma R et al (2012) Utility of GATA3 immunohistochemistry in differentiating urothelial carcinoma from prostate adenocarcinoma and squamous cell carcinomas of the uterine cervix, anus, and lung. Am J Surg Pathol 36:1472–1476
Clark BZ, Beriwal S, Dabbs DJ, Bhargava R (2014) Semiquantitative GATA-3 immunoreactivity in breast, bladder, gynaecologic tract, and other cytokeratin 7-positive carcinomas. Am J Clin Pathol 142:64–71
Acknowledgments
Nil.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Naik, M., Rao, B.V., Fonseca, D. et al. GATA-3 Expression in all Grades and Different Variants of Primary and Metastatic Urothelial Carcinoma. Indian J Surg Oncol 12 (Suppl 1), 72–78 (2021). https://doi.org/10.1007/s13193-019-01026-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13193-019-01026-0