Abstract
The objective of this study was to study the risk factors, management protocols, and the outcome of vulvar cancer cases over a period of 2 years in a tertiary care hospital. This is a case series of early-stage vulvar cancer in the Department of Surgical Oncology in BL Kapur Superspeciality Hospital from Jan 2016 to date. Five patients with histologically proven diagnosis of early-stage vulvar cancer were included. The mean age for the diagnosis of vulvar cancer was 58 years and the peak incidence was seen in postmenopausal age group. All of the cases were squamous cell carcinomas in stage IB except one which was a basisquamous variant. All cases were treated primarily with surgery and vulvar flap reconstruction. Adjuvant therapy was not given in any case. Cases were followed from 6 months to date, and no recurrence noted. The limitations of the study were rarity of disease and less number of cases. As all the cases in our study were in early stage of disease (stages I and II), surgical treatment in the form of modified radical vulvectomy with B/L inguinofemoral lymph node dissection and oncoplastic procedure was the treatment modality chosen for all the patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vulvar cancer is uncommon, representing about 4% of malignancies of the female genital tract and 0.6% of all cancers in women [1]. Squamous cell carcinoma accounts for about 90% of all primary vulvar malignancies.
Squamous cell carcinoma of the vulva is predominantly a disease of postmenopausal women. The mean age at diagnosis is about 65 years [2]. Most squamous carcinomas of the vulva occur on the labia majora and minora (60%), but the clitoris (15%) and perineum (10%) may be primary sites [2]. Treatment modalities depend on the stage of the disease and include surgery, radiotherapy (RT), and chemotherapy (CT) [3].
Being one of the less common types of genital cancer, there is a scarcity of literature about the changing trends in the methods of management, outcome of disease, and long-term survivals. Herein, we report a case series of vulvar cancer cases over a period of 2 years. This includes treatment modalities, and survival rates of vulvar cancer in our institute.
Methodology
This is a case series of early-stage vulvar cancer in the Department of Surgical Oncology in BL Kapur Superspeciality Hospital from Jan 2016 to date. Five patients with histologically proven diagnosis of early-stage vulvar cancer were included.
Information including the age, symptoms, size of tumor, location of tumor, surgical procedure performed, margin status, postoperative complications, recurrence, and survival were collected and analyzed and compared with the published data.
Result
All patients were postmenopausal with mean age at diagnosis of 58 years. Four of the five patients were multipara and one nullipara. One patient was on antiretroviral therapy for HIV, and four of the five patients were diabetic. Main presenting symptom was pruritis vulvae along with ulceroproliferative growth on vulval region. Duration of symptoms ranged from 2 months to 2 years.
Labia Majora is the most common site of involvement in our study, with majority of the lesions located centrally with involvement of clitoris. Two patients had multiple patches of leukoplakia which on biopsy showed severe dysplasia. The size of tumor ranged from 2 to 4 cm.
All patients were routinely investigated along with a whole body PET CT which was an integral part of work up; no patient had clinically palpable groin nodes. All five patients were planned for surgical management, three had modified radical vulvectomy with B/L inguinofemoral lymph node dissection and two had modified radical anterior hemivulvectomy with B/L groin node dissection taking a tumor margin of 2 cm in each case. Vulval reconstruction was done in all patients with perforator-based internal pudendal artey fasciocutaneous flap. The defects included only the skin and subcutaneous tissue loss; hence, no consideration was given to muscle based flap, e.g., grasilis flap.
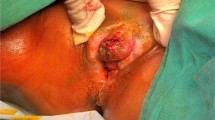
On final histopathology, all were in stage IB with squamous cell carcinoma as histological type with keratinizing variant except one whose histology was of a basisquamous variant. Depth of tumor invasion ranged from 0.5 to 0.9 cm. One patient had grade II disease and the rest had grade I histology. Of the two patients who had leukoplakic patches, one had severe dysplasia and other had bowenoid paulosis on final histology. Lymphovascular space invasion, positive margins, or positive lymph nodes were not seen in any case. None of the patients received adjuvant treatment. The preoperative lesion photograph, postoperative photograph, and post reconstruction photograph are shown in Figs. 1, 2, and 3.
Patients are followed up to date. All five patients had seroma collection in the inguinal region which was managed by compression and seroma aspiration, one patient had flap edema which was managed conservatively, and one patient had partial necrosis of reconstructed vulval flap along with DVT of the right leg which was managed medically. Inguinal flap necrosis was seen in one case which was managed by secondary suturing, and one patient had groin hematoma on the start of anticoagulant therapy which was managed by compression dressing and it subsided subsequently.
No recurrence or mortality reported till 6 months to 2 years of follow-up. As in all patients, vulval reconstruction was done with perforator-based fasciocutaneous flap; there are no complications like urethral stricture, vaginal stenosis, or perineal contracture. The results are tabulated in Table 1.
Discussion
Vulval cancer accounts for 4% of gynecological malignancies [1]. Based on data from the SEER database, 5-year survival ranges from 86% for stage I/II to 57% for stage III/IVA and finally to 17% with distant metastasis [4]. Ninety percent of vulvar cancers are of squamous cell carcinoma (SCC), other rare histologies are melanoma, extramammary Paget’s disease, Bartholin gland adenocarcinoma, verrucous carcinoma, basal cell carcinoma, and sarcoma [4]. Risk factors include increasing age, infection with HPV, cigarette smoking, inflammatory conditions of the vulva, and immunodeficiency. Most vulvar neoplasias are diagnosed at early stage [5].
Most squamous carcinomas of the vulva occur on the labia majora, but the labia minora, clitoris, and perineum also may be primary sites. A study from one university hospital in Germany reported a recent change in location of the tumor, with 37% of cases occurring between the clitoris and urethra [6]. Patients with HPV-positive tumor present with multifocal lesions and can have concurrent cervical neoplasia as compared to patients with HPV-negative tumors who present with unifocal tumor. Many patients are asymptomatic or may present with pain, pruritis, ulceration, or lump [1].
The management of vulvar cancer has evolved from primary surgical approach to chemoradiation. The surgical management has become more conservative due to the well-recognized morbidity with radical vulvectomy and extensive groin dissection and psychosexual sequelae. In this study, all the cases were early stage and so were primarily treated with surgery. The goal of primary tumor resection is complete removal with a 1–2 cm margin, and the deep margin should be the inferior fascia of the urogenital diaphragm, which is co-planar with the fascia lata and the fascia over the pubic symphysis [7].
According to the International Federation of Gynecology and Obstetrics guidelines, treatment of invasive vulvar cancer (stage IA) is wide local excision. Groin dissection is not necessary for such early lesions [8].
According to Luchini et al., lymph node metastasis is considered the most important prognostic factor and extracapsular spread has been linked to poorer prognosis [9]. Additional factors predictive of recurrence include depth of invasion, tumor thickness, and LVSI. In our study, all these factors were negative.
Resection of the primary tumor and groin lymph node dissection is recommended using separate groin and vulvar incisions (triple incision technique) to reduce morbidity and to improve primary healing [8]. This technique was utilized in all cases with groin dissection. Appropriate groin treatment is the single most important factor in reducing mortality from early vulvar cancer [8].
All patients with FIGO stage 1B or stage II lesions should have at least an ipsilateral inguinofemoral lymphadenectomy. It is recommended that both inguinal and femoral nodes should be removed, and appropriate dissection includes removal of at least 8–10 nodes [10].
The incidence of positive contralateral nodes in patients with small lateral lesions and negative ipsilateral nodes is less than 1%, so unilateral groin dissection is appropriate for such lesions [10].
Bilateral groin dissection should be performed for midline tumors and for those involving the anterior labia minora [10]. Large lateral tumors should probably also have bilateral dissection and definitely if the ipsilateral nodes are positive [10]. In our study, all patients had disease in close proximity to the midline, so B/L inguinofemoral lymphadenectomy was done.
Sentinel node excision is being increasingly practiced in many centers following the European multicenter observational study on sentinel node detection [11]. The study (GROINSS-V) involved 403 women with a unifocal tumor confined to the vulva less than 4 cm in diameter, stromal invasion more than 1 mm, and clinically negative lymph nodes [11]. Lymphadenectomy was omitted in sentinel node-negative women. Groin recurrences occurred in 2.3% of patients, with a median follow-up of 35 months.
The Gynecologic Oncology Group demonstrated superior results for pelvic and inguinal radiation compared with pelvic node dissection for patients who had an inguinal lymph node dissection with findings of grossly positive or more than one positive node [11]. A recent retrospective, multicenter German study also reported improved survival for patients with positive groin nodes who received adjuvant radiotherapy directed at the groins (positive/negative other fields) [12].
Patients with primary tumors extending beyond the vulva or bulky positive groin nodes are considered to have advanced vulvar cancer. For such patients, multimodality treatment planning is particularly important [13]. Chemoradiation has been used extensively for large lesions if surgical resection would damage central structures (anus, urethra) [13].
The major immediate morbidity is related to groin wound infection, necrosis, and breakdown.
With the separate-incision approach, the incidence of wound breakdown can be reduced to about 44%; major breakdown occurs in about 14% of patients). Other early postoperative complications include urinary tract infection, seromas in the femoral triangle, deep venous thrombosis, pulmonary embolism, myocardial infarction, and hemorrhage [14].
One major late complication is chronic lymphedema, which occurs in about 30% of patients [14].
The limitations of our study were less number of patients and a smaller follow-up period.
Conclusion
We acknowledge the limitations of our study. We had less number of patients with small follow-up period; however, the rarity of vulvar cancer makes it difficult to obtain a larger number.
As all the cases were in early stage of disease (stages I and II), surgical treatment in the form of modified radical vulvectomy with B/L inguinofemoral lymph node dissection and oncoplastic procedure was the treatment modality chosen for all the patients. The use of local flaps to overcome any tissue loss results in better functional and cosmetic outcomes with respect to sexual function and improved body image.
References
Siegel R, Ward E, Brawley O, Jemal A (2011) Cancer statistics, 2011. CA Cancer J Clin 61:212–236 http://cacancerjournal.org
Berek JS, Friedlander M, Hacker NF (2009) Epithelial ovarian, fallopiantube and peritoneal cancer. In: Berek and Hacker’s gynecologic oncology. 5th ed. Lippincott Williams & Wilkins, Philadelphia, pp 443–508
Royal College of Obstetrics and Gynaecology (2014) Guidelines for the diagnosis and management of vulvar carcinoma. British Gynaecological cancer society: Royal College of Obstetrics and Gynaecology
SEER Cancer Statistics Factsheets: Vulvar cancer. Bethesda, MD: National Cancer Institute, April 22, 2016
Stroup AM, Harlan LC, Trimble EL (2008) Demographic, clinical and treatment trends among women diagnosed with vulvar cancer in the United States. Gynecol Oncol 108:577–583
Hacker NF (2009) Vulvar cancer. In: Berek JS, Hacker NF (eds) Berek and Hacker’s gynecologic oncology. 5th ed. Lippincott Williams & Wilkins, Philadelphia, p 565
Chan JK, Sugniyama V, Pham H et al (2007) Margin distance and other clinic-pathological prognostic factors in vulvar carcinoma. A multivariate analysis. Gynecol Oncol 104:636–641
Singh N, Negi N, Srivastava K, Agarwal G (2016) A cohort study of vulvar cancer over a period of 10 years and review of literature. Indian J Cancer 53(3):412–415
Luchini C, Nottegar A, Solmi et al (2016) Prognostic implications of extragonadal extension in node positive squamous cell carcinoma of vulva: a systematic review and meta analysis. Surg Oncol 25:60–65
Hacker NF, Eifel PJ, van der Velden J (2015) Cancer of the vulva. Int J Gynecol Obstet. 131(S2)
Van der Zee AG, Oonk MH, De Hullu JA, Ansink AC, Vergote I, Verheijen RH et al (2008) Sentinel node dissection is safe in the treatment of early stage vulvar cancer. J Clin Oncol 26(6):884–889
Mahner S, Jueckstock J, Hilpert F, Neuser P, Harter P, de Gregorio N et al (2015) Adjuvant therapy in lymph node positive vulvar cancer: the AGO-CaRT-1 study. J Natl Cancer Inst 107(3):dju426
Beriwal S, Coon D, Heron DE, Kelley JL, Edwards RP, Sukumvanich P, Zorn KK, Krivak TC (2008) Preoperative intensity-modulated radiotherapy and chemotherapy for locally advance vulvar cancer. Gynecol Oncol 109(2):291–295
Gaarenstroom KN, Kenter GG, Trimbos JB, Agous I, Amant F, Peters AAW, Vergote I (2003) Postoperative complications after vulvectomy and inguinofemoral lymphadenectomy using separate groin incisions. Int J Gynecol Cancer 13:522–527
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Informed consent was taken from each patient and attendant.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lakhwani, P., Agarwal, P., Mahajan, J.A. et al. Surgical Management of Carcinoma Vulva—Case Series and Review of Literature. Indian J Surg Oncol 10, 324–328 (2019). https://doi.org/10.1007/s13193-019-00887-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13193-019-00887-9