Abstract
Despite efforts to increase the diversity of cancer clinical trial participants, African Americans are still underrepresented. While perceptions of participation have been studied, the objective of this study was to compare perceptions and decisional conflict towards clinical trials among African American cancer patients who have and have not participated in clinical trials to identify key areas for intervention. Post hoc analysis also looked at whether they had been asked to participate and how that group differed from those who did. Forty-one African American cancer patients were surveyed at two urban cancer centers and asked to agree/disagree to statements related to clinical trials perceptions (facilitators, barriers, beliefs, values, support, and helpfulness), and complete the O’Connor Decisional Conflict Scale. Independent-samples t tests compared participants by clinical trials participation status; 41% had participated in a clinical trial. Results revealed significant perceptual differences among the groups in three main areas: helpfulness of clinical trials, facilitators to participate in clinical trials, and barriers to participating in clinical trials. Post hoc analysis indicated that those who were not asked about clinical trials and had not participated differed significantly in all areas compared with participants. Additionally, clinical trial participants reported significantly lower decisional conflict in most items compared with both those who had and had not be asked to participate. These differences can give practitioners clues as to how to bridge the gap from non-participator to participator. Messages could then be infused in the clinician–patient dyad when introducing and discussing clinical trials, potentially providing a more effective strategy for communicating with African American patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cancer clinical trials are essential for testing the safety and effectiveness of potential treatment options and have introduced many of today’s standard therapies for cancer [1]. However, engaging patients to participate in clinical trials continues to be challenging; a recent systematic review estimates that only 8% of all adults diagnosed with cancer ever enroll in a clinical trial [2]. Under-enrollment presents an even greater challenge among ethnic and racial minorities, despite the requirements set forth by the 1993 NIH Revitalization Act for the inclusion of minorities in cancer clinical trials [3, 4]. This is especially true for African Americans. According to one estimate by the American Cancer Society of cancer patients registered in the Clinical Trials Matching Services, enrollment rates were substantially lower among African Americans than for any other racial/ethnic group [5]. Low participation in clinical trials among African Americans can result in failure to capture the effect of the proposed treatment in this sub-group which might lead to suboptimal assessment of therapies for African Americans [6]. Additionally, different racial/ethnic groups might have genetic variations that could affect the molecular targets of treatments, limiting the generalizability of and holding incorrect assumptions about effectiveness found in research done with Caucasian participants [7]. Because African Americans already carry a higher cancer burden, including mortality and diagnosis at advanced disease states compared with Caucasians, this disparity and inequality may be exacerbated by not participating in clinical trials [8].
Understanding perceptions towards cancer clinical trials and the decision-making process to participate may assist researchers in creating more effective recruitment strategies and clinicians in more appropriately addressing concerns, misperceptions, and needs when speaking to African American patients. An analysis of qualitative and quantitative studies grouped participation barriers into: protocol-related, patient-related, or physician-related [9]. Concerns with trial settings, a dislike of randomization, general discomfort with the research process, complexity and stringency of the protocol, presence of a placebo, potential side effects, fear of negative effects on the relationship with physicians, and physician’s attitudes towards the trial were some of the most common reasons cited [10]. This research study sought to discern potential differences in clinical trial perceptions and knowledge among African Americans who had and had not participated in a clinical trial, recognizing the heterogeneity of this underrepresented population. Additional post hoc analysis also looked at potential differences in those who had not participated but also had never been asked to participate. The goal was to gain a more nuanced understanding of potential barriers and facilitators of their participation in clinical trials and whether perceptual differences of clinical trials, as well as decisional conflict to participate in clinical trials were different between these groups of African American cancer patients.
Methods
Measures
Extensive literature review and findings from in-depth interviews with African American cancer patients [11] informed the development of a cross-sectional survey which included 64 items addressing a broad range of statements regarding perceptions of clinical trials (helpfulness, benefits, barriers, value), patient support, and beliefs about healthcare providers. These items asked participants how much they agreed or disagreed on a 0–10 scale (0 = strongly disagree, 10 = strongly agree) with statements, such as “I believe the benefits of being in a clinical trial outweigh the possible side effects,” and asked to rate their agreement (for all perceptual statements, see Table 2). In addition, the survey items included 11 standardized questions (e.g., demographics, technology use, health literacy, and cancer experience).
Participants were also asked to complete a modified version of the low-literacy version of O’Connor’s validated Decisional Conflict Scale [12], typically utilized to assess decisional conflict at a moment of decision. The original 10-item scale is composed of four subscales: uncertainty (2 items), feeling informed about options (3 items), values clarity (2 items), and support (3 items). Because some patients in our sample may never have had to actually decide on whether to participate in a clinical trial, we omitted four items that presume the respondent has made a decision. This left six items including the values clarity subscale (see Table 3). Responses are scored 0 = agree, 2 = uncertain, and 4 = disagree. The total score of the original scale is calculated by summing, dividing by the number of items, and multiplying by 25. The possible total score ranges between 0 and 100, with higher total scores denoting higher decisional conflict. Scoring of the modified scale in this study followed the exact original scoring method [12]. Three consultants in health communication and health disparity, along with members of a community advisory council, reviewed the survey instrument for content and face validity.
Participants
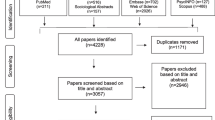
Participants were recruited from two cancer centers in the Northeast United States representing a diverse socio-economic population of African Americans. Patients were eligible if they (1) were 21 years or older; (2) self-identified as African American; and (3) were a current patient at one of the sites. To ensure that a balanced combination of patients who had and had not participated in a clinical trial, there were additional eligibility criteria so that we recruited an equal number for each of the following groups of patients: (1) patients who were currently enrolled in a clinical trial or had been in a clinical trial within the past 9 months based on medical records and (2) patients who had not participated in a clinical trial and were newly diagnosed or had a recurrence within the past 9 months (which may include those who declined or were never asked about a clinical trial).
In one site, oncologists and clinical trial nurses were asked to provide patients who they deemed eligible with written information about the study during their scheduled appointments. Eligible patients were then asked by their oncologists and nurses about their interest to participate in the study. Study research staff called interested patients to screen for eligibility, obtain verbal consent, and schedule the survey (either in person or by telephone) prior to their next scheduled appointment. At the second site, patients’ clinical trial participation statuses were identified using the hospital’s database. Research staff then reviewed the medical oncology appointment schedules to identify eligible patients with an appointment scheduled within the next 6 weeks. Consent for contact was obtained from patients’ treating physicians verbally or in-writing to research staff. Once consent was granted, research staff called patients by phone and provided information about the study. If interested, participants were scheduled to take the survey (either in person or by telephone) prior to the next scheduled appointment. Scheduling accommodations were offered for all patients as needed. The survey was designed to be completed in less than 30 min. Questions in the survey were organized by content, with similar items grouped together (e.g., facilitators followed by barriers). All patients who participated provided written consent and received a $15 gift card. The study was approved by the Institutional Review Boards at the two sites. Approval allowed us to only capture data from those who participated in the study.
Statistical Analyses
Independent-samples t tests were conducted to assess the significant differences in perceptions of clinical trials and decisional conflict between participants by clinical trial participation status (had or had not participated), with p = 0.05. Cohen’s d was used to calculate the effect size of the standardized difference between the means, where d = 0.2, d = 0.5 and d > 0.8 denoting small, medium, and large effect sizes, respectively [13]. Given that the survey contained a question regarding whether or not a clinical trial was discussed with participants as a treatment option, we were able to determine that there was a small subgroup that had not participated in a clinical trial but had been asked. Therefore, we also conducted additional post hoc analysis using three groups: (1) those who were asked to participate in clinical trials and did; (2) those who were asked to participate in clinical trials but declined; and (3) those who did not participate in clinical trials and were not asked to participate. To account for the smaller cell size in each group in the post hoc analysis, a non-parametric Kruskal–Wallis one-way analysis of variance was used to assess perceptions and attitudes. Bonferroni correction method was used to account for multiple tests. All analyses were performed using SPSS version 24.0 [14].
Results
Sample Description
A total of 41 cancer patients participated. A little over half (51%) were female. The average age was 60.5 (SD = 12.6), and 46% of the total sample had high school or less education. A comparison of demographic characteristics was conducted with the three groups, and the only significant difference was in having a smart phone (Table 1) with only 12% of those in the “not asked, not participated” group having a smart phone.
Statistical Results
Independent-samples t tests of the two primary groups (had and had not participated) revealed significant perceptual differences between individuals who had participated in a clinical trial and those who had not in three main areas: helpfulness of clinical trials, facilitators to participating in clinical trials, and barriers to participating in clinical trials (Table 2). There were no differences found in perceived patient support, general beliefs about health, or overall perceived value of clinical trials. In the helpfulness of clinical trials, participants who had participated more strongly agreed on one item: “My doctor gave me enough information to make a decision about being part of a clinical trial” (M = 7.8 vs. 3.8; d = 1.1; p = 0.001).
Of the seven statements related to facilitators of participating in clinical trials, those who had participated in clinical trials were significantly more likely to agree with five. These included “I have a better chance of living longer if I am part of a clinical trial” (M = 7.4 vs. 4.0; d = 1.1; p = 0.002), “Being part of a clinical trial improves my quality of life” (M = 6.3 vs. 4.2; d = 0.7; p = 0.041), “I believe the benefits of being in a clinical trial outweigh the possible side effects” (M = 6.7 vs. 4.4; d = 0.76; p = 0.016), “Being part of a clinical trial offers the best treatment available for my cancer” (M = 7.0 v. 4.5; d = 0.79; p = 0.015), and “If my doctor said a clinical trial was the best option for me, I would follow their advice” (M = 8.7 v. 6.1; d = 0.9; p = 0.008).
Non-clinical trial participants were significantly more likely to agree with five of the 17 statements related to barriers to clinical trial participation. These included “I am afraid of being part of a clinical trial” (M = 5.0 vs. 3.1; d = 0.77; p = 0.02), “I am worried that my health insurance won’t pay for me to be part of a clinical trial” (M = 5.8 v. 2.5; d = 1.04; p = 0.006), “I believe that taking part in a clinical trial will make me sicker than I am now” (M = 3.7 vs. 1.7; d = 0.78; p = 0.019), and “No one talked to me about being part of a clinical trial” (M = 5.3 vs. 0.6; d = 1.32; p = 0.001). Finally, this group more strongly agreed that “I’m too upset about my cancer diagnosis to think about being part of a clinical trial” (M = 3.8 vs. 1.2; d = 0.81; p = 0.016).
Independent-samples t tests for the modified O’Connor’s Decisional Conflict Scale revealed that clinical trial participants reported significantly lower decisional conflict in four of the six items, including benefits of being in a clinical trial (M = 0.7 vs. 2.2; d = 0.62; p = 0.001), risks of being in a clinical trial (M = 1.1 vs. 2.6; d = 0.67; p = 0.001), support from others to make a choice (M = 0.6 vs. 1.6; d = 0.41; p = 0.028), and clarity about which benefits are most important (M = 0.9 vs. 2.1; d = 0.24; p = 0.021). Overall decisional conflict was also significantly lower (M = 19 vs. 46; d = 1.21; p = 0.001) (Table 3). Similarly, for the values clarity subscale, clinical trial participants also reported significantly lower conflict than non-participants (M = 27.9 vs. 54.4; d = 0.73, p = 0.030) (Table 3).
The post ad hoc analysis (using Kruskal–Wallis test) of perceptions and attitudes in the three groups (participated, did not participate, did not participate and were not asked) revealed significant differences regarding facilitators, barriers, and decisional conflict. The post hoc analysis showed that these differences were primarily driven by differences in clinical trial participants versus non-participants who were not asked to participate in a clinical trial. This was especially true in perceived concerns or barriers with participating, with those who had not been asked to be significantly more likely to agree that they were afraid of being in a clinical trial (p = 0.042) and they worried more their health insurance will not pay for clinical trials (p = 0.008). However, not knowing the benefit of being in a trial was significantly different between those who participated versus those who were asked to participate and declined (p = 0.47) (Table 4).
Discussion
This study was unique in exploring the perceptions of African American patients who had and had not participated in cancer clinical trials to more fully explore differences and similarities to guide the development of more salient interventions at both the patient and provider levels. Post hoc analysis then also allowed us to investigate possible differences in those who had not been asked to participate in a clinical trial. As described in the ConNECT Framework, “within-group disparities are less often measured and therefore inadequately understood” [15, p.5], and although the participation rate in clinical trials is lower in African Americans than most other racial and ethnic groups, there are African Americans who do participate; understanding their attitudes, perceptions and decision making may provide insights to increase participation overall.16, 17
Our findings highlight that disparities in clinical trial participation may be even more pronounced for African Americans with less education. Those who had not participated were two times more likely to have only a high school education or less compared with trial participants (61% vs. 29%, respectively). Moreover, although we recruited African American patients from both a more suburban comprehensive cancer center and an urban safety net hospital, we found no significant difference in trial participation between the sites (p = .5, not shown in tables). Our findings suggest that education may be more highly associated with participation than other socio-economic factors and interventions need to be accessible (more video based, limited medical jargon and use of plain language, addressing myths and perceptions) to address the needs of those with more limited education [e.g., ]. The only other demographic variables significant between the two primary groups were related to technology. Although the overwhelming majority of both groups had mobile phone access, similar to studies showing the proliferation of mobile phone use [18], those who had participated were over three times more likely to own a smart phone, which may indicate that those who had participated were at a higher socioeconomic status and suggests that technology-based (e.g., DVD) and web-based interventions compared with smartphone apps may be more appropriate to reach this group of patients [19, 20]. This disparity was also seen in the post hoc analysis where the group who had not been asked to participate were significantly less likely to own a smartphone.
One of the major barriers that are often cited in studies of clinical trial participation of racial and ethnic groups is the lack of trust in research and the medical community [21]. However, the results of our study show no difference between the primary groups or in the post hoc analysis on beliefs that they would “be a guinea pig” if they participated in a trial, that important information would be withheld, that doctors mislead patients, or agreement that they lack trust in medical researchers. The groups reported that they had high levels of trust of their treating doctor. This could be a reflection of the institutions where the survey was conducted, but it is an important finding that should be tested in other diverse African American populations. Another barrier often cited [2] is African Americans may not believe in the value of clinical trials. In this study, those who had participated in a clinical trial reported that they felt the trial gave them a better chance of living longer and improved their quality of life. They also believed that the benefits of participating outweighed the risks. More qualitative research is needed to more fully understand the foundation of these values and beliefs to guide the development of interventions and messages to facilitate discussion with patients and improve informed decision making.
Significantly, findings of this study highlight that those who had not participated expressed more overall fear of clinical trials and concerns that the trial will make them sicker. They also reported that they were overwhelmed with their diagnosis and they had concerns about insurance coverage if they participated. These are the types of concerns that may not be routinely addressed in clinical trial materials or discussions and where patient-focused interventions, such as mychoice [11], and patient navigation could be impactful approaches to communicate strategies and address these individual concerns, especially for those with more limited education and socio-economic statuses.
As a retrospective study, it might be expected that those patients who had participated in a clinical trial had lower decisional conflict scores and that these patients understood the risks and benefits. We do not know if these attitudes preceded their participation or are a reflection of having made a decision to participate. However, there was a large effect size in decision conflict between the primary groups showing that those who had participated were more likely to indicate that their physician gave them adequate information which may have facilitated a more informed decision-making process. Clinical trial participation is a patient-centered choice and these findings suggest that clinical trial education needs to directly address the benefits, risks, and individual patient’s values to ensure an informed decision-making process.
Obviously, the role of providers in clinical trial education and patient decision making is critical [22, 23] and strongly supported by these results. African American patients often cite that they were not asked and would consider if offered [24]. What is not known is if these patients are not in institutions that participate in clinical trials or if there is actually an implicit bias in healthcare that fails to adequately recruit African American patients. In our sample, which came from a large cancer center and an affiliated hospital, only a small proportion of our sample (26%) of those who had not participated stated they had been asked, but clearly this significantly affected those participants’ perceptions of clinical trials overall. Respondents who had participated in a clinical trial were more likely to agree that “their doctor gave them enough information to make a decision,” were more likely to say they had support in making a decision, were more clear about benefits, risks and side effects, and were more likely to state that they would follow the advice of their physician if they recommended a trial. The role of physicians and others in the healthcare team in providing information about clinical trials as a treatment option at diagnosis and throughout the treatment process is highly valued by patients and facilitates the decision-making process if a trial is offered. Moreover, these encounters need to go beyond the didactic information (e.g., what is a clinical trial or details of a specific trial) and address these more patient-centered concerns.
Our findings support a number of recommendations by ASCO-NCI at both the patient and provider levels [25]. As recommended, patients’ points of view need to be represented, and intervention strategies should be multi-faceted, including educational and marketing tools, patient navigation programs, and community partnership [26]. Providers should be offered training on the unique concerns and issues facing underrepresented groups and the use of information technology to identify patients. The potential for shared learning between providers and patients is important to explore, and our research indicates that patients do value their provider’s viewpoint and look to them for more information about clinical trials.
Finally, Dennicoff and colleagues point to the need for future research in clinical trial decision making that focuses on different demographic groups [25]. Again, our findings highlight that there is significant heterogeneity among African American patients and to ensure that we do not unintentionally increase the disparities in clinical trial participation, we need to continue to explore differences within racial and ethnic groups. These deeper insights into how some patients have overcome these barriers to participation serve as a foundation to deliver more personalized approaches to address the complex issues patients face when making decisions about participating in clinical trials, particularly in those populations that are underrepresented.
Limitations
The cross-sectional survey precludes conclusions about causality of the relationship between attitudes and participation in clinical trials. This study was not designed to more deeply explore the reasons why patients chose not to participate in a clinical trial and we therefore were not able to explore some of the factors reported in the literature, such as interactions with study recruiters and staff of diverse characteristics, communication skills, and levels of personal investment in the research. However, significant differences in the survey items between those who were asked and did not participate suggest that attitudes towards barriers and benefits may drive these decisions. Future research should use a longitudinal design to support this supposition. The small sample size might also restrict the generalizability of the study findings. To address this potential issue, data were collected at two cancer institutes that serve African American patients from all socioeconomic backgrounds. The significant differences found between the groups, even in such a small sample, indicate that a larger study is warranted. Finally, we are unable to know what potential differences existed in those patients who either were not provided an invitation to the study or who did not participate. Therefore, it was not possible to compare them with the 41 patients successfully recruited.
Conclusions
Understanding both barriers and facilitators to participation in clinical trials for African American cancer patients is important to address in clinical practice, but despite a number of studies elucidating these decisional antecedents, participation rates still lag behind. This study importantly compares these perceptions between participators and in non-participators in a sample from diverse socio-cultural and economic strata. This can give practitioners clues as to how to bridge the gap from non-participator to participator by focusing on those perceptions that are significantly different between the two groups. These messages could then be infused in the clinician–patient dyad when introducing and discussing clinical trials, potentially providing a more effective strategy for communicating with African American patients.
References
(2016) What Are Cancer Clinical Trials? Retrieved September 30, 2019, from https://www.cancer.gov/about-cancer/treatment/clinical-trials/what-are-trials
Unger JM, Vaidya R, Hershman DL, Minasian LM, Fleury ME (2019) Systematic review and meta-analysis of the magnitude of structural, clinical, and physician and patient barriers to cancer clinical trial participation. J Natl Cancer Inst 111(3):245–255
Al-Refaie WB, Vickers SM, Zhong W, Parsons H, Rothenberger D, Habermann EB (2011) Cancer trials versus the real world in the United States. Ann Surg 254(3):438–443
(n.d.) NIH Policy and Guidelines on The Inclusion of Women and Minorities as Subjects in Clinical Research. Retrieved September 30, 2019, from https://grants.nih.gov/policy/inclusion/women-and-minorities/guidelines.htm
Gansler T, Jin M, Bauer J, Dahlquist K, Tis L, Sharpe K, Comis R, Naples K, Kepner J (2012) Outcomes of a cancer clinical trial matching service. J Cancer Educ 27(1):11–20
Goss E, Lopez AM, Brown CL, Wollins DS, Brawley OW, Raghavan D (2009) American society of clinical oncology policy statement: disparities in cancer care. J Clin Oncol 27(17):2881–2885
Ahaghotu C, Tyler R, Sartor O (2016) African American participation in oncology clinical trials—focus on prostate cancer: implications, barriers, and potential solutions. Clin Genitourin Cancer 14(2):105–116
Aizer AA, Wilhite TJ, Chen MH, Graham PL, Choueiri TK, Hoffman KE, Martin NE, Trinh QD, Hu JC, Nguyen PL (2014) Lack of reduction in racial disparities in cancer-specific mortality over a 20-year period. Cancer 120(10):1532–1539
Mills EJ, Seely D, Rachlis B, Griffith L, Wu P, Wilson K, Ellis P, Wright JR (2006) Barriers to participation in clinical trials of cancer: a meta-analysis and systematic review of patient-reported factors. Lancet Oncol 7(2):141–148
Wujcik D, Wolff SN (2010) Recruitment of African Americans to National Oncology Clinical Trials through a clinical trial shared resource. J Health Care Poor Underserved 21(1 Suppl):38–50
Fleisher L, Bass SB, Shwarz M, Washington A, Nicholson A, Alhajji M, Geynisman DM, Maurer L, Greener J, Kenny C (2020) Using theory and user-centered design in digital health: The development of the mychoice communication tool to prepare patients and improve informed decision making regarding clinical trial participation. Psycho-Oncology 29(1):114–122. https://doi.org/10.1002/pon.5254
O'Connor AM (1995) Validation of a decisional conflict scale. Med Decis Mak 15(1):25–30
Cohen J (1988) Statistical power analysis for the behavioral sciences. Lawrence Erlbaum Associates
(n.d.) Downloading IBM SPSS Statistics 24. Retrieved September 30, 2019, from https://www-01.ibm.com/support/docview.wss?uid=swg24041224
Alcaraz KI, Sly J, Ashing K, Fleisher L, Gil-Rivas V, Ford S, Yi JC, Lu Q, Meade CD, Menon U, Gwede CK (2017) The ConNECT Framework: a model for advancing behavioral medicine science and practice to foster health equity. J Behav Med 40(1):23–38
Du W, Mood D, Gadgeel S, Simon MS (2008) An educational video to increase clinical trials enrollment among lung cancer patients. J Thorac Oncol 3(1):23–29
Strevel EL, Newman C, Pond GR, MacLean M, Siu LL (2007) The impact of an educational DVD on cancer patients considering participation in a phase I clinical trial. Support Care Cancer 15(7):829–840
(2019) Pew Research: Mobile fact sheet. Retrieved November 7, 2019, from https://www.pewresearch.org/internet/fact-sheet/mobile/
Unger JM, Hershman DL, Albain KS, Moinpour CM, Petersen JA, Burg K, Crowley JJ (2013) Patient income level and cancer clinical trial participation. J Clin Oncol 31(5):536–542
Harris Y, Gorelick PB, Samuels P, Bempong I (1996) Why African Americans may not be participating in clinical trials. J Natl Med Assoc 88(10):630–634
Jacobs EA, Rolle I, Ferrans CE, Whitaker EE, Warnecke RB (2006) Understanding African Americans’ views of the trustworthiness of physicians. J Gen Intern Med 21(6):642–647
Howerton MW, Gibbons MC, Baffi CR, Gary TL, Lai GY, Bolen S et al (2007) Provider roles in the recruitment of underrepresented populations to cancer clinical trials. Cancer 109(3):465–476
Albrecht TL, Eggly SS, Gleason ME, Harper FW, Foster TS, Peterson AM et al (2008) Influence of clinical communication on patients’ decision making on participation in clinical trials. J Clin Oncol 26(16):2666–2673
Brown RF, Cadet DL, Houlihan RH, Thomson MD, Pratt EC, Sullivan A, Siminoff LA (2013) Perceptions of participation in a phase I, II, or III clinical trial among African American patients with cancer: what do refusers say? J Oncol Pract 9(6):287–293
Denicoff AM, McCaskill-Stevens W, Grubbs SS, Bruinooge SS, Comis RL, Devine P et al (2013) The National Cancer Institute–American Society of Clinical Oncology cancer trial accrual symposium: summary and recommendations. J Oncol Pract 9(6):267–276
Vuong I, Wright J, Nolan MB, Eggen A, Bailey E, Strickland R, Traynor A, Downs T (2019) Overcoming barriers: evidence-based strategies to increase enrollment of underrepresented populations in cancer therapeutic clinical trials— a narrative review. J Cancer Educ. https://doi.org/10.1007/s13187-019-01650-y
Acknowledgments
Thanks to the patients and staff who contributed to this study.
Funding
This study was funded by Temple University-Fox Chase Cancer Center Nodal Grant Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in this study that involved human participants were in accordance with the ethical standards of the institutional research committee at Fox Chase Cancer Center, Philadelphia, PA, USA (IRB# 14-811) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alhajji, M., Bass, S.B., Nicholson, A. et al. Comparing Perceptions and Decisional Conflict Towards Participation in Cancer Clinical Trials Among African American Patients Who Have and Have Not Participated. J Canc Educ 37, 395–404 (2022). https://doi.org/10.1007/s13187-020-01827-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13187-020-01827-w