Abstract
Introduction
Acetaminophen protein adducts in the circulation are a specific biomarker of acetaminophen oxidation, and may be a more sensitive measure of impending hepatic injury following overdose than alanine transaminase (ALT). We performed an exploratory analytical substudy of adducts during a clinical trial (NACSTOP) of abbreviated (12-hour) versus control (20-hour) acetylcysteine to identify any signal of diminished antidotal effectiveness with shortened therapy.
Methods
We measured adducts at 0, 12, and 20 hours from a convenience sample of subjects enrolled in the cluster-controlled NACSTOP trial evaluating a 12-hour (“abbreviated”; 200 mg/kg over 4 hours, 50 mg/kg over 8 hours) vs 20-hour acetylcysteine regimen (“control”; 200 mg/kg over 4 hours, 100 mg/kg over 16 hours). Adducts were assayed using high-performance liquid chromatography/mass spectrometry.
Results
Median ALT 20 hours after the initiation of acetylcysteine was 12 U/L (IQR 8,14) in the abbreviated 12-hour regimen group (N = 8), compared with the control group 16 U/L (IQR 11,21; N = 21) (p = 0.46). Adduct concentrations were similarly low in both groups: abbreviated [(0.005 μmol/L, IQR (0,0.14)] and control [(0.005 μmol/L, IQR (0,0.05)] (p = 0.61).
Conclusions
There were minimal to no acetaminophen protein adducts detected. These findings further support discontinuing acetylcysteine when acetaminophen concentrations are low and liver function tests normal after 12 hours of treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acetaminophen is one of the most frequent causes of hospitalisation for antidotal treatment, fulminant liver failure and death following intentional pharmaceutical overdose worldwide. Given the frequency and potential severity, there is considerable interest in optimising antidotal therapy, especially individualising, simplifying and abbreviating the standard 3-bag, 20- to 21-hour treatment for lower risk patients, and intensifying it for higher risk patients [1,2,3,4,5]. Research in this area has been hampered by reliance on the relatively blunt outcome measure of hepatotoxicity defined as a peak alanine transaminase (ALT) > 1000 IU/L or death. These outcomes are also fortunately uncommon in the typical scenario of an acute overdose patient treated with acetylcysteine within 12 hours of ingestion [6, 7]. Among candidate biomarkers with greater sensitivity for subclinical hepatic injury, circulating acetaminophen protein adducts show considerable promise as a more direct measure of acetaminophen oxidation. Given that acetaminophen oxidation is a necessary step in the pathway that leads to hepatic injury [8], acetaminophen protein adducts may be both more specific and perhaps more sensitive biomarkers of impending toxicity following acetaminophen overdose [9, 10]. Furthermore, the mechanism of action of the antidote, acetylcysteine, is widely accepted to reduce this deleterious oxidation and thus generation of adducts, allowing insight into the protective effect of a given dose.
A two-bag acetylcysteine regimen consisting of 200 mg/kg infused over 4 hours followed by a further 100 mg/kg infused over 16 hours decreases adverse reactions and simplifies the traditional three-bag regimen [11,12,13]. We have recently demonstrated that stopping this newer dosing regimen after 12 hours was not more likely to cause a rise in ALT or AST compared with the full 20-hour infusion (the NACSTOP trial) in selected patients [1]. Subjects were eligible for early cessation if, after 12 hours of acetylcysteine, serum acetaminophen concentration was < 132 μmol/L (20 mg/L) or lower and the alanine transaminase (ALT) remained normal (< 40 U/L), regardless of the time delay to treatment following overdose. Other groups are actively studying abbreviated acetylcysteine regimens of 8- to 12-hour duration [2, 14].
The aim of this substudy of the NACSTOP trial was to investigate whether acetaminophen protein adduct concentrations differed between the abbreviated and non-abbreviated (control) acetylcysteine treatment groups, in particular after the antidote was discontinued. This exploratory analysis was intended to identify any safety signal which might suggest that early cessation of acetylcysteine would allow the additional generation of deleterious oxidation products of acetaminophen metabolism. Given the limited data surrounding adducts in general, we also included as positive controls a convenience sample of contemporaneous acetaminophen overdose patients treated with acetylcysteine but who were ineligible for the NACSTOP trial due to overt acute liver injury (ALI) or hepatotoxicity at presentation.
Methods
Study Population
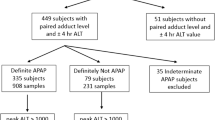
Subjects treated with acetylcysteine following intentional, mainly acute single ingestion acetaminophen overdose were potentially eligible for the parent, cluster-controlled NACSTOP trial, described in detail elsewhere [1]. For the present analytical substudy, we selected all subjects enrolled from February 2016 to July 2017 at three of the six enrolling sites, all three emergency departments at Monash Health, Victoria, Australia (1 intervention, 2 control) [1]. The decision to treat with acetylcysteine was based on criteria set out in the Australian and New Zealand acetaminophen guidelines [15], including the acetaminophen nomogram treatment threshold of 1000 μmol/L (150 mg/L) at 4 hours post single ingestion. Consecutive patients with a normal serum ALT at presentation (ALT < 40 U/L) and who subsequently had a low acetaminophen concentration (< 132 μmol/L [20 mg/L]) and normal ALT after 12 hours of acetylcysteine infusion were enrolled in the parent study on an intention-to-treat basis. The control group came from sites using the full 20-hour course of acetylcysteine (200 mg/kg over 4 hours, 100 mg/kg over 16 hours), while the intervention sites stopped acetylcysteine after at least 12 hours of infusion (i.e. 200 mg/kg over 4 hours, followed by at least half of the 100 mg/kg over 16-hour infusion). The protocol stipulated that all subjects were to be kept in hospital and have blood tested at both 12 and 20 hours following the start of acetylcysteine.
We defined acute liver injury (ALI) to be an ALT > 50 U/L at presentation or after 12 hours of treatment, and hepatotoxicity to be peak ALT > 1000 U/L. Fully informed consent from participants was obtained to participate in the study [1].
Two additional groups of patients also had blood used for adduct testing. Intended to represent positive controls, these were patients who presented with (or developed within 12 hours) either ALI or hepatotoxicity due to acetaminophen overdose, and were thus ineligible for the NACSTOP trial. Patients with ALI or hepatotoxicity often receive prolonged acetylcysteine infusions beyond the standard 20 hours, usually until serum ALT has peaked and is falling and International Normalised Ratio (INR) is < 2.0. As a result, additional blood samples were available and analysed in these groups. We report in this study four groups of patients: NACSTOP subjects receiving (i) the abbreviated treatment regimen, or (ii) full treatment regimen (control group), and non-trial patients (iii) with ALI and (iv) with hepatotoxicity. Ethics approval was obtained from the Monash Health Ethics Research Committee.
By study protocol, timed blood samples were collected at presentation (pre-treatment), and again after 12 and 20 hours of acetylcysteine in all NACSTOP subjects. Additional blood samples were typically collected beyond 20 hours in those with ALI and hepatotoxicity until ALT had peaked and was falling as per usual clinical practice. Serum was separated and stored at – 80 °C for batch analysis using high-performance liquid chromatography–tandem mass spectrometry (HPLC/MS) at a single, experienced laboratory (Department of Pathology, University of Utah, Utah, USA). The acetaminophen protein adduct assay methodology has been described previously [16]. The lower limit of quantification for the acetaminophen adduct assay was 0.01 μmol/L. If trace amounts of adducts were detected (i.e. between the limits of quantification and of detection), this was recorded as a concentration of 0.005 μmol/L (half the lower limit of quantification) for analysis and graphing using logarithmic transformation, and undetectable concentrations as 0.001 μmol/L.
Statistical Analysis
Presentation, peak, and end of acetylcysteine infusion concentrations of adducts were compared.
The a priori statistical null hypothesis compared the 20-hour adduct concentration between the NACSTOP study arms. No covariates were used for statistical adjustment given the small number of subjects. We compared groups using the non-parametric Mann-Whitney U test. Additional exploratory analyses compared the timed and the peak adduct concentrations between all four groups, and by nomogram-adjusted acetaminophen concentration and delay to treatment post acute overdose. GraphPad Prism (Version 7, California, USA) was used to perform statistical analysis.
Results
One hundred thirty blood samples were collected from 40 patients receiving acetylcysteine after acetaminophen overdose. There were eight patients (20%) who were enrolled at the intervention site and had received the 12-hour treatment regimen (abbreviated group), and twenty-one (53%) from the control sites who received 20 hours of acetylcysteine (control group). Nine (23%) other patients had ALI, all at presentation, and two (5%) hepatotoxicity, also at presentation. All received acetylcysteine. The overall median age was 22 years (IQR 18,32). The majority of patients were female (70%) (Table 1). The median ALT after 20 hours of acetylcysteine was 12 U/L (IQR 8,14) in the abbreviated NACSTOP group, compared with 16 U/L (IQR 11,21) in the NACSTOP control group (p = 0.46). No subjects in the NACSTOP trial developed any abnormality of ALT. In the ALI group, median ALT after 20 hours of acetylcysteine was 62 U/L (IQR 49,87); in the hepatotoxicity group, it was 1572 U/L (IQR 1249, 2947). The median time interval to starting acetylcysteine post-overdose was 6 hours (IQR 5.5,12), 6.5 (5.6,11), 9 (5.5,16) and 21 (12,31) in the abbreviated, control, ALI and hepatotoxicity groups, respectively. Median acetylcysteine duration was 13 hours (IQR 13,13) for the abbreviated group, substantially shorter than the controls (20 hours [20]) (p < 0.001), ALI (20 hours [16, 20]) and hepatotoxicity groups.
Acetaminophen adduct concentrations were compared between the study groups (Figs. 1 and 2). Adduct concentrations at presentation were low and similar between the abbreviated (0 μmol/L, IQR (0,0.13)) and control groups (0 μmol/L, IQR (0,0.05)) (p = 0.56). In the primary analysis, there was no significant difference in acetaminophen adduct concentration after 20 hours when comparing the abbreviated (median 0.005 μmol/L, IQR (0,0.14)) to the control ((0.005 μmol/L, IQR (0,0.05)) NACSTOP groups (p = 0.61). The highest individual patient adduct concentrations recorded in the abbreviated, control, ALI and hepatotoxicity groups were 0.19, 0.2, 0.43 and 12.09 μmol/L, respectively. In the two patients with overt hepatotoxicity, the initial adduct concentrations were 1.75 μmol/L (24 hours post overdose) and 0.23 μmol/L (31 hours post overdose), and peaked 44 to 51 hours post-ingestion. The ALT peak was seen several hours later in these patients at 53 (ALT 15601 IU/L) and 66 hours (ALT 2365 IU/L) post-overdose, respectively.
Acetaminophen adduct concentrations in NACSTOP patients (i) abbreviated and (ii) control groups; and comparative groups with (iii) acute liver injury (ALI) and (iv) hepatotoxicity from acetaminophen overdose. Different scale for y-axis in hepatotoxicity group used to identify values outside the range of other comparative groups.
Adduct concentration approximately correlated with the concurrent serum aminotransferase, especially when ALT exceeded 100 IU/L (Fig. 3). At lower aminotransferase activity, the correlation was poor and many patients had very low to undetectable adducts despite mildly elevated and abnormal ALT. In NACSTOP and ALI patients whose serum acetaminophen concentration could be interpreted using the traditional treatment nomogram (i.e. obtained within 4 to 20 hours following acute overdose at known time), there appeared to be little association between the highest measured adduct concentration and the distance above the nomogram treatment threshold or delay to presentation (Fig. 4).
Serum ALT activity and simultaneous acetaminophen (APAP) adduct concentrations. Every measured adduct concentration is plotted against the simultaneous ALT. Samples from both NACSTOP patients and those who developed acute liver injury are represented with circles, while patients with hepatotoxicity (peak ALT > 1000 IU/L) are shown with x’s.
Bubble plot showing the highest observed acetaminophen adduct concentration relative to the initial serum acetaminophen (APAP) post ingestion. The area of the bubble represents the adduct concentration (scale shown in boxed legend), and is plotted on the treatment nomogram based on the first serum acetaminophen concentration obtained at least 4 hours post acute overdose. The dashed line represents the usual threshold for antidotal treatment with acetylcysteine.
Adduct concentrations were detectable in 3 (38%) patients in the abbreviated group and 9 (43%) patients in the control group on presentation (Table 2). Most adduct concentrations in these two groups were in trace amounts only. Twelve (50%) samples from the abbreviated and thirty-one (49%) samples from the control group had no detectable acetaminophen adducts at presentation. Those that had detectable adducts in these groups were all below 0.3 μmol/L at 20 hours post initiation of acetylcysteine. All samples in the two patients who developed hepatotoxicity had detectable adducts. Adducts were detected in most cases with ALI (5 of 9, 55%) on presentation, and all samples (including at presentation) in patients with hepatotoxicity.
Discussion
Circulating acetaminophen protein adducts are best characterised in the setting of acute liver failure attributed to acetaminophen overdose [9]. Often these presentations to hospital are delayed post-overdose, most or all of the acetaminophen has been cleared from the serum, and evidence of liver injury is already apparent. Adducts show considerable promise with establishing or exculpating acetaminophen overdose as contributory [9, 17]. The presence of adducts following therapeutic or subtoxic dosing has also been described by some investigators [10, 18,19,20]. However, relatively little is known about acetaminophen protein adduct concentrations in the first hours following overdose treated with acetylcysteine, or the effect of lower doses of antidotal therapy on these adducts.
With therapeutic use, acetaminophen is primarily metabolised via glucuronidation and sulfation pathways, with less than 5% oxidised via the cytochrome P450 system to the toxic metabolite N-acetyl-para-benzoquinoneimine (NAPQI) [21]. NAPQI is typically conjugated with glutathione, then further metabolised to APAP-cysteine and APAP-mercapturate and renally excreted [22]. After acetaminophen overdose, glucuronidation and sulfation pathways become saturated, leading to greater oxidative metabolism and increased NAPQI formation. The increase in NAPQI rapidly depletes glutathione stores and unconjugated NAPQI binds to sulfhydryl groups on cellular proteins. Binding of NAPQI to cellular proteins results in formation of high molecular mass acetaminophen protein adducts which can be detected in the protein fraction of serum. These protein adducts are distinct from the much smaller APAP-cysteine metabolite resulting from glutathione conjugation (and presumably from acetylcysteine rescue) [23], and are present in the non-protein fraction of serum. Therefore, once glutathione is depleted acetaminophen protein adduct formation will increase. As such, this biomarker may estimate the toxic exposure following overdose incorporating both dose ingested and delay to acetylcysteine.
Moreover, acetaminophen protein adducts can be quantified albeit at low concentrations even after therapeutic use of acetaminophen [10, 20]. Furthermore, protein adducts would be expected to arise primarily from perivenous cells (i.e. zone I in the acinus) which have the greatest CYP activity and which correspond histologically to the most vulnerable area of the liver lobule following acetaminophen overdose. ALT activity, on the other hand, is much higher in the periportal or zone III zone, and serum ALT activity rarely changes when patients are treated with acetylcysteine within several hours of overdose [24]. Taken together, these properties would suggest that the release of acetaminophen protein adducts into the circulation might be a more sensitive marker of hepatic injury following acetaminophen overdose than ALT.
While about half of the samples in both groups from the NACSTOP trial had detectable adduct concentrations throughout their admission, we did not identify a late rise in adducts after acetylcysteine was discontinued. The peak concentration in both groups was less than 0.3 μmol/L. This low rate of adduct production, taken with the normal ALT concentrations in both groups after 20 hours of acetylcysteine and low levels of miR-122 (another putative biomarker of hepatic injury [25], provides some reassurance that the abbreviated 12-hour protocol delivers sufficient acetylcysteine for most patients deemed to be at low risk of developing hepatotoxicity based on the trial inclusion criteria [26] (i.e. ALT < 40 U/L and low acetaminophen concentrations (< 20 mg/L)) after at least 12 hours of acetylcysteine. These data support the ongoing use of these low-risk criteria used to continue to use and to study the 12-hour acetylcysteine regimen after acetaminophen overdose.
Similarly, the ALI comparison group demonstrated similar acetaminophen adduct concentrations over the 20-hour treatment course of acetylcysteine compared with the NACSTOP patients. This coincides with the minimal degree of liver injury in this group, and the stable ALT activities observed during antidotal therapy. While these patients were ineligible for NACSTOP, we suspect that in most cases acetaminophen was not the cause of their abnormal ALT activities at presentation. However, the hepatotoxicity comparison group clearly had extremely high adduct concentrations, with much longer periods of detection compared with the other groups. This is in keeping with previous studies measuring acetaminophen protein adducts in the setting of acute liver failure secondary to acetaminophen ingestion, when adduct concentrations over 1 μmol/L are typical when peak ALT values exceed 1000 IU/L [17].
Previous studies have examined the formation of acetaminophen protein adducts in the setting of therapeutic ingestion of acetaminophen over several days. Heard (2016) et al. reported on adduct formation in patients taking 4 g/day of acetaminophen [10]. There was an increase in adduct production after consecutive days of acetaminophen consumption, peaking on day 7 (median peak adduct concentration 0.14 μmol/L, max 1.06 μmol/L) and levelled off at 0.1 μmol/L for day 7 to 16. Increases in circulating adducts coincided with minor elevation in serum ALT after repeated consumption and may have been the result of increased NAPQI production; however, adduct concentrations and ALT were uncorrelated [27]. In addition, James (2013) et al. reported detectable adduct concentrations (peaked at 8 hours post ingestion and values mostly < 0.1 nmol/mL) after single ingestion of 80 mg/kg of acetaminophen [18]. Importantly, acetylcysteine was not administered in either study by Heard or James et al. [10, 18]. Acetylcysteine, used as a glutathione donor, may prevent acetaminophen adduct formation and increase acetaminophen metabolism by the non-toxic pathways [23]. This could explain the negligible adduct concentrations detected in the NACSTOP trial patients.
For those with hepatotoxicity (i.e. ALT > 1000 IU/L), we confirm the good association between ALT and contemporaneous adduct concentrations recently reported by Curry et al. [17]. We were unable to demonstrate a clear association between peak adduct concentrations and the nomogram-adjusted initial acetaminophen concentration, a traditional measure of risk (and a surrogate measure of ingested dose) following acute acetaminophen overdose. This variability at the lower end of adduct concentrations may be related to interpersonal variation such as race, gender, alcohol use, genetic polymorphisms and nutritional status, which have long been speculated to explain the variability in outcomes beyond dose and delay to treatment [19]. Biomarkers such as protein adducts and miR-122 may eventually shed light on the importance of such elusive covariates, but much larger studies will be needed.
There are several limitations of this study. The stated ingestion times of acetaminophen were based on patient recollection but were asked on multiple occasions to minimise any inaccuracies. The number of participants was small, but suitable for an exploratory substudy, and future studies should aim to analyse adducts from a larger trial population. In addition, the study offers novel insights into the wide range of acetaminophen protein adduct concentrations and changes in the first hours following acute overdose in a distinct and important clinical population. Future studies should test for biomarkers beyond ALT, including acetaminophen adducts, when comparing different acetylcysteine treatment regimens in the setting of acetaminophen overdose.
Conclusions
Acetaminophen adduct formation was similarly negligible in selected low-risk subjects when acetylcysteine was discontinued after 12 hours when compared with those receiving the full, standard 20-hour regimen. These observations support continued efforts to abbreviate antidotal therapy when serial testing is reassuringly normal, and specifically acetaminophen concentrations are therapeutic to undetectable and liver transaminases remain normal after 12 hours of treatment. Acetaminophen protein adducts following acute overdose are generally very low but also highly variable, which may reflect human variability in metabolism and detoxification of acetaminophen.
Sources of Funding
AW has received a NHMRC Early Career Research Fellowship ID 1159907.
References
Wong A, McNulty R, Taylor D, Sivilotti M, Greene S, Gunja N, et al. The NACSTOP trial: a multicenter, cluster-controlled trial of early cessation of acetylcysteine in acetaminophen overdose. Hepatology. 2019;69(2):774–84.
Pettie J, Caparrotta TM, Hunter RW, et al. Safety and efficacy of the SNAP 12-hour acetylcysteine regimen for the treatment of paracetamol overdose. EClinicalMed. 2019.
Johnson MT, McCammon CA, Mullins ME, Halcomb SE. Evaluation of a simplified N-acetylcysteine dosing regimen for the treatment of acetaminophen toxicity. Ann Pharmacother. 2011;45(6):713–20.
Chiew AL, Isbister GK, Kirby KA, Page CB, Chan BSH, Buckley NA. Massive paracetamol overdose: an observational study of the effect of activated charcoal and increased acetylcysteine dose (ATOM-2). Clin Toxicol. 2017;55(10):1055–65.
Dart RC, Rumack BH. Patient-tailored acetylcysteine administration. Ann Emerg Med. 2007;50(3):280–1.
Rumack BH. Acetaminophen hepatotoxicity: the first 35 years. J Toxicol Clin Toxicol. 2002;40(1):3–20.
Sivilotti ML, Yarema MC, Juurlink DN, Good AM, Johnson DW. A risk quantification instrument for acute acetaminophen overdose patients treated with N-acetylcysteine. Ann Emerg Med. 2005;46(3):263–71.
Mitchell JR, Jollow DJ, Potter WZ. Acetaminophen induced hepatic necrosis. I. Role of drug metabolism. J Pharmacol Exp Ther. 1973;187(1):185–94.
Davern ITJ, James LP, Hinson JA, Polson J, Larson AM, Fontana RJ, et al. Measurement of serum acetaminophen-protein adducts in patients with acute liver failure. Gastroenterology. 2006;130(3):687–94.
Heard K, Green JL, Anderson V, Bucher-Bartelson B, Dart RC. Paracetamol (acetaminophen) protein adduct concentrations during therapeutic dosing. Br J Clin Pharmacol. 2016;81(3):562–8.
Wong A, Graudins A. Simplification of the standard three-bag intravenous acetylcysteine regimen for paracetamol poisoning results in a lower incidence of adverse drug reactions. Clin Toxicol. 2016;54(2):115–9.
McNulty R, Lim JME, Chandru P, Gunja N. Fewer adverse effects with a modified two-bag acetylcysteine protocol in paracetamol overdose. Clin Toxicol. 2018;56(7):618–21.
Schmidt LE, Rasmussen DN, Petersen TS, Macias-Perez IM, Pavliv L, Kaelin B, et al. Fewer adverse effects associated with a modified two-bag intravenous acetylcysteine protocol compared to traditional three-bag regimen in paracetamol overdose. Clin Toxicol. 2018:1–7.
Wong A, Gunja N, McNulty R, Graudins A. Analysis of an 8-hour acetylcysteine infusion protocol for repeated supratherapeutic ingestion (RSTI) of paracetamol. Clin Toxicol. 2018;56(3):199–203.
Chiew A, Fountain JS, Graudins A, Isbister GK, Reith D, Buckley NA. Summary statement: new guidelines for the management of paracetamol poisoning in Australia and New Zealand. Med J Aust. 2015;203(5):215–8.
Cook SF, King AD, Chang Y, Murray GJ, Norris HR, Dart RC, et al. Quantification of a biomarker of acetaminophen protein adducts in human serum by high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry: clinical and animal model applications. J Chromatogr B Analyt Technol Biomed Life Sci. 2015;985:131–41. https://doi.org/10.1016/j.jchromb.2015.01.028.
Curry SC, Padilla-Jones A, Ruha AM, O'Connor AD, Kang AM, Wilkins DG, et al. The relationship between circulating acetaminophen-protein adduct concentrations and alanine aminotransferase activities in patients with and without acetaminophen overdose and toxicity. J Med Toxicol. 2019.
James LP, Chiew A, Abdel-Rahman SM, Letzig L, Graudins A, Day P, et al. Acetaminophen protein adduct formation following low-dose acetaminophen exposure: comparison of immediate-release vs extended-release formulations. Eur J Clin Pharmacol. 2013;69(4):851–7.
Court MH, Zhu Z, Masse G, Duan SX, James LP, Harmatz JS, et al. Race, gender, and genetic polymorphism contribute to variability in acetaminophen pharmacokinetics, metabolism, and protein-adduct concentrations in healthy African-American and European-American volunteers. J Pharmacol Exp Ther. 2017;362(3):431–40.
Heard KJ, Green JL, James LP, Judge BS, Zolot L, Rhyee S, et al. Acetaminophen-cysteine adducts during therapeutic dosing and following overdose. BMC Gastroenterol. 2011;11:20.
Prescott LF. Paracetamol: past, present, and future. Am J Ther. 2000;7(2):143–7.
Prescott LF. Kinetics and metabolism of paracetamol and phenacetin. Br J Clin Pharmacol. 1980;10(Suppl 2):291S–8S.
Wong A, Homer N, Dear JW, Choy KW, Doery J, Graudins A. Paracetamol metabolite concentrations following low risk overdose treated with an abbreviated 12-h versus 20-h acetylcysteine infusion. Clin Toxicol. 2019;57(5):312–7.
Kietzmann T. Metabolic zonation of the liver: the oxygen gradient revisited. Redox Biol. 2017;11:622–30.
Wong A, Nejad C, Gantier M, Choy KW, Doery J, Graudins A. MicroRNA from a 12-h versus 20-h acetylcysteine infusion for paracetamol overdose. Hum Exp Toxicol. 2019;38(6):646–54.
Wong A, Graudins A. Risk prediction of hepatotoxicity in paracetamol poisoning. Clin Toxicol. 2017;55(8):879–92.
Heard K, Green JL, Anderson V, Bucher-Bartelson B, Dart RC. A randomized, placebo-controlled trial to determine the course of aminotransferase elevation during prolonged acetaminophen administration. BMC Pharmacol Toxicol. 2014;15:39.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Fully informed consent from participants was obtained to participate in the study. Ethics approval was obtained from the Monash Health Ethics Research Committee.
Conflicts of Interest
None.
Additional information
Supervising Editor: Michael Levine, MD
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wong, A., Heard, K., Graudins, A. et al. Adducts Post Acetaminophen Overdose Treated with a 12-Hour vs 20-Hour Acetylcysteine Infusion. J. Med. Toxicol. 16, 188–194 (2020). https://doi.org/10.1007/s13181-020-00757-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13181-020-00757-9