Abstract
While reliable detection of illicit drug use is paramount to the field of addiction, current methods involving self-report and urine drug screens have substantial limitations that hinder their utility. Wearable biosensors may fill a void by providing valuable objective data regarding the timing and contexts of drug use. This is a preliminary observational study of four emergency department patients receiving parenteral opioids and one individual using cocaine in a natural environment. A portable biosensor was placed on the inner wrist of each subject, to continuously measure electrodermal activity (EDA), skin temperature, and acceleration. Data were continuously recorded for at least 5 min prior to drug administration, during administration, and for at least 30 min afterward. Overall trends in biophysiometric parameters were assessed. Injection of opioids and cocaine use were associated with rises in EDA. Cocaine injection was also associated with a decrease in skin temperature. Opioid tolerance appeared to be associated with a blunted physiologic response as measured by the biosensor. Laterality may be an important factor, as magnitude of response varied between dominant and nondominant wrists in a single patient with bilateral wrist measurements. Changes in EDA and skin temperature are temporally associated with intravenous administration of opioids and cocaine; the intensity of response, however, may vary depending on history and extent of prior use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The detection of illicit drug use is of great interest to the field of addiction medicine given that relapse is a key indicator of outcomes. At present, detecting drug use relies upon data obtained from testing biological specimens (e.g., qualitative screens of urine for drugs of abuse) or self-report. While these approaches are useful, they have methodological shortcomings [1–4]. First, both methods obtain discontinuous data that cannot provide real-time information about drug using behaviors. Second, neither method allows researchers to reliably discern drug use patterns over time in order to better evaluate the time course of treatment effects. Third, neither method has been adapted into smartphones or other mobile health devices, which could integrate drug use data into the affective and environmental contexts surrounding drug use [1, 2]. Finally, neither method currently provides an opportunity for real-time intervention or analysis of triggers, which is currently a substantial limitation of addiction treatment.
Portable biosensors have the potential to revolutionize the field of addiction by providing continuous data streams to detect the timing, location, contexts, and durations of drug use in individuals under treatment. These small, unobtrusive electronic devices are easily applied and worn, can detect important physiologic parameters that are expected to change with substance use (such as electrodermal activity [EDA], physical movement of the body in three dimensions [acceleration], and skin temperature) and can wirelessly stream information to a smartphone to trigger behavioral interventions precisely at the moment of greatest need [1, 4]. The use of portable biosensors to enhance monitoring/intervention strategies have been described in other at-risk populations, including HIV positive individuals, epilepsy patients, and those diagnosed with PTSD [1, 4, 5]. Few data, however, describe the biometric profile of individuals actively using recreational drugs, and this is imperative to define before biosensors can be utilized in a meaningful way in drug treatment. In this pilot study, we sought to evaluate the biometric changes associated with the injection of cocaine and opioids.
Methods
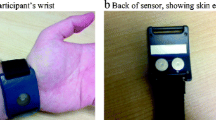
All protocols were approved by the appropriate institutional review board. A Q sensor™ (Affectiva, Waltham, MA) was utilized for all data collection. The sensor is approximately 4 by 5 cm; it is worn on the volar aspect of the wrist and secured by a Velcro band (Fig. 1). The Q sensor continuously records EDA measures in microsiemens (μS), skin temperature in degree Celsius, and acceleration in units g (SI unit of acceleration). Recordings were taken at a sample rate of 8 readings per second. To assess for measurable physiologic changes and to examine the feasibility of technology deployment with data collection in vivo, two separate protocols were used to recruit emergency department (ED) patients and a field subject, respectively, as outlined below.
Emergency Department Protocol: Biometrics of Opioid Use
The University of Massachusetts Institutional Review Board approved the experimental protocol for measurement of biometric profiles of opioid administration in ED patients. Inclusion criteria were (1) age 18–90 years old, (2) chief complaint of acute pain, (3) a clinician’s written order for intravenous opioid analgesic, and (4) ability to provide consent. Exclusion criteria were (1) inability to wear a biosensor, (2) musculoskeletal causes of pain, (3) pregnancy, (4) inability to consent, or (5) upper extremity amputation. Potential subjects were informed that the purpose of the study was to determine the utility of the device to detect opioid use by measuring physiologic change after known exposure. After obtaining informed consent, research staff placed a portable biosensor first on the nondominant wrist unless this interfered with medical treatment (i.e., intravenous lines), then it was placed on the dominant wrist if such interference was identified. EDA, skin temperature, and acceleration were continuously recorded before, during, and 30 min after intravenous opioid administration. Demographic data including age, gender, hand dominance, and history of opioid use, in addition to biosensor responses to drug administration were recorded. Opioid use history was ascertained from patient report, and supplemental prescription history data from the institution’s electronic medical record was included as well.
Field Deployment Protocol: Biometrics of Cocaine Use
The Massachusetts Institute of Technology Committee on the Use of Humans as Experimental Subjects and the University of Massachusetts Medical School Institutional Review Board approved the experimental protocol. Recruitment was performed with the assistance of a community outreach program that referred individuals with active substance abuse. Because IRB approval was granted for application of biosensors to individuals pursuing normal activities in their daily lives, only subjects who were disinterested in seeking drug treatment were referred. Inclusion criteria were (1) age 18–90 years old and (2) ability to provide consent. Exclusion criteria were (1) inability to wear a biosensor, (2) pregnancy, (3) inability to consent, or (4) upper extremity amputation. Potential subjects were informed that the purpose of the study was to measure physiologic changes associated with normal daily activities and to determine the feasibility of procedures surrounding deployment of the sensor; they were shown the sensor prior and briefed on the device capabilities. After an IRB-mandated encouragement to avoid drug use and other unsafe activities, the sensor was placed on the subject’s dominant wrist. The subject was instructed to come back to the community outreach site in 4 h to return the sensor, or sooner if any concerns arose.
At the end of data collection, a timeline follow back interview was conducted to determine activities at various points during the period the subject wore the sensor.
Results
Emergency Department Data
During this portion of the study, the ambient hospital temperature and humidity were maintained at approximately 65 °F and 50 %, respectively. Four subjects who met the inclusion criteria were approached, and all consented to enrollment. Demographic characteristics are listed in Table 1. All subjects were supine in gurneys and none engaged in any significant physical activity during the recording period. The subjects denied any discomfort associated with the sensors. One subject questioned whether the sensor would be perceived by staff as a court-ordered electronic monitoring device, but nonetheless chose to participate in the study. The remaining three subjects did not perceive any stigma associated with the device.
The q sensor tracing and summary data are presented in Figs. 2, 3, 4, 5, and 6 and Table 1.
EDA scale varies between tracings to emphasize the shape of the curve and is labeled on the upper right portion of the tracing. Each tracing consist of three panels: top panel: electrodermal activity in μS; middle panel: temperature in °C; and lower panel: Acceleration in unit g. Participant 1. Please note, time of day is shown on the top axis, and an event marker along the top of the screen image delineates the time of intravenous opioid administration (where applicable)
In subject 1, an opioid naïve 82-year-old man, a rise in EDA from a baseline of 4.5 μS was noted immediately following the administration of 4 mg IV morphine; the EDA peaked at approximately 60 μS at 6 min post-intervention, then gradually declined toward baseline. This patient’s EDA level remained elevated at the end of the recording period. The sensor electrode temperature remained constant throughout the session with the exception of the typical rise from room temperature to skin temperature shortly after the sensor was placed on the patient. The patient reported almost complete relief in pain after opioid administration (Fig. 2).
Subject 2, a 47-year-old man, reported an approximately 2-week history of oxycodone use following a recent surgical procedure, but denied other prior opioid use history. After 1 mg IV hydromorphone administration, his measured EDA rose from a baseline of 3.4 μS to a peak of 12.2 μS approximately three minutes post administration. No change in body temperature was noted. The patient reported only a small decrease in pain, and a second dose of 1 mg IV hydromorphone was administered 25 min later (Fig. 3).
Subject 3 was a 43-year-old woman who reported chronic use of opioid analgesics. She presented with abdominal pain, and treating clinicians expressed concern that she distorted her symptoms to ensure narcotic administration. Intravenous administration of 1 mg hydromorphone produced no change in EDA, but a gradual 4 °C upward trend in skin temperature occurred over a 10-min period after administration. The patient reported no change in her pain following hydromorphone administration and requested additional medication (Fig. 4).
Subject 4 was a 72-year-old man with a history of chronic opioid use including oral oxycodone 1–3 times daily over the past year, with sporadic use of hydrocodone in the preceding years. Given the dramatic intersubject differences in the early portion of the study, combined with new data emerging from other ongoing studies, the protocol was adjusted to include bilateral wrist sensors during this subject’s participation. Following the administration of 4 mg of morphine, a small increase in EDA was noted in both wrists. A smooth, gradual increase was noted in the dominant wrist from a baseline of 0.5 to 0.8 μS. A more stepwise increase was seen in the nondominant wrist from a baseline of 0.9 μS to a peak of 1.6 μS over 16 min. Skin temperature also increased, more prominently in the nondominant wrist. The patient reported minimal improvement in his pain (Fig. 5).
Field Data
Because of concerns that the device might reveal an individual’s location to law enforcement, only one person agreed to participate in the pilot field study out of the two potential subjects approached. The field study subject was a left-hand-dominant 53-year-old man with a 20-year history of daily parenteral cocaine use. He reported no medical problems other than drug use; he denied taking any prescription or over-the-counter medications. He wore the sensor on his left wrist for a continuous 5-hour period. Data were collected between 10 a.m. and 3 p.m.; the air temperature averaged 78 °F with humidity of 40 %. During the timeline follow-back interview, the subject reported that he had eaten a snack before injecting cocaine about 40 min after leaving the community outreach site. He did not report engaging in physical activity aside from walking for approximately 0.5 mi during the data collection period.
The study subject reported that he did not perceive any stigma associated with the biosensor even though he was wearing a short-sleeved shirt and was around familiar and unfamiliar persons during the data collection period. He also reported that he received no queries about the purpose of the device even though he was in locations where illegal activities occurred (e.g., the buying and using of drugs).
Data from the sensor was downloaded and a portion of the 5-hour recording is presented in Fig. 6. Review of the data demonstrates a dramatic increase in electrodermal activity of approximately 10 μS accompanied by decrease in skin temperature of approximately 1–2 °F and an increase in acceleration (i.e., increased physical movement). This upsweep in electrodermal activity is consistent with the reported time of cocaine injection as identified in the timeline follow-back interview.
Discussion
In this small pilot study, both intravenous cocaine and opioid administration were temporally associated with notable biometric changes, primarily an increase in EDA, that were detectable by wearable biosensors in relatively controlled environments. Skin temperature and acceleration parameters also showed some change, although less pronounced, particularly in our field participant using cocaine. These findings supports the ability of mobile biosensors to detect episodes of drug use in real time, with the ultimate goal of detecting drug use as it occurs in natural environments. Having a direct, continuous, and real-time measure of drug use episodes offers an advantageous supplement to existing methods by providing the opportunity for real-time feedback and intervention. When linked to environmental and behavioral events surrounding relapsing drug use, mobile biosensors may allow drug abuse researchers and clinicians to tailor interventions to highly specific contexts, including the delivery of such interventions precisely at the moment of greatest need.
It is important to underscore that any psychological or physiological stress is expected to produce an increase in SNS activity, and thus alterations in EDA, skin temperature, and acceleration. These phenomena have been documented in numerous studies evaluating the measurable stress responses in humans during driving, public speaking, or simple everyday activities that people report as stressful [6–8]. These responses are notably less dramatic that those associated with drug administration. Figure 7 provides an example of a person with PTSD who is exposed to a stimulus known to elicit stress and anxiety. In comparison, the biometric pattern from subject 5 demonstrates a more robust decrease in skin temperature that is consistent with the vasoconstrictive effects of cocaine. Because of the temporal association of physiologic change with drug administration, the magnitude of the EDA response, the graded biometric response in tolerant individuals, and the inverse relationship between skin temperature changes in drug use and stress, these sensors appear capable of detecting isolated opioid and cocaine use in human subjects.
Compared with the robust increases in EDA that occurred in opioid-naïve persons, diminished biometric responses were observed among individuals with self-described chronic opioid use. Variation in biometric response to opioids among the four ED subjects highlights the importance of confounding variables in interpretation of physiologic data. Of particular interest is the apparent relationship between previous opioid use and perception of clinical effectiveness (as indicated by patient-reported analgesia) with EDA response. Subjects with extensive opioid use, lower levels of reported pain relief, and, presumably, tolerance to this class of drug’s pharmacologic effects showed little to no EDA change at the doses prescribed, while opioid-naïve or less experienced patients demonstrated more dramatic changes. This finding raises the possibility of developing an objective measure of opioid tolerance using biosensors that could guide clinician prescribing.
Cerebral lateralization and resultant differences in EDA measured in bilateral limbs of the same individual has been well documented [6, 9] Complex differences in EDA measurements with respect to laterality have been observed in normal subjects performing a variety of physical and cognitive tasks [9, 10]. Furthermore, studies looking at stress responses and EDA demonstrate conflicting results, with some demonstrating exaggeration of bilateral differences and others showing decreased variability or even pattern reversal [11]. Striking asymmetries in bilateral EDA measurements appear to be more pronounced specifically in periods of high anxiety or perceived threat [6]. Interestingly, the presence of stereotyped lateralizing behaviors that are considered indicative of nigrostriatal asymmetry has been linked with differential patterns of drug abuse in animal models [12], but laterality in EDA response to substance use has not been documented. Factors that may influence magnitude of response include hand dominance, gender, and preexisting CNS pathology, such as traumatic brain injury [11, 13]. It is hypothesized that multiple areas of the CNS contribute to the EDA at a given location in the body, some of which discharge in an ipsilateral fashion and others in a contralateral fashion, potentially leading to differences in bilateral expression patters with various physiologic stimuli [6]. In the single ED patient wearing bilateral sensors, comparable trends were observed bilaterally (e.g., the EDA increased in both wrists), but this degree of change was greater in the nondominant wrist measurement. Cerebral hemispheric dominance and laterality of EDA expression will undoubtedly add a layer of complexity to the application of this technology to drug detection and will require further exploration.
Clinical trials for substance abuse interventions that employ wearable biosensors require that this equipment be distributed to drug users who may lose these expensive devices or may have an incentive to sell them. The distribution, use, and recovery of a wearable biosensor from an active drug user in this study is consistent with the findings of previous research using cellphones and personal data assistants as successful data collection devices for opioid and cocaine abusers [14–17]. In contrast to smartphones, personal data assistants, and beepers, wearable biosensors are potentially stigmatizing mobile electronic devices. Although the avidity with which active drug users accept wearable biosensors cannot be ascertained from this study, these data suggest the feasibility of recovering an expensive ubiquitous computing device such as the biosensor.
Limitations
The main limitation of this study is the inability to generalize these findings due to small sample size in this pilot and the fact that only a single subject was recorded in a natural setting outside of a healthcare facility. Also, the dosing of opioid analgesic based on providers’ discretion likely differs from subject initiated recreational use. Unilateral data was obtained on four of the five patients and may have missed a more brisk response on the contralateral side. Lack of prolonged data recording on individuals prior to drug administration makes it difficult to interpret how much these changes would differ from baseline variability in EDA.
Conclusion
In this small pilot study, wearable biosensors were able to detect the administration of opioids as well as cocaine, were acceptable to our subjects and were feasible to use in this capacity. The nature and intensity of patients’ biometric response to drug administration, in a small sample of patients, suggests that the physiologic response to opioids may vary inversely with opioid tolerance. Wearable biosensors show promise as adjuncts to drug abuse treatment research to help determine the efficacy of relapse prevention interventions. Follow-up studies are underway to explore both the feasibility and accuracy of this approach using larger sets of data, natural use settings, and various levels of drug exposure history.
References
Boyer EW, Smelson D, Fletcher R et al (2010) Wireless technologies, ubiquitous computing and mobile health: application to drug abuse treatment and compliance with HIV therapies. J Med Toxicol 6:212–216. doi:10.1007/s13181-010-0080-z
Boyer EW, Fletcher R, Fay RJ et al (2012) Preliminary efforts directed toward the detection of craving of illicit substances: the iHeal project. J Med Toxicol 8:5–9. doi:10.1007/s13181-011-0200-4
Harrison L.The validity of self-reported drug use in survey research: an overview and critique of research methods 1997.
Fletcher RR, Tam S, Omojola O, et al. Wearable sensor platform and mobile application for use in cognitive behavioral therapy for drug addiction and PTSD. 2011 33rd Annual International Conference of the IEEE Engineering in Medicine and Biology Society. 2011; 1802–1805. doi: 10.1109/IEMBS.2011.6090513
Poh M-Z, Loddenkemper T, Reinsberger C et al (2012) Convulsive seizure detection using a wrist-worn electrodermal activity and accelerometry biosensor. Epilepsia 53:e93–e97. doi:10.1111/j.1528-1167.2012.03444.x
Picard RW, Fedor S, Ayzenberg Y. Multiple arousal theory and daily-life electrodermal activity asymmetry. Emotion Review. 2014.
Adams P, Rabbi M, Rahman T, et al. Towards personal stress informatics: comparing minimally invasive techniques for measuring daily stress in the wild. pac.cs.cornell.edu
Healey JA, Picard RW (2005) Detecting stress during real-world driving tasks using physiological sensors. Intell Transp Syst. doi:10.1109/TITS.2005.848368
Schulter G, Papousek I (1998) Bilateral electrodermal activity: relationships to state and trait characteristics of hemisphere asymmetry. Int J Psychophysiol 31:1–12. doi:10.1016/S0167-8760(98)00027-0
Schulter G, Papousek I (1992) Bilateral electrodermal activity: reliability, laterality and individual differences. Int J Psychophysiol 13:199–213. doi:10.1016/0167-8760(92)90070-R
Světlák M, Bob P, Roman R, Ježek S. Stress-induced alterations of left-right electrodermal activity coupling indexed by pointwise transinformation. Physiological research. 2013.
Glick SD, Hinds PA (1985) Differences in amphetamine and morphine sensitivity in lateralized and non-lateralized rats: locomotor activity and drug self-administration. Eur J Pharmacol 118:239–244. doi:10.1016/0014-2999(85)90134-7
Dubé A-A, Duquette M, Roy M et al (2009) Brain activity associated with the electrodermal reactivity to acute heat pain. NeuroImage 45:169–180. doi:10.1016/j.neuroimage.2008.10.024
Freedman MJ, Lester KM, McNamara C et al (2006) Cell phones for ecological momentary assessment with cocaine-addicted homeless patients in treatment. J Subst Abuse Treat 30:105–111. doi:10.1016/j.jsat.2005.10.005
Epstein DH, Willner-Reid J, Vahabzadeh M et al (2009) Real-time electronic diary reports of cue exposure and mood in the hours before cocaine and heroin craving and use. Arch Gen Psychiatry 66:88–94. doi:10.1001/archgenpsychiatry.2008.509
Preston KL, Epstein DH (2011) Stress in the daily lives of cocaine and heroin users: relationship to mood, craving, relapse triggers, and cocaine use. Psychopharmacology 218:29–37. doi:10.1007/s00213-011-2183-x
Epstein DH, Marrone GF, Heishman SJ et al (2010) Tobacco, cocaine, and heroin: craving and use during daily life. Addict Behav 35:318–324. doi:10.1016/j.addbeh.2009.11.003
Acknowledgments
Authors would like to acknowledge the generous financial support from the University of Massachusetts Department of Emergency Medicine and the National Institute of Health.
Funding Acknowledgements
This project was funded by the National Institute on Drug Abuse, National Institutes of Health through Grant Number R01DA033769-01 and a grant from the University of Massachusetts Emergency Medicine Research Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carreiro, S., Smelson, D., Ranney, M. et al. Real-Time Mobile Detection of Drug Use with Wearable Biosensors: A Pilot Study. J. Med. Toxicol. 11, 73–79 (2015). https://doi.org/10.1007/s13181-014-0439-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13181-014-0439-7