Abstract
Background/aims
The reciprocal promotion of cancer and stroke occurs due to changes in shared risk factors, such as metabolic pathways and molecular targets, creating a “vicious cycle.” Cancer plays a direct or indirect role in the pathogenesis of ischemic stroke (IS), along with the reactive medical approach used in the treatment and clinical management of IS patients, resulting in clinical challenges associated with occult cancer in these patients. The lack of reliable and simple tools hinders the effectiveness of the predictive, preventive, and personalized medicine (PPPM/3PM) approach. Therefore, we conducted a multicenter study that focused on multiparametric analysis to facilitate early diagnosis of occult cancer and personalized treatment for stroke associated with cancer.
Methods
Admission routine clinical examination indicators of IS patients were retrospectively collated from the electronic medical records. The training dataset comprised 136 IS patients with concurrent cancer, matched at a 1:1 ratio with a control group. The risk of occult cancer in IS patients was assessed through logistic regression and five alternative machine-learning models. Subsequently, select the model with the highest predictive efficacy to create a nomogram, which is a quantitative tool for predicting diagnosis in clinical practice. Internal validation employed a ten-fold cross-validation, while external validation involved 239 IS patients from six centers. Validation encompassed receiver operating characteristic (ROC) curves, calibration curves, decision curve analysis (DCA), and comparison with models from prior research.
Results
The ultimate prediction model was based on logistic regression and incorporated the following variables: regions of ischemic lesions, multiple vascular territories, hypertension, D-dimer, fibrinogen (FIB), and hemoglobin (Hb). The area under the ROC curve (AUC) for the nomogram was 0.871 in the training dataset and 0.834 in the external test dataset. Both calibration curves and DCA underscored the nomogram’s strong performance.
Conclusions
The nomogram enables early occult cancer diagnosis in hospitalized IS patients and helps to accurately identify the cause of IS, while the promotion of IS stratification makes personalized treatment feasible. The online nomogram based on routine clinical examination indicators of IS patients offered a cost-effective platform for secondary care in the framework of PPPM.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The PPPM approach is vital for the treatment and clinical management of occult cancers in IS patients
The global burden of stroke continues to increase with the aging of the population and the prevalence and poor control of risk factors such as obesity, diabetes, hypertension, and hyperlipidemia. In 2020, the direct and indirect economic burden of stroke worldwide will reach $891 billion [1]. In the last three decades (from 1990 to 2020), the absolute number of strokes has doubled [2]. Stroke is the second leading cause of death worldwide, and the World Stroke Organization (WSO) predicts that stroke mortality and disability-adjusted life years (DALYs) will continue to increase significantly over the next thirty years (2020 to 2050) [1]. Additionally, stroke and cancer often coexist in clinical practice, with one-tenth of ischemic stroke (IS) patients suffering from cancer [3]. As stroke and cancer treatment methods continue to improve, patients’ median survival rates are expected to increase. However, individuals who are dealing with both cancer and stroke concurrently have a notably unfavorable prognosis. This is characterized by increased disability rates and elevated healthcare costs compared to those without cancer [4,5,6,7,8,9]. Moreover, in some cases, stroke may be a first manifestation of an underlying occult cancer. A recent comprehensive review highlighted that the cumulative diagnosis rate of cancer during the initial year subsequent to an IS was 13.6 per thousand (95% confidence interval (CI) 5.6–24.8). This incidence was markedly higher in cases with cryptogenic stroke (62.0 per thousand, 95% CI 13.6–139.3), as well as after cancer screening (39.2 per thousand, 95% CI 16.4–70.6) [10]. A registry study from Switzerland found that 5.4% of acute IS patients suffer from active cancer, with a quarter diagnosed during or within 12 months of hospitalization for index stroke [6].
The reactive medical approach used in the treatment and clinical management of IS patients makes it difficult to detect underlying occult cancer during the first admission. The Suboptimal Health Study Consortium and European Association for Predictive, Preventive and Personalized Medicine proposed that predictive, preventive, and personalized medicine (PPPM/3PM) strategies could benefit stroke patients, particularly those with unknown etiology [11]. Once hospitalized IS patients are initially stabilized, there is an urgent need to accurately identify the cause of IS so that appropriate secondary prevention measures can be initiated. Based on this, applying the PPPM approach to identify underlying occult cancers in patients with IS as early as possible, and carrying out appropriate secondary prevention measures, such as cancer treatment, based on specific cancer and stroke conditions, may not only help prolong the survival time of cancer patients, and also contribute to the personalized treatment and clinical management of cancer-associated stroke patients.
The mechanisms of the reciprocity between IS and cancers
Some metabolic pathways and molecular targets are common risk factors for IS and cancer, and changes in these factors can lead to irreversible damage, such as silent brain microinfarction (SBI), retinal microvascular abnormalities, systemic inflammation, mitochondrial impairments, pre-cancerous lesions, pre-metastatic niches, which in turn can lead to activated metalloproteinases, blood–brain barrier (BBB) breakdown, and ultimately altogether leading to the reciprocal cancer-stroke promotion in a “vicious cycle” [12]. On the other hand, there are multiple direct or indirect factors significantly associated with the occurrence of IS in cancer patients, including compression, embolism, or invasion of blood vessels, cancer induced activation of the coagulation cascade and fibrinolysis-system hyperactivity, treatment-related side effects, and non-bacterial thrombotic endocarditis [3, 13]. It is precisely based on the aforementioned mechanisms of the reciprocity between IS and cancer that clinical practice simultaneously faces the challenges of underlying occult cancer in IS patients and underlying occult IS in cancer patients.
Challenges in predicting occult cancers of IS patients
Prior investigations have elucidated several factors independently linked with active cancer in IS patients. These include undetermined etiology stroke, inflammatory markers, hypercoagulability indicators, smoking history, and the presence of lesions spanning multiple vascular territories, as evident through diffusion-weighted imaging (DWI) [14,15,16]. Although multiple clinical risk scores have been proposed, the corresponding clinical scores have not been compared with different statistical methods and are mostly single center studies, lacking sufficient internal validation and external data validation. It is crucial to develop and validate a reliable model for identifying occult cancers in hospitalized patients with IS, and to use more intuitive and convenient methods to assist clinical decision-making, thereby translating PPPM into clinical practice.
Working hypothesis in the framework of PPPM
We hypothesized that a multiparameter model based on routine clinical examination indicators of IS patients would enable identification of occult cancer and stratification of hospitalized IS patients. Therefore, based on multiparameter analysis, this current study endeavors to devise a nomogram intended for the discreet detection of occult cancers among patients afflicted by IS, thereby achieving patient stratification within the PPPM framework to promote a shift from reactive medicine to predictive diagnosis and targeted interventions. To be more precise, we embarked on an investigation to identify suitable biomarkers and, through comparative analysis of diverse prediction models, construct and autonomously validate a dependable instrument that can be readily utilized within clinical practice in the context of PPPM.
Methods
Data collection and study population
A matched case–control investigation was conducted involving patients admitted with a diagnosis of IS. The recruitment spanned from January 2014 to January 2019, with participants enrolled at the Stroke Center of the First Affiliated Hospital of Xiamen University. For the training dataset, a cohort of 136 IS patients with active cancer was meticulously selected and juxtaposed against a control group, establishing a 1:1 ratio based on age and sex. Additionally, 239 cases (121 in the cancer group vs. 118 in the control group) were procured from six other centers for external validation. Comprehensive details concerning the six research centers can be found within the supplementary materials.
Inclusion criteria were delineated as follows: (1) IS cases adhering to the Baltimore-Washington Cooperative Young Study Criteria, and (2) brain magnetic resonance imaging (MRI) scans consistently reflecting infarctions in line with clinical presentations. Exclusion criteria encompassed: (1) the presence of risk factors for cardioembolic stroke, such as atrial fibrillation, valvular heart disease, and prosthetic valve replacement; (2) aortic stenosis responsible for cerebral infarction exceeding 50%; (3) substantial brain parenchymal atrophy; and (4) hematological malignancies and primary intracranial malignancies or intracranial metastases, given their anticipated distinct underlying mechanisms for cerebral infarction [17].
The definition of active cancer entailed: (1) previously ascertained malignancy, (2) recent cancer diagnosis, and (3) ongoing cancer treatment, all occurring within 12 months preceding or following the index stroke. These details were discerned from the electronic medical records of the patients.
Predicated on pertinent literature and expert consensus, the following factors were meticulously gleaned from the electronic medical record system to serve as observational markers: (1) socio-demographics: gender and age; (2) habits: smoking and alcohol consumption; (3) medical history: diabetes, hypertension, and hyperlipidemia; (4) stroke features: regions of ischemic lesions and involvement of multiple vascular territories; (5) type of cancer; and (6) laboratory indicators: D-dimer, fibrinogen (FIB), hemoglobin (Hb), platelet count (PLT), lactic dehydrogenase (LDH). All the indicators mentioned above were collected upon admission.
Blinding of validation data
The validation data for external assessment was supplied in a concealed manner, withholding information regarding the diagnosis of active cancer. Comprehensive particulars of the groupings only emerged subsequent to the culmination of the prediction analysis.
Patient and public involvement statements
The design, execution, reporting, and dissemination of our research did not encompass the involvement of patients or the general public.
Statistical analyses
In the initial phase, descriptive statistics were utilized to ascertain disparities in the observation indicators between the cancer group and the control group within the training data. This was accomplished through chi-square tests for categorical variables and the Wilcoxon rank-sum test for numeric variables. It is noteworthy that laboratory examination indicators were regarded as both numeric variables and categorical variables (normal vs. abnormal). Local medical references were employed to classify laboratory examination indicators such as D-dimer (< 0.5 μg/mL), FIB (2–4 g/L), Hb (115–150 g/L in females, 130–175 g/L in males), PLT (125–350 109/L), and LDH (< 240 U/L) as normal.
Following the exclusion of non-significant variables, multivariable logistic regression was engaged to uncover the factors influencing the occurrence of IS in conjunction with cancer. This entailed estimation of odds ratios (ORs) alongside corresponding 95% confidence intervals (CIs). Subsequently, significant variables were retained for subsequent analysis.
In the subsequent phase, the risk associated with IS in patients with cancer was assessed through the implementation of logistic regression, Lasso regression, linear discriminant analysis (LDA), Naïve Bayes, support vector machine (SVM), and Random Forest models. These models, being conventional tools for classification within machine learning, were trained using the data of 272 IS patients as delineated earlier. Internal validation entailed a ten-fold cross-validation, yielding IS-cancer probabilities for evaluating predictive performance. To quantify predictive accuracy, the area under the receiver operating characteristic curves (AUC-ROC) was computed for each of the six aforementioned statistical approaches. Pairwise comparisons were conducted to discern distinctions between the various models. The ultimate model was chosen by jointly considering predictive accuracy and model interpretability. Subsequently, this chosen model underwent independent external validation using data from an additional 239 IS patients sourced from other centers.
Four, a nomogram was formulated utilizing the definitive prediction model. Visual scrutiny was directed towards calibrating the final model.
Five, we conducted a comparative analysis between the ultimate prediction model and two models documented in prior investigations. The assessment of their predictive efficacy was undertaken through the analysis of AUC-ROC. The three prediction models were expressed as follows: [Model 1]: logit (IS with cancer) ~ Ischemic lesions regions + Multiple vascular territories + Hypertension + D-dimer + FIB + Hb; [Model 2]: logit (IS with cancer) ~ Hyperlipidemia + D-dimer + FIB; [Model 3]: logit (IS with cancer) ~ Smoke + D-dimer + Hb.
Lastly, two sensitivity analyses were executed to scrutinize any disparities in predictive performance. Firstly, laboratory examination indicators within the final model were appraised as numeric variables to assess any shifts in model performance. Secondly, the final model was further subjected to validation within the following subset: (1) omission of IS patients whose cancer was identified prior to the incidence of IS and (2) exclusion of external centers contributing a sample size of fewer than 20.
All P-values were two-tailed. P-values < 0.05 were deemed statistically significant. The entirety of statistical analyses was executed employing R software (R version 4.2.1, USA).
Results
Patient characteristics
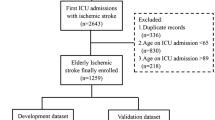
A succinct representation of the study's procedural flow is depicted in Fig. 1, while Table 1 provides an overview of demographic data encompassing the cancer and control groups within the training dataset. Given the matched age and sex distribution, no statistically significant distinctions in these attributes were noted. In an overarching context, the cancer group exhibited a predominance of elderly participants (median age of 69 years) and a higher proportion of males (cancer group: 65.44%, control group: 61.03%). The analysis of ischemic lesion regions indicated a higher incidence of “both Internal Carotid Artery (ICA) and Vertebral-Basilar Artery (VBA)” involvement in the cancer group compared to the control group (40.44% vs. 13.97%, P < 0.001). Furthermore, the cancer group displayed a greater prevalence of involvement across multiple vascular territories in contrast to the control group (61.03% vs. 30.15%, P < 0.001). Noteworthy differences in demographic variables were observed, with the cancer group exhibiting lower rates of alcohol consumption (P = 0.022), hypertension (P < 0.001), diabetes (P < 0.001), and hyperlipidemia (P = 0.003) relative to the control group. Irrespective of classification criteria, the cancer group consistently demonstrated elevated levels of D-dimer (P < 0.001), FIB (P < 0.001), PLT (P < 0.001), and LDH (P < 0.05) compared to the control group, while concurrently presenting lower levels of Hb (P < 0.05). Within the subset of 257 IS patients diagnosed with cancer, the distribution of cancer types is delineated in Supplementary Fig. 1. Notably, the top three cancers identified are lung cancer (24.32%), bowel cancer (14.29%), and genitourinary system cancers (13.13%), findings consistent across both the training and validation datasets.
Multivariable logistic regression
The forest plot (Supplementary Fig. 2) comprehensively illustrates the assessment conducted through a multivariable logistic regression model encompassing all observation indicators (excluding age and sex). Subsequent to multivariate analysis, several attributes emerged as significant independent factors associated with active cancer among IS patients. Notable associations include ischemic lesion regions (vertebral-basilar artery (VBA) vs. Internal Carotid Artery (ICA)) (OR = 0.423, 95% CI 0.184–0.972), involvement of multiple vascular territories (OR = 2.691, 95% CI 1.165–6.215), presence of hypertension (OR = 0.398, 95% CI 0.18–0.879), abnormal D-dimer (OR = 11.034, 95% CI 5.063–24.046), abnormal FIB (OR = 6.121, 95% CI 2.842–13.185), and abnormal Hb (OR = 3.424, 95% CI 1.684–6.959)—all exhibiting statistical significance (all P < 0.05). Consequently, these variables, encompassing ischemic lesion regions, involvement of multiple vascular territories, hypertension, D-dimer, FIB, and Hb, underwent further scrutiny across six distinct prediction models, as delineated below.
Internal cross validation and final model
The comparison of prediction performance among six prediction models is outlined in Table 2. Employing a ten-fold cross-validation procedure on the training set (comprising 272 IS patients), the computation of the AUC was undertaken for each statistical method in prognosticating cancer occurrence in IS patients. The AUC, a gauge of predictive accuracy, ranges between 0.5 (signifying random prediction) and 1 (representing perfect prediction). Following the ten-fold cross-validation, all models showcased robust predictive performance (all AUC > 0.84). Among the sextet of models evaluated, the multivariable logistic regression model (AUC = 0.854, 95% CI 0.806–0.894) exhibited no statistically significant distinction in comparison to other models (pairwise comparison P > 0.05). In light of these outcomes, the multivariable logistic regression model was subsequently employed for the formulation of a nomogram, and its independent validation was executed using an external test dataset encompassing 239 IS patients.
Nomogram and independent validation
The construction of a nomogram for the estimation of cancer risk probabilities in IS patients was accomplished utilizing the training dataset comprising 272 IS patients. Subsequent to formulation, the nomogram underwent validation using an independent external test dataset, encompassing 239 IS patients. The AUC for the nomogram in the training dataset registered at 0.871 (95% CI 0.825–0.908), while the external test dataset yielded an AUC of 0.834 (95% CI 0.781–0.879). In both datasets, the calibration curves, embodying three lines (Apparent, Bias-corrected, and Ideal), evinced a close alignment, affirming sound calibration (refer to Fig. 2). Additionally, the validation of the nomogram using data distinct to each center yielded AUC values spanning from 0.766 to 1.00 (detailed in Supplementary Table 1).
Nomogram for cancer risk in IS patients and predictive performance. A Nomogram depicting the estimation of occult cancer risk in IS patients. Based on the 6 variables in the nomogram, the individual clinical characteristics of the patient are inputted and the total score of the patient is calculated. Each total score corresponds to a probability of IS with the occurrence cancer. B Assessment of the nomogram’s predictive performance in the training data. C Evaluation of the nomogram’s predictive performance in the validation data. The diagonal dashed line and solid line in the calibration curve represent the perfect prediction of the ideal model and the performance of the column chart, respectively. The solid line near the dashed line represents better prediction
Comparative analysis with two other studies
As demonstrated in Fig. 3, juxtaposed against the two prediction models propounded by Selvik and Jiang in their respective studies, our nomogram exhibited markedly superior performance, boasting significantly elevated AUC values across both the training data (Fig. 3A) and validation data (Fig. 3B). Additionally, to underscore the clinical applicability of the nomogram, we deployed DCA analysis to juxtapose it with the aforementioned models. Evidently, clinical interventions guided by our nomogram yielded greater net benefit than the models proposed by Selvik and Jiang across diverse threshold probabilities (as depicted in Fig. 3C, D).
ROC curves and DCA analysis for three different models. A AUC comparison among three models in the training data. B AUC comparison among three models in the validation data. AUC: area under the ROC curve. The higher the AUC of the prediction model, the better the predictive performance. Model 1 (the nomogram) exhibited markedly superior performance, boasting significantly elevated AUC values across both the training data and validation data. C Clinical utility comparison among three models in the training data. D Clinical utility comparison among three models in the validation data. The further the model curve is from these two lines (the horizontal line and slashes), the better the clinical value of the nomogram. Model 1 (the nomogram) exhibited markedly superior clinical value across both the training data and validation data
Sensitivity analysis
By integrating numeric laboratory examination indicators as variables within the final prediction model, the model demonstrated commensurate performance (AUC = 0.865 in training data, AUC = 0.812 in validation data, refer to Supplementary Fig. 3A). Moreover, in the subset of IS patients where cancer was identified subsequent to the incidence of IS, the predictive prowess of the current nomogram remained largely unaffected (AUC = 0.874, as evidenced in Supplementary Fig. 3B Subset 1). Notably, even after the exclusion of the center contributing a smaller sample, the predictive performance of the prevailing nomogram within the external validation dataset exhibited marginal variation (AUC = 0.823, illustrated in Supplementary Fig. 3B Subset 2).
Discussion
Overview of research findings
In this study, we considered the PPPM strategy for predictive diagnosis occult cancer in patients with IS. The primary aim of this study encompassed the formulation and subsequent validation, both within and beyond the study cohort, of an individualized cancer risk nomogram designed for patients recently diagnosed with IS. The inclusion criteria for the prediction model entailed attributes such as ischemic lesions regions, multiple vascular territories, hypertension, D-dimer, FIB, and Hb. In contrast to alternative prediction models, the multivariable logistic regression model emerged as the most fitting choice when evaluated within the internal dataset sourced from the Stroke Center of the First Affiliated Hospital of Xiamen University (comprising 272 IS patients). Notably, the model’s efficacy was further established through autonomous external validation, utilizing an external dataset culled from six distinct centers (encompassing 239 IS patients).
A comprehensive overview of patient characteristics, spanning both the training data and the external validation data, has been meticulously catalogued in Supplementary Table 2. Noteworthy dissimilarities surfaced in the MRI results and laboratory examination outcomes. This discernible asymmetry could potentially be attributed to variations in patient origins and differing hospital tiers across the diverse centers. Addressing this imbalance would indeed enhance the model's extrapolative efficacy, thereby rendering it a more robust tool for evaluation.
Multiparametric analysis reveals the mechanisms of the reciprocity between IS and cancers
Cancer patients with brain malignancies and metastases to the brain (e.g., triple-negative breast cancer) and cancer patients receiving vasculo-toxic therapies, demonstrating extensive vasoconstriction and systemic hypoxic-ischemic lesions including SBI [18, 19]. Notably, the inclusion of ischemic lesion regions spanning both the ICA and VBA, as well as those confined solely to the ICA, was indicative of an escalated cancer risk, thereby surpassing the corresponding risk associated with lesions situated solely within the VBA. Furthermore, IS patients manifesting infarctions across multiple vascular territories exhibited an amplified cancer risk. Intriguingly, these two distinct MRI markers independently correlated with cancer in IS patients within our study. This aligns with analogous findings observed in prior investigations [20, 21].
Antecedent studies have corroborated that escalated D-dimer levels, augmented FIB, and diminished Hb levels can serve as prognosticators of occult cancer amidst stroke patients [22, 23]. IS patients with concomitant cancer often present an augmented hypercoagulable state, instigating abnormal D-dimer, FIB, and Hb readings [24]. Evidence from the Acute STroke Registry and Analysis of Lausanne registry study indicated that nearly half of cancer-associated IS patients are attributed to hypercoagulability [6]. Our study fortifies the utility of these three laboratory examination indicators for prognosticating cancer in IS patients.
Our comprehensive analyses further illuminated a noteworthy finding: the absence of a history of hypertension emerged as an independent correlate of active cancer presence in individuals afflicted with IS. This intriguing observation aligns with earlier investigations [25], even though a majority of studies tend to dismiss any association between hypertension and IS. Most infarcts in IS are lacunes, of which hypertensive small-vessel disease is thought to be the main cause [26]. And the intricate interplay between hypertension and cancer risk has yielded conflicting outcomes in prior research [27,28,29]. Given the prevailing global prevalence and elevated fatality rates attributed to hypertension, a renewed emphasis on dissecting the mechanistic role of hypertension in IS patients with concurrent cancer warrants earnest consideration.
Cancer screening in hospitalized IS patients and benefit from the proposed approach
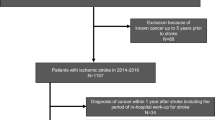
The increasing prevalence of both stroke and cancer highlights the significant impact of changing socioeconomic conditions and an aging population [30, 31]. In the contemporary landscape, these two maladies reign as principal contributors to mortality. In response, evolving guidelines advocate for cancer screening among individuals afflicted with stroke [32, 33]. The trajectory ahead portends a landscape where a burgeoning cohort of stroke patients will likely harbor occult cancers. In view of the discernible disparage in healthcare standards among diverse medical institutions, coupled with persisting scarcity of medical resources in numerous regions across China, the pragmatic application of the nomogram delineated within our study offers a potent tool. Its judicious employment stands to empower neurologists, particularly those operating within resource-scarce domains, to effectively unveil concealed malignancies within stroke patients. This, in turn, augments clinical decision-making abilities and serves to alleviate the overall disease burden. With the aid of the nomogram furnished by this study, we advocate for a diagnostic flowchart to identify potential occult cancers in stroke patients (Fig. 4). We proffer the proposition that hospitalized stroke patients be simultaneously evaluated for their propensity towards occult cancers. For those garnering a prediction probability exceeding 0.5, further investigative measures such as chest and lung CT, abdomen ultrasound, cancer biomarker assessments, and PET-CT scans stand imperative, facilitating precise diagnosis and subsequent follow-up endeavors.
Proposed flowchart for diagnosing occult cancer in IS patients. Based on online nomogram tools, rapid screening of newly admitted IS patients can be achieved, followed by clear diagnosis and targeted secondary prevention measures. A readily accessible online platform has been devised for cancer screening in hospitalized IS patients (https://stroke-cancer.shinyapps.io/dynnomapp/)
Strengths
Golubnitschaja et al. researchers have highlighted the underestimated risks associated with cryptogenic strokes and have called for predictive and in-depth diagnostics to enable targeted primary and secondary prevention [12]. In response to this call, our research explores the application of prediction tools tailored to convenient biomarkers for screening occult cancers among stroke patients, particularly those with cryptogenic stroke. Furthermore, strokes in young individuals with unclear etiology have been hypothetically associated with characteristic symptoms of the Flammer syndrome phenotype, including low body weight, abnormal stress reactions, and vascular dysregulation [12, 34]. Given the interaction between cancer and stroke, as well as the high incidence rate of cancer following stroke diagnosis, our research focuses on the early screening of occult cancer and its relationship with IS to facilitate the initiation of appropriate secondary prevention measures. Additionally, various biomarkers, such as cell-free nucleic acid, tears, blood, and others, have been applied to stroke management [35, 36]. Our study utilized only a few necessary and timely examination indicators for stroke patients during admission to construct a predictive model, without involving additional processes like sample preservation, mass spectrometry analysis, and sequencing technology application. This approach is more conducive to the clinical application of screening tools.
Limitations
To our knowledge, this study marks the first attempt to provide a nomogram specifically designed to offer personalized cancer risk predictions for patients who have been newly diagnosed with IS. We developed and validated a routine model for screening occult cancer in hospitalized IS patients in the context of PPPM. However, it is important to acknowledge the limitations inherent in our current study. Firstly, the study may be infiltrated by inherent selection bias due to the matched case–control design, which could potentially introduce imprecision and reduce statistical power. Fortunately, this concern is somewhat mitigated by the fact that the external dataset does not strictly match age and sex, which strengthens the stability and reliability of the predictive performance of our final model. Secondly, it is noteworthy that our exhaustive exploration of risk factors for IS co-occurring with cancer remained elusive in the current iteration. For example, it should be noted that certain cancer markers, such as Carbohydrate Antigen (CA) 125, have been reported to be independently associated with cancer-related IS [37]. However, it should be mentioned that the variables included in our final model are routine assessments that are mandatory during the initial admission of stroke patients, which is a crucial aspect for early detection of IS with concurrent cancer. Thirdly, for the sake of operational ease pertaining to the nomogram, our final model subdivided laboratory examination indicators into a binary categorization of “normal” and “abnormal.” This, indeed, may entail certain fluctuations in predictive performance, given that benchmarks for normalcy exhibit variations across diverse regions and nations. However, our sensitivity analysis reveals that the inclusion of numerical laboratory indicators did not improve predictive accuracy compared to categorical laboratory indicators in the final model. Finally, it should be noted that a detailed exploration including subgroup analyses focusing on different types of cancer has not been conducted. In our study, the largest group was comprised of lung cancer patients (24.32%). Different types of malignancies may have different characteristics in IS patients, highlighting the need to develop customized predictive tools in future research.
Conclusion and expert recommendations
IS and cancer are two of the most prevalent and deadly diseases in the world. In particular, cancer-related stroke is frequently discussed, and identifying IS patients with concurrent cancer is of significant clinical importance. Current reactive medical approaches to disease management do not enable timely identification and treatment of IS patients with underlying occult cancer. Therefore, we utilized basic clinical information obtained from the admission examinations of IS patients to develop and validate a risk prediction model for occult cancer. A nomogram tool constructed based on the model assists clinicians and patients in predicting their own occult cancer and making informed decisions regarding whether to undergo detailed, targeted cancer screening. Additionally, the model facilitates precise prevention and personalized management of patients with cancer-related stroke, thus supporting a paradigm shift in stroke management from reactive therapy to PPPM.
Predictive medical approach
We propose that occult cancer screening be conducted for IS patients based on PPPM. In our current study, we have developed and validated a nomogram that allows for individualized cancer risk assessment for IS patients. This innovative instrument propounds an estimation of cancer risk that is uniquely personalized, as opposed to a more generic collective appraisal predicated upon specific patient-centric attributes. This nomogram has significant implications, as it can aid both patients and healthcare practitioners in determining the need for cancer screening at the early stages of IS admission. Additionally, it can greatly enhance treatment decisions, prognostic evaluation, and ultimately reduce the burden of disease. To facilitate the clinical implementation of this nomogram, we have created an easily accessible online platform (https://stroke-cancer.shinyapps.io/dynnomapp/).
Targeted prevention and personalized treatments
Given the high risk of occult cancers in IS patients, it is crucial to conduct regular cancer screening for early detection [6, 10, 38, 39]. Our proposed nomogram can be used as a population-based screening tool for occult cancers in hospitalized IS patients, particularly those with cryptogenic IS. If the prediction probability from the nomogram exceeds 0.5, additional investigative procedures such as chest and lung CT scans, abdomen ultrasound, cancer biomarker assessments, and PET-CT scans should be conducted to accurately diagnose and follow up with the patient. Moreover, for IS patients with comorbid occult cancers, personalized interventions and treatments can be developed based on the progression of both the cancer and IS. This nomogram facilitates early diagnosis of occult cancers in hospitalized IS patients and helps identify the underlying cause of IS. By promoting IS stratification, personalized treatment becomes feasible. Therefore, we believe that in the context of PPPM, the nomogram prediction model presented in this study offers the best strategy for personalized management of patients with cancer-related stroke.
Data availability
The datasets upholding the outcomes of this study can be accessed through a judicious solicitation to the corresponding author.
Code availability
All software applications used are included in this article.
Abbreviations
- AUC:
-
Area under the ROC curve
- AUC-ROC:
-
Area under the receiver operating characteristic curves
- BBB:
-
Blood-brain barrier
- CA:
-
Carbohydrate antigen
- CI:
-
Confidence interval
- DALYs:
-
Disability-adjusted life years
- DCA:
-
Decision curve analysis
- DWI:
-
Diffusion-weighted imaging
- FIB:
-
Fibrinogen
- Hb:
-
Hemoglobin
- ICA:
-
Internal carotid artery
- IS:
-
Ischemic stroke
- LDA:
-
Linear discriminant analysis
- LDH:
-
Lactic dehydrogenase
- MRI:
-
Magnetic resonance imaging
- ORs:
-
Odds ratios
- PLT:
-
Platelet count
- PPPM/3PM:
-
Predictive, preventive, and personalized medicine
- ROC:
-
Receiver operating characteristic
- SBI:
-
Silent brain microinfarction
- SVM:
-
Support vector machine
- VBA:
-
Vertebral-basilar artery
- WSO:
-
World Stroke Organization
References
Feigin VL, Owolabi MO. Pragmatic solutions to reduce the global burden of stroke: a world stroke organization-lancet neurology commission. Lancet Neurol. 2023;22(12):1160–206. https://doi.org/10.1016/s1474-4422(23)00277-6.
Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20(10):795–820. https://doi.org/10.1016/s1474-4422(21)00252-0.
Navi BB, Iadecola C. Ischemic stroke in cancer patients: a review of an underappreciated pathology. Ann Neurol. 2018;83(5):873–83. https://doi.org/10.1002/ana.25227.
Chae WH, Vössing A, Li Y, et al. Treatment of acute ischemic stroke in patients with active malignancy: insight from a comprehensive stroke center. Ther Adv Neurol Disord. 2023;16:17562864231207508. https://doi.org/10.1177/17562864231207508.
Seystahl K, Hug A, Weber SJ, et al. Cancer is associated with inferior outcome in patients with ischemic stroke. J Neurol. 2021;268(11):4190–202. https://doi.org/10.1007/s00415-021-10528-3.
Costamagna G, Hottinger A, Milionis H, et al. Clinical and demographic characteristics, mechanisms, and outcomes in patients with acute ischemic stroke and newly diagnosed or known active cancer. Neurology. 2023;100(24):e2477–89. https://doi.org/10.1212/wnl.0000000000207341.
Dearborn JL, Urrutia VC, Zeiler SR. Stroke and cancer- a complicated relationship. J Neurol Transl Neurosci. 2014;2(1):1039.
Castro HHG, Alencar AP, Bensenor IM, Lotufo PA, Goulart AC. Multimorbidities are associated to lower survival in ischaemic stroke: results from a Brazilian Stroke Cohort (EMMA Study). Cerebrovasc Dis. 2017;44(3–4):232–9. https://doi.org/10.1159/000479827.
Lin J, Wu S, Xu R, et al. Clinical Characteristics and risk factors of lung cancer-associated acute ischemic stroke. Biomed Res Int. 2019;2019:6021037. https://doi.org/10.1155/2019/6021037.
Rioux B, Touma L, Nehme A, Gore G, Keezer MR, Gioia LC. Frequency and predictors of occult cancer in ischemic stroke: a systematic review and meta-analysis. Int J Stroke. 2021;16(1):12–9. https://doi.org/10.1177/1747493020971104.
Wang W, Yan Y, Guo Z, et al. All around suboptimal health - a joint position paper of the suboptimal health study consortium and european association for predictive preventive and personalised medicine. EPMA J. 2021;12(4):403–33. https://doi.org/10.1007/s13167-021-00253-2.
Golubnitschaja O, Potuznik P, Polivka J Jr, et al. Ischemic stroke of unclear aetiology: a case-by-case analysis and call for a multi-professional predictive, preventive and personalised approach. EPMA J. 2022;13(4):535–45. https://doi.org/10.1007/s13167-022-00307-z.
Navi BB, Kasner SE, Elkind MSV, Cushman M, Bang OY, DeAngelis LM. Cancer and embolic stroke of undetermined source. Stroke. 2021;52(3):1121–30. https://doi.org/10.1161/strokeaha.120.032002.
Jiang J, Shang X, Zhao J, et al. Score for predicting active cancer in patients with ischemic stroke: a retrospective study. Biomed Res Int. 2021;2021:5585206. https://doi.org/10.1155/2021/5585206.
Selvik HA, Bjerkreim AT, Thomassen L, Waje-Andreassen U, Naess H, Kvistad CE. When to screen ischaemic stroke patients for cancer. Cerebrovasc Dis. 2018;45(1–2):42–7. https://doi.org/10.1159/000484668.
Seystahl K, Gramatzki D, Wanner M, et al. A risk model for prediction of diagnosis of cancer after ischemic stroke. Sci Rep. 2023;13(1):111. https://doi.org/10.1038/s41598-022-26790-y.
Grisold W, Oberndorfer S, Struhal W. Stroke and cancer: a review. Acta Neurol Scand. 2009;119(1):1–16. https://doi.org/10.1111/j.1600-0404.2008.01059.x.
Kubatka P, Mazurakova A, Koklesova L, et al. Antithrombotic and antiplatelet effects of plant-derived compounds: a great utility potential for primary, secondary, and tertiary care in the framework of 3P medicine. EPMA J. 2022;13(3):407–31. https://doi.org/10.1007/s13167-022-00293-2.
Polivka J Jr, Kralickova M, Polivka J, Kaiser C, Kuhn W, Golubnitschaja O. Mystery of the brain metastatic disease in breast cancer patients: improved patient stratification, disease prediction and targeted prevention on the horizon? EPMA J. 2017;8(2):119–27. https://doi.org/10.1007/s13167-017-0087-5.
Nouh AM, Staff I, Finelli PF. Three Territory Sign: an MRI marker of malignancy-related ischemic stroke (Trousseau syndrome). Neurol Clin Pract. 2019;9(2):124–8. https://doi.org/10.1212/CPJ.0000000000000603.
Gon Y, Okazaki S, Terasaki Y, et al. Characteristics of cryptogenic stroke in cancer patients. Ann Clin Transl Neurol. 2016;3(4):280–7. https://doi.org/10.1002/acn3.291.
Grazioli S, Paciaroni M, Agnelli G, et al. Cancer-associated ischemic stroke: a retrospective multicentre cohort study. Thromb Res. 2018;165:33–7. https://doi.org/10.1016/j.thromres.2018.03.011.
Quintas S, Rogado J, Gullon P, et al. Predictors of unknown cancer in patients with ischemic stroke. J Neurooncol. 2018;137(3):551–7. https://doi.org/10.1007/s11060-017-2741-0.
Lee MJ, Chung JW, Ahn MJ, et al. Hypercoagulability and mortality of patients with stroke and active cancer: the OASIS-CANCER study. J Stroke. 2017;19(1):77–87. https://doi.org/10.5853/jos.2016.00570.
Kono T, Ohtsuki T, Hosomi N, et al. Cancer-associated ischemic stroke is associated with elevated D-dimer and fibrin degradation product levels in acute ischemic stroke with advanced cancer. Geriatr Gerontol Int. 2012;12(3):468–74. https://doi.org/10.1111/j.1447-0594.2011.00796.x.
Vermeer SE, Longstreth WT Jr, Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurol. 2007;6(7):611–9. https://doi.org/10.1016/s1474-4422(07)70170-9.
Han H, Guo W, Shi W, et al. Hypertension and breast cancer risk: a systematic review and meta-analysis. Sci Rep. 2017;7:44877. https://doi.org/10.1038/srep44877.
Liang Z, Xie B, Li J, et al. Hypertension and risk of prostate cancer: a systematic review and meta-analysis. Sci Rep. 2016;6:31358. https://doi.org/10.1038/srep31358.
Copland E, Canoy D, Nazarzadeh M, et al. Antihypertensive treatment and risk of cancer: an individual participant data meta-analysis. Lancet Oncol. 2021;22(4):558–70. https://doi.org/10.1016/S1470-2045(21)00033-4.
Ahmad FB, Anderson RN. The leading causes of death in the US for 2020. JAMA. 2021;325(18):1829–30. https://doi.org/10.1001/jama.2021.5469.
Vandenberghe D, Albrecht J. The financial burden of non-communicable diseases in the European Union: a systematic review. Eur J Public Health. 2020;30(4):833–9. https://doi.org/10.1093/eurpub/ckz073.
Simonetto M, Rutrick S, LeMoss NM, et al. Adherence to guideline-recommended cancer screening in stroke survivors: a nationwide analysis. J Stroke Cerebrovasc Dis. 2022;31(3):106297. https://doi.org/10.1016/j.jstrokecerebrovasdis.2021.106297.
Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021;52(7):e364–467. https://doi.org/10.1161/STR.0000000000000375.
Golubnitschaja O, Liskova A, Koklesova L, et al. Caution, “normal” BMI: health risks associated with potentially masked individual underweight-EPMA Position Paper 2021. EPMA J. 2021;12(3):243–64. https://doi.org/10.1007/s13167-021-00251-4.
Kropp M, De Clerck E, Vo TKS, Thumann G, Costigliola V, Golubnitschaja O. Short communication: unique metabolic signature of proliferative retinopathy in the tear fluid of diabetic patients with comorbidities - preliminary data for PPPM validation. EPMA J. 2023;14(1):43–51. https://doi.org/10.1007/s13167-023-00318-4.
Crigna AT, Samec M, Koklesova L, et al. Cell-free nucleic acid patterns in disease prediction and monitoring-hype or hope? EPMA J. 2020;11(4):603–27. https://doi.org/10.1007/s13167-020-00226-x.
Nezu T, Hosomi N, Naito H, et al. Clinical characteristics and tumor markers in ischemic stroke patients with active cancer. Intern Emerg Med. 2022;17(3):735–41. https://doi.org/10.1007/s11739-021-02862-1.
Wilbers J, Sondag L, Mulder S, Siegerink B, van Dijk EJ. Cancer prevalence higher in stroke patients than in the general population: the Dutch String-of-Pearls Institute (PSI) Stroke study. Eur J Neurol. 2020;27(1):85–91. https://doi.org/10.1111/ene.14037.
Verhoeven JI, Fan B, Broeders MJM, et al. Association of stroke at young age with new cancer in the years after stroke among patients in the Netherlands. JAMA Netw Open. 2023;6(3):e235002. https://doi.org/10.1001/jamanetworkopen.2023.5002.
Funding
We acknowledge the substantial support rendered by the Natural Science Foundation of China (Grants nos. 82072777), the Key Project of Natural Science Foundation of Fujian Province (Grants nos. 2021J02057), and the Natural Science Foundation of Fujian Province (Grants nos. 2022J011358, 2022J011365, 2020J02063). Additionally, we extend our gratitude to the Medical and Health Key Project of Xiamen (Grants nos. 3502Z20204006), as well as the Xiamen Municipal Health Commission and the Xiamen Municipal Bureau of Science and Technology (Grants nos. 3502Z20209005).
Author information
Authors and Affiliations
Contributions
We present a comprehensive summary of the roles undertaken by the authors in this scholarly endeavor. The conceptualization was primarily orchestrated by J.F. and Q.M., whereas the study design was meticulously crafted by J.F. and J.W. In consonance, data collection and quality control were executed by the entire team. The analytical facet of data was proficiently managed by J.W. Subsequently, the initial manuscript draft was skillfully composed by J.W., with subsequent iterations and editing being jointly administered by J.F. and J.W. Lastly, the ultimate manuscript was reviewed and endorsed by all authors, underscoring their collective commitment. This collaborative effort underscores their involvement in writing, substantiates their engagement with the manuscript, and attests to their consensus on its finalization.
Corresponding authors
Ethics declarations
Ethics approval
Approval for the study was duly granted by the Ethics Committee of the First Affiliated Hospital of Xiamen University (ID: XMYY-2021KYSB074).
Consent to participate
Given the retrospective, non-interventional nature of this study wherein the personal data of patients were not gathered, the requirement for informed consent was duly obviated.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Disclaimer
It is pertinent to note that the funders played no role in shaping the study’s inception and execution, encompassing aspects such as design, data collection, analysis, and manuscript preparation, review, and final endorsement.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fang, J., Wu, J., Hong, G. et al. Cancer screening in hospitalized ischemic stroke patients: a multicenter study focused on multiparametric analysis to improve management of occult cancers. EPMA Journal 15, 53–66 (2024). https://doi.org/10.1007/s13167-024-00354-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13167-024-00354-8