Abstract
Background/aims
The papillomacular bundle (PMB) area is an important anatomical site associated with central vision. As preventive medicine and health screening examinations are now becoming commonplace, the incidental detection of papillomacular bundle defect (PMBD) on fundus photography has been increasing. However, clinical significance of incidental PMBD has not been well documented to date. Thus, through long-term and longitudinal observation, we aimed to investigate the risk factors for the development and progression of PMBD and its predictive role associated with systemic diseases and glaucoma.
Methods
This longitudinal study included subjects who had undergone standardized health screening. We retrospectively reviewed patients for whom PMBD had been detected in fundus photography and followed up for more than 5 years. For a comparative analysis, non-PMBD groups of age- and gender-matched healthy controls were selected.
Results
A total of about 67,000 fundus photographs were analyzed for 8.0 years, and 587 PMBD eyes were found. Among them, 234 eyes of 234 patients who had had fundus photographs taken for more than 5 years were finally included. A total of 216 eyes (92.3%) did not progress during the 8.1 ± 2.7 years, whereas 18 eyes (7.7%) showed progression at 7.6 ± 2.9 years after initial detection. A multivariate logistic regression analysis using 224 non-PMBD healthy controls revealed low body mass index (BMI < 20 kg/m2), systemic hypertension, and sclerotic changes of retinal artery as the significant risk factors for the development of PMBD. Regarding PMBD progression, low BMI, concomitant retinal nerve fiber layer defect (RNFLD) at non-PMB sites, optic disc hemorrhage, and higher vertical cup/disc ratio were individual significant risk factors.
Conclusion
PMBD is associated with ischemic effects. Although the majority of PMBD do not progress, some of cases are associated with glaucomatous damage in a long-term way. PMBD might be a personalized indicator representing ischemia-associated diseases and a predictive factor for diagnosis and preventive management of glaucoma.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, the prevalence of the cardiovascular disease, metabolic disease, and cancer has rapidly increased in Korea [1, 2]. To address this problem, preventive medicine as for early diagnosis of disease and prediction of development has received increasing attention and a comprehensive approach, for example, health screening examinations are being recommended [3, 4]. In line with this issue, Korea is actively operating a health screening system or healthcare center, and is suggesting predictive, preventive, and personalized strategies according to individual characteristics [5, 6]. In the future, this supply and demand in medicine are expected to continue to increase worldwide.
Fundus photography is one of representative ophthalmic examinations in health screening programs and is becoming more popular. In parallel, early detection of vision-threatening diseases such as age-related macular degeneration, diabetic retinopathy, and glaucoma has been increasing [7,8,9]. Moreover, the retina is the only human tissue allowing direct visualization of vessels and nerve fibers. Retinal microvascular changes have been known as independent predictors for systemic diseases including diabetes, hypertension, coronary artery disease, and stroke, while significant associations between alterations of retinal nerve fiber layers and neurodegenerative diseases have been reported [10].
The papillomacular bundle (PMB) is a collection of retinal nerve fibers that carry the information from the macula, and papillomacular bundle defect (PMBD) is not infrequently discovered by chance in screening fundus photography. Although it has been reported that PMBD is likely to be caused by ischemic events [11] and the PMB area is usually spared until the end stage of glaucoma [12], clinical significance of incidental PMBD has not been well documented so far. In fact, focal and solitary PMBD may develop even in non-glaucomatous eyes [11, 13,14,15]. Therefore, knowledge of the development and progression of PMBD is essential for establishing its predictive, preventive, and personalized value.
Accordingly, through long-term and longitudinal observation, we investigated the individual risk factors related to the development and progression of incidentally detected PMBD in fundus photography and suggested customized guidelines to individuals.
Methods
This investigation was approved by the Institutional Review Board of Seoul National University Hospital (No. 1906-141-1043) and was conducted in accordance with all Declaration of Helsinki requirements.
Study population
This study enrolled individuals who had been attending a healthcare screening program for general check-ups at Seoul National University Hospital Healthcare System Gangnam Center during the period from 2010 to 2018 and were aged >18 years at the time of the initial examination. A total of about 67,000 fundus photographs were initially checked for the purposes of the present study’s analysis (H.J.C.).
Inclusion and exclusion criteria
In this study, patients for whom PMBD had been incidentally detected on fundus photography and who met the following inclusion criteria were consecutively enrolled: (1) follow-up longer than 5 years; (2) at least five consecutive fundus photographs measured. The exclusion criteria were (1) history of intraocular surgery other than uncomplicated cataract surgery or of diseases that could affect the retinal nerve fiber layer (RNFL) (e.g., diabetic retinopathy, retinal vein occlusion, ischemic optic neuropathy, pituitary lesions, or demyelinating diseases), (2) optic disc pallor, and (3) media opacity rendering fundus reading difficult for diagnosis (significant cataract, asteroid hyalosis, or vitreous opacity). In cases where both eyes of a patient were eligible for inclusion, one eye with a larger PMBD size was selected as study eye.
Health screening examination
The health screening examination consists of two parts: a health interview survey and a health screening program including an ophthalmologic examination. The health interview survey, administered by trained research technicians, included standardized questionnaires on demographic variables as well as current and past medical conditions (e.g., diabetes mellitus, systemic hypertension, coronary heart disease, asthma, and hyperlipidemia) and health-influencing behaviors (e.g., smoking and alcohol consumption). The health screening program included measurement of body height, weight, waist circumference, and average systolic and diastolic blood pressures, as well as blood tests (e.g., complete blood cell counts, glucose, lipid profile, kidney function, liver enzyme, and thyroid function), routine urinalysis, and an ophthalmologic examination.
Height and body weight were measured on anthropometry. Waist circumference was measured at the midpoint between the lowest rib and the iliac crest during exhalation. The body mass index (BMI) was calculated as weight (kg) divided by height (m) squared. The BMI was categorized into 3 groups: BMI of less than 20 kg/m2, BMI of 20~25 kg/m2, and BMI of 25 kg/m2 or more. The World Health Organization defines obesity as a BMI of 30 kg/m2 or more and overweight status as a BMI of 25 kg/m2 or less than 30 kg/m2. However, because of the lower prevalence rates for overweight status and obesity in Asian countries compared with Western ones [16], we set the standard as a BMI of 25 kg/m2 instead of 30 kg/m2. Systolic blood pressure and diastolic blood pressure were measured in the right arm after a 5-min stabilization period using a standard mercury sphygmomanometer (Baumanometer; Baum, NY, USA). Further, the level of smoking was categorized as “have never smoked,” “previously smoked but no longer smoking,” or “currently smoking,” and the level of alcohol consumption was categorized as “do not drink at all,” “less than once a month,” “2–3 times a month,” “1–2 times a week,” “3–4 times a week,” or “almost every day.” Of these, two or more drinks per week, considered to correspond to consumption of more than 140 g, were classified as “excessive consumption.”
The ophthalmic screening examination included visual acuity (Snellen chart), measurements of intraocular pressure (IOP) using a non-contact tonometer (CT-80 or CT-1P; Topcon Inc., Tokyo, Japan), and fundus photographs using a 45° digital non-mydriatic fundus camera (CR6-45NW; Canon Inc., Utsunomiya, Japan, or TRC-NW8, Topcon Inc., Tokyo, Japan). The cup to disc ratio was evaluated both horizontally and vertically. The optic disc ovality was calculated by dividing the vertical disc diameter by the horizontal disc diameter. The peripapillary atrophy was defined as a peripapillary area that consisted of a zone with chorioretinal atrophy and visible large choroidal vessels and an outlining zone with irregular retinal pigment epithelium [17]. The severity of arterial sclerosis was graded by adopting Scheie’s classification system [18] to assess each subject’s ischemic status of the retina indirectly.
Definition of papillomacular bundle defect
Two independent glaucoma specialists (S.U.B. and H.J.C.) evaluated the presence and progression of PMBD on photographs. All of the fundus photography images were exported to ImageJ software (ImageJ version 1.50i; National Institutes of Health, Bethesda, MD, USA; available at https://imagej.nih.gov/ij/index.html) for analysis and rescaled to a unified scale for measurement of degree of PMBD as defined by its angular width and pattern of involvement.
The specific criteria for PMBD, as based on the previous literature [19,20,21], were as follows. The temporal region of the disc was divided evenly into six sectors of 30° and the PMB area was defined in this study as the angular location within −30.0~+30.0° (sector “c” or “d”) of the reference line connecting the optic nerve disc and the macula (Fig. 1). Then, the location of the retinal nerve fiber layer defect (RNFLD) was described by sectors. A RNFLD was considered to be PMBD when the proximal border of the nearest defect was located in the PMB area (sector “c” or “d”) [11, 19]. Additionally, the focality of the defects was assessed according to the detectability of their boundary.
Definition of papillomacular bundle defect (PMBD). The red line is a straight line from the center of the optic disc to the foveal center and is termed the “reference line.” Draw a line (solid blue line) that runs perpendicular to the reference line and passes through the center of the optic disc. The dotted blue line is a 3.46-mm-diameter circle centered on the optic nerve head and including the reference line and vertical line. As a result, the hemisphere can be divided into six equal sectors (a~f sections). Among the six sectors, the central upper and lower sectors surrounding the reference line (c + d section) from −30 to +30° were defined as the PMB area. A retinal nerve fiber layer defect was considered to be PMBD (2 white arrows) when the proximal border of the nearest defect was located within “sector c or d”
Concomitant other RNFLDs found outside the PMB area were also checked and analyzed in both eyes. Localized RNFLD was defined as a well-outlined, dark wedge-shaped area in the bright striated pattern of the surrounding healthy RNFL with its tip touching the optic disc border [22].
Grouping according to papillomacular bundle defect
The PMBD group included cases where PMBD existed from the first baseline or did not exist initially but newly developed during the follow-up. In all cases, each PMBD group included only those observed continuously for at least 5 years after the first detection of PMBD.
For comparative analysis with the PMBD group, healthy individuals who had visited the same health screening center were enrolled for a non-PMBD group. In detail, the healthy subjects were age (performed to within 1 year of age)- and gender-matched with the PMBD group and had had a minimum of 5 years of follow-up. These non-PMBD group members were randomly registered using a randomization program without knowledge of any clinical information.
Definition of papillomacular bundle defect progression and grouping
Two observers (S.U.B. and H.J.C.) masked to the clinical information independently classified the pattern of progressive PMBD into one of the following categories [23]: (1) deepening or (2) widening of the pre-existing PMBD defect. First, deepening of the PMBD was defined as the presence of significant change overlapping with the pre-existing defect (Fig. 2a). Second, widening of the PMBD was defined as the presence of significant change to the edge of the pre-existing defect (Fig. 2b). Progressive PMBDs were confirmed by the same two experienced glaucoma specialists (S.U.B. and H.J.C.), each of whom was masked to the subject’s identity and to all other test results. Any disagreements were resolved through discussion, and, if necessary, a third grader (K.H.P.) was consulted.
Determination of progression of papillomacular bundle defect (PMBD). Patients with PMBD were classified into “progressors” and “non-progressors” based on the following criteria. (A) deepening or (B) widening of PMBD. a Deepening of PMBD was defined as the presence of significant change overlapping with a pre-existing defect. b Widening of PMBD was defined as a significant change to the edge of a pre-existing defect
For the subsequent analysis, the PMBD group was subdivided into progressors and non-progressors according to progression of PMBD.
Statistical analysis
The baseline demographics and clinical variables were summarized by means and standard deviations or frequencies and percentages, as appropriate. The clinical characteristics of the PMBD group versus non-PMBD group and progressors versus non-progressors were compared using unpaired t-tests or Mann-Whitney’s U tests for continuous values and the chi-square test for categorical variables.
The inter-observer reliability of the presence and progression of PMBD was assessed with fundus photography of 50 randomly selected eyes by 2 observers (S.U.B. and H.J.C.), and was calculated using the kappa statistic (poor agreement, <0.20; fair, 0.21–0.40; moderate, 0.41–0.60; good 0.61–0.80, excellent; 0.81–100) [24]. The agreement for presence of PMBD was 0.89 (95% CI, 0.82–0.93), and that for PMBD progression was 0.77 (95% CI, 0.68–0.87).
Univariate and multivariate logistic regression analyses employing a forward conditional method were performed to determine the prediction of individual factors with presence and progression of PMBD; hazard ratios (HRs) and 95% confidence intervals (CI) were reported. To avoid multi-collinearity, variables correlated significantly with each other were not analyzed simultaneously. Instead, the variable with the highest significance among correlated variables was chosen. If significances were similar between correlated variables, multiple analyses were conducted separately using each variable. Kaplan-Meier survival analysis was used to compare the inter-group cumulative probability of sparing of the PMBD without progression, as stratified by the significant variables derived from multivariate logistic regression. All of the statistical analyses were performed using SPSS version 21.0 (SPSS, Chicago, IL, USA). All of the P values were two-sided and were considered significant when <0.05.
Results
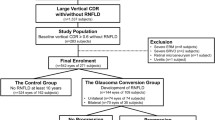
From 2010 to 2018, about 67,000 fundus photographs were checked and 587 eyes with PMBD were detected during the health screening examination. Among them, 234 eyes of 234 patients who had been identified as having PMBD for more than 5 years through fundus photography and who met the inclusion and exclusion criteria were finally enrolled in this study (Fig. 3). In particular, 224 age- and gender-matched subjects who had visited the same health screening center were enrolled in the non-PMBD group.
Clinical patterns of papillomacular bundle defect
Initially, PBMDs were observed bilaterally in 11 patients (4.7%). In each eye, a solitary PMBD was observed in 171 eyes (69.4%), whereas 63 eyes (30.6%) showed multiple PMBDs. During the follow-up, 8 eyes (3.4%) showed a new PMBD at different sites. The cotton wool spot (CWS) was identified in 32 eyes (13.7%) at the same site before or at the same time when PMBD occurred. Ipsilateral and contralateral concomitant RNFLDs at non-PMB area were observed in 57 eyes (24.4%) and 27 eyes (11.5%), respectively, and optic disc hemorrhages (DHs) were shown in 14 eyes (5.9%).
Clinical characteristics and risk factors associated with development of papillomacular bundle defect
The demographics and baseline characteristics of the PMBD and non-PMBD groups are summarized in Table 1. The PMBD group showed significantly higher proportions of low BMI (<20 kg/m2), systemic hypertension, and sclerotic changes of retinal vessels in fundus photographs (P ≤ 0.001, 0022, and 0.003, respectively) and higher level of aspartate aminotransferase (AST) (P = 0.041).
With regard to the risk factors for the presence of PMBD, low BMI (<20 kg/m2), systemic hypertension, AST, peripapillary atrophy, and sclerotic change on fundus photography were significantly different variables between the two groups by the univariate logistic regression model (Table 2). The subsequent multivariate logistic regression analysis revealed low BMI (HR = 2.602, 95% CI, 1.044–6.488; P = 0.040), systemic hypertension (HR = 1.574, 95% CI, 1.071–2.025; P = 0.027), and sclerotic change of retinal vessels (HR = 3.240, 1.600–6.560; P = 0.001) as the personalized risk factors for the presence of PMBD.
Clinical characteristics and risk factors associated with progression of papillomacular bundle defect
During the average 8.0 years of follow-up, 18 (7.7%) of 234 eyes showed progression of PMBD (progressors), while the majority of PBMDs in 216 eyes (92.3%) remained stationary until the last follow-up (non-progressors). In detail, the progressors showed widening (14 eyes) and deepening (4 eyes) of PMBD. Figure 4 shows representative cases of progressors and non-progressors.
Representative a non-progressor and b progressor cases in papillomacular bundle defect (PMBD) group. a A 49-year-old man with systemic hypertension and hyperlipidemia demonstrated cooper wire-like sclerosis on the retinal vessels in fundus photography (black arrow). A PMBD (white arrow) began to be observed with cotton wool spot (green arrow) in the right eye since 2005 and remained stationary for 14 years until 2019. b A 40-year-old female patient with no underlying disease initially had a suspicion of PMBD on her right eye (white arrow) in 2011, which continued to increase in size until 2017. During the follow-up, retinal nerve fiber layer defect developed with optic disc hemorrhage on the left eye (red arrow)
The systemic and ophthalmic characteristics of the two groups are listed in Table 3. Among the progressors, subjects showed higher proportions of low BMI (<20 kg/m2) and systemic hypertension as well as higher levels of white blood cell (WBC) counts, alanine aminotransferase (ALT), and triglycerides. Among the ophthalmic parameters, the proportions of co-occurrence of ipsilateral RNFLD at non-PMB area, newly developed PMBD at different sites, and DH on fundus photography were more frequent (P = 0.034, 0.039, and <0.001, respectively), and mean IOP and vertical cup to disc ratio (VCDR) on fundus photography were significantly higher (P = 0.032 in both) in the progressors.
Regarding the risk factors for the progression of PMBD, the multivariate analysis indicated that PMBD progression was significantly associated with low BMI (HR = 3.895, 1.618–8.376; P = 0.003), ipsilateral RNFLD at non-PMB area (HR = 2.990, 1.618–8.376; P = 0.003), DH (HR = 12.205, 2.879–45.114; P = 0.001), and VCDR (HR = 20.526, 1.356–103.359; P = 0.025), while the result of the univariate analysis revealed higher mean IOP as significant variable in addition to aforementioned parameters (Table 4). Figure 5 reflects the estimation and comparison of the cumulative probability of PMBD progression according to each variable. Higher proportion of low BMI (<20 kg/m2), ipsilateral RNFLD at non-PMB area, DH, and larger VCDR all showed greater cumulative probability of progression of PMBD (P = 0.003, <0.001, 0036, and 0.041 by log-rank test, respectively).
Kaplan-Meier survival analysis of stationary papillomacular bundle defect (PMBD). The subgroups were stratified as a body mass index (BMI) < 20 or ≥ 20 kg/m2, b presence or absence of optic disc hemorrhage, c concomitant retinal nerve fiber layer defect on ipsilateral eye, and d vertical cup to disc ratio ≥ 0.40 or < 0.40
Discussion
Through long-term longitudinal observation, the present study reported the clinical course of PMBD incidentally detected in a health screening examination. We found that most of PMBDs remained stationary during the mean follow-up period of 8.1 years, whereas some PMBDs can progress. In particular, the risk factors for PMBD development as analyzed by systemic and ophthalmic factors were ischemic components such as low BMI, systemic hypertension, and sclerotic change on retinal vessels. Meanwhile, the risk factors for PMBD progression were glaucomatous components such as co-occurrence of RNFLD at non-PMB area, DH, and large VCDR. To the best of our knowledge, the current study is the first to analyze natural course of incidental PMBD and suggest its clinical significance as a predictive, preventive, and personalized indicator based on large volumes of health examination data.
Since the PMB has been known to be an important structure for determining central vision and retinal sensitivity, PMBD can significantly affect a patient’s quality of life [25, 26]. Therefore, the role of PMBD in alleged ocular diseases has been researchers’ major concern so far. Glaucoma patients with PMBD have been shown to suffer central scotoma even at the early stage of the disease [27, 28], although the PMB area is usually impaired at the end stage of glaucoma [12]. Furthermore, ischemic injury to the PMB has been reported to be a predictive marker for poor vision in eyes with branch retinal artery occlusion [29] or non-arteritic anterior ischemic optic neuropathy [30]. However, there have been no reports on clinical significance of incidentally detected solitary PMBD.
In this study, the comparative analysis with the non-PMBD group revealed that the risk factors for the development of PMBD were low BMI, systemic hypertension, and sclerotic change on retinal arteries. Interestingly, these factors already have been identified as the personalized profile associated with ophthalmic ischemic conditions [31, 32]. A poor vascular supply in the temporal region of the optic disc can contribute to the development of PMBD [20]. Chihara et al. classified PMBD as the focal type of RNFLD, which resembles the presumed ischemic defects observed along with CWS [11]. CWS is the result of acute non-perfusion to the retina causing blockage of axoplasmic transport [33]. In this study, we also observed CWS in 13.7% of eyes with PMBD before or at the same time of the development of PMBD. These suggest that PMBD might be one of presentations of ocular ischemic events, and thus, a predictor for ocular ischemic diseases. Expanding the related meaning, it is well known that there is a link between eye and cardiovascular diseases [34]. Atherosclerosis in the retinal artery has been well documented to be related with atherosclerosis in the coronary and carotid arteries [35]. Therefore, incidentally detected PMBD also might be associated with systemic atherosclerosis, which causes systemic ischemic diseases such as coronary artery disease and stroke.
The present analysis of the predictive factors of PMBD progression showed clinical differences with those of PMBD development. With regard to PMBD progression, concomitant RNFLD at non-PMB area, DH, and increased optic disc cupping were associated with increased risk of progression. Indeed, DH is an important individual predisposition for the development and progression of glaucoma [36,37,38]. Glaucomatous optic disc, defined as increased VCDR and running counter to the ISNT rule, has been significantly associated with glaucoma progression [39]. Considering that the essential pathologic process of glaucoma is the loss of retinal ganglion cells and their axons, progressive PMBD is likely to be a positional variant that occurs in the PMB area instead of the superotemporal or inferotemporal sectors. In other words, progressive PMBD might be an important clinical clue for early diagnosis of glaucoma and reasonable evidence to start preventive medicine. Therefore, when PMBD is accidentally observed in screening fundus photography, meticulous evaluation of concomitant RNFLD at non-PMB area and optic disc morphology should be done and close follow-up examination is needed, because such risks may lead to a high probability of developing central scotoma through PMBD progression.
Especially in the present study, low BMI, a core component of the Flammer syndrome (FS) [40], was found to be the predictive factor involved in both PMBD development and progression. Low BMI is associated with the paracentral visual field loss POAG subtype [41] and this relationship could result from impaired endothelium-dependent vasodilation [42]. Additionally, low BMI is known to be an element of primary vascular dysregulation, which is associated with ischemic damage in glaucoma [31, 32]. Dysfunctional vascular autoregulation of the eye produces impaired ocular blood flow, and this phenomenon may cause RNFLD-associated diseases such as glaucoma, retinal CWS, and ischemic optic neuropathy in the PMB area [43, 44]. Therefore, progression of PMBD as observed in this study can be explained by the interaction between ischemic damage (microinfarction) and the glaucomatous susceptibility of the optic nerve head itself. Meanwhile, the microinfarctions in different organs have known to be typical for subjects with the FS [43]. This explains two extremes; cardiovascular disease and the FS can lead to similar disease patterns [45]. The choroidal infarcts and occlusion of the cilioretinal vessels are common in such people with low BMI [46]. In addition, depending on the location, such microinfarction can probably induce autoimmunity such as multiple sclerosis [47]. Further follow-up studies on the link between PMBD and other elements of FS such as migraine, cold extremities, personality, and stress [40, 43, 48] are thought to be meaningful.
Health screening facilitates early detection of diseases and allows for early access to proper treatment, which in turn leads to reduced incidence and overall morbidity. A paradigm shift from post-diagnosis disease care to early management of comorbidities and targeted prevention is warranted to deliver a cost-effective medical services and desirable healthcare economy [49]. In that sense, the current study also would be valuable as it showed clinical significance of incidental PMBDs in the aspect of a predictive, preventive, and personalized medicine (PPPM). Although most of these PMBDs do not progress, the presence of PMBD itself suggests ischemic changes that require ocular and systemic assessment. Such a diagnostic clue may be especially useful for identifying individual health profiles in subjects who have not been diagnosed with specific diseases such as diabetes mellitus, hypertension, ischemic heart disease, or stroke. Meanwhile, some PMBDs have additional meaning especially when they progress as time goes on. In these rare cases, each PMBD would be considered an atypical presentation of RNFLDs observed in glaucoma and might be one of predictive markers for early diagnosis and severity assessment of glaucoma like red blood cell distribution width and axial length [50, 51]. Therefore, anti-glaucoma medication might be started based on the individual benefit-risk assessment to prevent further glaucomatous damage.
Some points need to be considered when interpreting the results of the current study. First, this study was not population-based but healthcare center-based, and we recruited only patients who had been followed up for more than 5 years. These imply that there is a possibility that subjects who are willing to take care of their own health mostly participated in this study, which might have resulted in selection bias. Second, optical coherence tomography (OCT) and VF tests could not be used to evaluate structural and functional damages associated with PMBD. OCT and visual field tests might have provided more information in case it was difficult to decide whether PMBD had progressed or not. Third, we used a non-contact tonometer instead of the Goldmann applanation tonometer. Although a previous study reported that there was no statistically significant difference between two tonometers within the normal IOP range [52], the variation of IOP values obtained with the non-contact tonometer is usually greater than that with the Goldmann applanation tonometer. Actually, IOP showed marginal significance (multivariate analysis P = 0.072) as a risk factor for PMBD progression in the current study. Further analysis using the Goldmann applanation tonometer may suggest more confident relationship between IOP and PMBD progression. Finally, we cannot predict individual diseases based on the presence or progression of PMBD. Further study will be needed to clarify the role of incidentally detected PMBD as an independent predictor for ocular or systemic ischemic diseases.
Conclusions and expert recommendations
We found that the presence of PMBD through fundus photography in a health screening examination was associated with ocular and systemic ischemic components. Thus, careful assessment of fundus photography and confirmation of PMBD might be a noninvasive and useful tool to identify the individual’s cardiovascular profile which can be easily incorporated in health screening examinations. The present study also showed that most incidentally detected PMBDs do not progress. Therefore, personalized observation with regular screening fundus photography and preventive check-up of ocular and systemic diseases would be sufficient for the majority of the patients. Meanwhile, some PMBDs can progress especially when they are accompanied by RNFLD at non-PMB area and glaucomatous optic disc, which means they might be atypical presentations of glaucomatous damages. Therefore, monitoring of detected PMBD could be a personalized-preventive strategy for glaucomatous damage. In those cases, clinicians would be in a better position to consider detailed work-up for early diagnosis of glaucoma and establish a customized treatment plan to prevent further glaucomatous damage. In summary, the PMBD would be a useful biomarker in the context of PPPM.
Abbreviations
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- BMI:
-
Body mass index
- CI:
-
Confidence intervals
- CWS:
-
Cotton wool spot
- DH:
-
Optic disc hemorrhage
- FS:
-
Flammer syndrome
- HR:
-
Hazard ratios
- IOP:
-
Intraocular pressure
- PMB:
-
Papillomacular bundle
- PMBD:
-
Papillomacular bundle defect
- PPPM:
-
Predictive, preventive, and personalized medicine
- RNFL:
-
Retinal nerve fiber layer
- RNFLD:
-
Retinal nerve fiber layer defect
- VCDR:
-
Vertical cup to disc ratio
- WBC:
-
White blood cell
References
Beaglehole R, Bonita R, Horton R, Adams C, Alleyne G, Asaria P, et al. Priority actions for the non-communicable disease crisis. Lancet. 2011;377(9775):1438–47.
Kim HC, Oh SM. Noncommunicable diseases: current status of major modifiable risk factors in Korea. J Prev Med Public Health. 2013;46(4):165–72.
Breslow L, Somers AR. The lifetime health-monitoring program: a practical approach to preventive medicine. N Engl J Med. 1977;296(11):601–8.
Jameson JL, Longo DL. Precision medicine—personalized, problematic, and promising. Obstet Gynecol Surv. 2015;70(10):612–4.
Alyabsi M, Alhumaid A, Allah-Bakhsh H, Alkelya M, Aziz MA. Colorectal cancer in Saudi Arabia as the proof-of-principle model for implementing strategies of predictive, preventive, and personalized medicine in healthcare. EPMA Journal. 2019:1–13.
Lee JH, Yu SE, Kim K-H, Yu MH, Jeong I-H, Cho JY, et al. Individualized metabolic profiling stratifies pancreatic and biliary tract cancer: a useful tool for innovative screening programs and predictive strategies in healthcare. EPMA Journal. 2018;9(3):287–97.
Muramatsu C, Hayashi Y, Sawada A, Hatanaka Y, Hara T, Yamamoto T, et al. Detection of retinal nerve fiber layer defects on retinal fundus images for early diagnosis of glaucoma. J Biomed Opt. 2010;15(1):016021.
Abràmoff MD, Reinhardt JM, Russell SR, Folk JC, Mahajan VB, Niemeijer M, et al. Automated early detection of diabetic retinopathy. Ophthalmology. 2010;117(6):1147–54.
Pirbhai A, Sheidow T, Hooper P. Prospective evaluation of digital non-stereo color fundus photography as a screening tool in age-related macular degeneration. Am J Ophthalmol. 2005;139(3):455–61.
Wagner SK, Fu DJ, Faes L, Liu X, Huemer J, Khalid H et al. Insights into systemic disease through retinal imaging-based oculomics. Translational Vision Science & Technology. 2020;9(2):6-.
Chihara E, Matsuoka T, Ogura Y, Matsumura M. Retinal nerve fiber layer defect as an early manifestation of diabetic retinopathy. Ophthalmology. 1993;100(8):1147–51.
Ogden TE. Nerve fiber layer of the primate retina: morphometric analysis. Invest Ophthalmol Vis Sci. 1984;25(1):19–29.
Barr CC, Glaser JS, Blankenship G. Acute disc swelling in juvenile diabetes: clinical profile and natural history of 12 cases. Arch Ophthalmol. 1980;98(12):2185–92.
Chihara E, Honda Y. Topographic changes in the optic disc in eyes with cotton-wool spots and primary open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol. 1991;229(1):13–8.
Sheets C, Grewal D, Greenfield DS. Ocular toxoplasmosis presenting with focal retinal nerve fiber atrophy simulating glaucoma. J Glaucoma. 2009;18(2):129–31.
Kim KE, Kim MJ, Park KH, Jeoung JW, Kim SH, Kim CY, et al. Prevalence, awareness, and risk factors of primary open-angle glaucoma: Korea National Health and Nutrition Examination Survey 2008–2011. Ophthalmology. 2016;123(3):532–41.
Uchida H, Yamamoto T, Tomita G, Kitazawa Y. Peripapillary atrophy in primary angle-closure glaucoma: a comparative study with primary open-angle glaucoma. Am J Ophthalmol. 1999;127(2):121–8.
Scheie HG. Evaluation of ophthalmoscopic changes of hypertension and arteriolar sclerosis. AMA archives of ophthalmology. 1953;49(2):117–38.
Airaksinen PJ, Mustonen E, Alanko HI. Optic disc hemorrhages: analysis of stereophotographs and clinical data of 112 patients. Arch Ophthalmol. 1981;99(10):1795–801.
Chihara E, Tanihara H. Parameters associated with papillomacular bundle defects in glaucoma. Graefes Arch Clin Exp Ophthalmol. 1992;230(6):511–7.
Kimura Y, Hangai M, Morooka S, Takayama K, Nakano N, Nukada M, et al. Retinal nerve fiber layer defects in highly myopic eyes with early glaucoma. Invest Ophthalmol Vis Sci. 2012;53(10):6472–8.
Kim DM, Seo JH, Kim SH, Hwang S-S. Comparison of localized retinal nerve fiber layer defects between a low-teen intraocular pressure group and a high-teen intraocular pressure group in normal-tension glaucoma patients. J Glaucoma. 2007;16(3):293–6.
Shin JW, Sung KR, Park S-W. Patterns of progressive ganglion cell–inner plexiform layer thinning in glaucoma detected by OCT. Ophthalmology. 2018;125(10):1515–25.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. biometrics. 1977:159-74.
Black AA, Wood JM, Lovie-Kitchin JE. Inferior visual field reductions are associated with poorer functional status among older adults with glaucoma. Ophthalmic Physiol Opt. 2011;31(3):283–91.
Sumi I, Shirato S, Matsumoto S, Araie M. The relationship between visual disability and visual field in patients with glaucoma. Ophthalmology. 2003;110(2):332–9.
Aspinall PA, Johnson ZK, Azuara-Blanco A, Montarzino A, Brice R, Vickers A. Evaluation of quality of life and priorities of patients with glaucoma. Invest Ophthalmol Vis Sci. 2008;49(5):1907–15.
Blumberg DM, De Moraes CG, Prager AJ, Yu Q, Al-Aswad L, Cioffi GA, et al. Association between undetected 10-2 visual field damage and vision-related quality of life in patients with glaucoma. JAMA ophthalmology. 2017;135(7):742–7.
Cho KH, Ahn SJ, Jung C, Han MK, Park KH, Woo SJ. Ischemic injury of the papillomacular bundle is a predictive marker of poor vision in eyes with branch retinal artery occlusion. Am J Ophthalmol. 2016;162:107–20.e2.
Rebolleda G, Sánchez-Sánchez C, González-López JJ, Contreras I, Munoz-Negrete FJ. Papillomacular bundle and inner retinal thicknesses correlate with visual acuity in nonarteritic anterior ischemic optic neuropathy. Invest Ophthalmol Vis Sci. 2015;56(2):682–92.
Flammer J, Konieczka K, Flammer AJ. The primary vascular dysregulation syndrome: implications for eye diseases. EPMA Journal. 2013;4(1):14.
Konieczka K, Choi HJ, Koch S, Fankhauser F, Schoetzau A, Kim DM. Relationship between normal tension glaucoma and Flammer syndrome. EPMA Journal. 2017;8(2):111–7.
McLEOD D, Marshall J, Kohner E, Bird AC. The role of axoplasmic transport in the pathogenesis of retinal cotton-wool spots. Br J Ophthalmol. 1977;61(3):177–91.
Flammer J, Konieczka K, Bruno RM, Virdis A, Flammer AJ, Taddei S. The eye and the heart. Eur Heart J. 2013;34(17):1270–8. https://doi.org/10.1093/eurheartj/eht023.
Song Y-J, Cho K-I, Kim S-M, Jang H-D, Park J-M, Kim S-S, et al. The predictive value of retinal vascular findings for carotid artery atherosclerosis: are further recommendations with regard to carotid atherosclerosis screening needed? Heart Vessel. 2013;28(3):369–76.
Sugiyama K, Tomita G, Kitazawa Y, Onda E, Shinohara H, Park KH. The associations of optic disc hemorrhage with retinal nerve fiber layer defect and peripapillary atrophy in normal-tension glaucoma. Ophthalmology. 1997;104(11):1926–33.
Suh MH, Park KH. Period prevalence and incidence of optic disc haemorrhage in normal tension glaucoma and primary open-angle glaucoma. Clin Exp Ophthalmol. 2011;39(6):513–9.
Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol. 2003;121(1):48–56.
Zheng Y, Cheung CY, Wong TY, Mitchell P, Aung T. Influence of height, weight, and body mass index on optic disc parameters. Invest Ophthalmol Vis Sci. 2010;51(6):2998–3002.
Konieczka K, Ritch R, Traverso CE, Kim DM, Kook MS, Gallino A, et al. Flammer syndrome. EPMA J. 2014;5(1):11. https://doi.org/10.1186/1878-5085-5-11.
Kang JH, Loomis SJ, Rosner BA, Wiggs JL, Pasquale LR. Comparison of risk factor profiles for primary open-angle glaucoma subtypes defined by pattern of visual field loss: a prospective study. Invest Ophthalmol Vis Sci. 2015;56(4):2439–48.
Higashi Y, Sasaki S, Nakagawa K, Kimura M, Noma K, Sasaki S, et al. Low body mass index is a risk factor forimpaired endothelium-dependent vasodilation in humans: role of nitric oxide and oxidative stress. J Am Coll Cardiol. 2003;42(2):256–63.
Konieczka K, Erb C. Diseases potentially related to Flammer syndrome. EPMA J. 2017;8(4):327–32. https://doi.org/10.1007/s13167-017-0116-4.
Flammer J, Konieczka K. The discovery of the Flammer syndrome: a historical and personal perspective. EPMA J. 2017;8(2):75–97. https://doi.org/10.1007/s13167-017-0090-x.
Barthelmes J, Nägele MP, Ludovici V, Ruschitzka F, Sudano I, Flammer AJ. Endothelial dysfunction in cardiovascular disease and Flammer syndrome-similarities and differences. EPMA J. 2017;8(2):99–109. https://doi.org/10.1007/s13167-017-0099-1.
Terelak-Borys B, Grabska-Liberek I, Piekarniak-Wozniak A, Konieczka K. Choroidal infarction in a glaucoma patient with Flammer syndrome: a case report with a long term follow-up. BMC Ophthalmol. 2017;17(1):23. https://doi.org/10.1186/s12886-017-0416-4.
Konieczka K, Koch S, Binggeli T, Schoetzau A, Kesselring J. Multiple sclerosis and primary vascular dysregulation (Flammer syndrome). EPMA J. 2016;7(1):13. https://doi.org/10.1186/s13167-016-0062-6.
Sabel BA, Wang J, Fähse S, Cárdenas-Morales L, Antal A. Personality and stress influence vision restoration and recovery in glaucoma and optic neuropathy following alternating current stimulation: implications for personalized neuromodulation and rehabilitation. EPMA J. 2020;11(2):177–96. https://doi.org/10.1007/s13167-020-00204-3.
Berger JS, Haskell L, Ting W, Lurie F, Chang SC, Mueller LA, et al. Evaluation of machine learning methodology for the prediction of healthcare resource utilization and healthcare costs in patients with critical limb ischemia-is preventive and personalized approach on the horizon? EPMA J. 2020;11(1):53–64. https://doi.org/10.1007/s13167-019-00196-9.
Chen Q, Zhao B, Wang MY, Chen XY, Li D, Jiang XQ, et al. Associations between the red blood cell distribution width and primary angle-closure glaucoma: a potential for disease prediction. EPMA J. 2019;10(2):185–93. https://doi.org/10.1007/s13167-019-00166-1.
Li S, Shao M, Wan Y, Tang B, Sun X, Cao W. Relationship between ocular biometry and severity of primary angle-closure glaucoma: relevance for predictive, preventive, and personalized medicine. EPMA J. 2019;10(3):261–71. https://doi.org/10.1007/s13167-019-00174-1.
Lee JS, Lee SH, Oum BS, Chung JS, Cho BM, Hong JW. Relationship between intraocular pressure and systemic health parameters in a Korean population. Clin Exp Ophthalmol. 2002;30(4):237–41.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2019R1F1A1058426).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The project was approved by Institutional Review Board of Seoul National University Hospital (No. 1906-141-1043)
Statement of informed consent
The requirement to obtain written informed consent was waived by the Institutional Review Board, because our study was retrospective research based on medical records, and also because this research presented no more than minimal risk of harm to subjects
Statement of human and animal rights
The study was carried out according to the Declaration of Helsinki
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Meeting presentation
None
Rights and permissions
About this article
Cite this article
Baek, S.U., Lee, W.J., Park, K.H. et al. Health screening program revealed risk factors associated with development and progression of papillomacular bundle defect. EPMA Journal 12, 41–55 (2021). https://doi.org/10.1007/s13167-021-00235-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13167-021-00235-4
Keywords
- Papillomacular bundle defect
- Ophthalmology
- Health screening examination
- Program
- Low body mass index
- Ischemia-associated diseases
- Risk assessment
- Risk factors
- Screening
- Cardiovascular disease
- Longitudinal study
- Disease development and progression
- Systemic effects and characteristics
- Glaucoma
- Predictive factors
- Personalized indicator
- Preventive management
- Predictive preventive personalized medicine (PPPM/3PM)