Abstract
The world acreage of organic crop production systems is increasing, but the soil microbial dynamics in these systems are not fully understood. We studied the composition and functioning of soil microbial communities in 4-year organic or conventional rotations. The organic systems were tilled to control weeds, and N2-fixing legumes or compost supplied nutrients. The conventional systems were managed under no-till; herbicides controlled weeds, and compost or fertilizers supplied nutrients. Soil microbial biomass C (MBC), the diversity and composition of bacterial communities, and activities of enzymes that mediate C, N, P, and S cycling were determined. The bacterial classes Gemmatimonadetes, C0119 (phylum Chloroflexi), and Thermomicrobia (phylum Chloroflexi) were more abundant in organic than conventional cropping systems, so were some genera from the class Actinobacteria. The bacterial β-diversity showed similar cropping system differences. However, acid phosphomonoesterase activity was greater in conventional than organic cropping systems, presumably because the soil P from the large amounts of compost applied in the organic system suppressed this enzyme. MBC, bacterial α-diversity, the relative abundances of the bacterial classes δ-Proteobacteria, γ-Proteobacteria, and Bacilli (phylum Firmicutes) were all greater in compost than no-compost treatments. The relative abundances of three genera from Actinobacteria class were negatively correlated with acid phosphomonoesterase activity due to the high relative abundances of these genera, but low acid phosphomonoesterase activities, where compost was applied in the organic system. Therefore, there were soil bacterial compositional differences between organic and conventional cropping systems, but only differences in the activities of a P cycling enzyme were detected.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The acreage devoted to organic production systems in agriculture is increasing throughout the world. In Canada, there were 0.98 million hectares under organic production in 2015, representing 1.5% of total agricultural land (Statistics Canada 2017). These systems consist of operations that are sustainable and harmonious with the environment. In crop production systems, soil fertility is managed mainly by promoting soil biological activity through organic soil inputs that add organic C to the soil because synthetic fertilizers are not allowed. These organic inputs include animal manures/compost, green manures, and cover crops that increase soil organic C (Gopinath et al. 2008; Li et al. 2015; Chen et al. 2020). Because most soil microorganisms depend on organic C for their metabolism, organic manures increase soil microbial characteristics including biomass, diversity, and activity (Lupwayi et al. 2017a; Denbi et al. 2018; Tully and McAskill 2020). Another difference between organic and conventional production systems is that synthetic pesticides are not allowed in organic systems, and weeds are controlled by mechanical tillage. Whereas organic soil inputs boost soil microbial communities, the tillage used to control weeds accelerates loss of soil organic C by breaking soil aggregates that protect it from oxidation and soil erosion (Li et al. 2015; Kibet et al. 2016). This tillage-induced loss of soil organic C could adversely affect soil microbial communities if it exceeds C additions through organic inputs. Because of the soil conservation and soil health advantages of no-till systems (Nunes et al. 2020), organic researchers and growers are seeking ways of reducing tillage in weed control (Fernandez et al. 2019; Alba et al. 2020).
On the Canadian prairies, Li et al. (2012) reported greater relative abundance of Proteobacteria in organic crop production than conventional production, but the reverse for Actinobacteria and Chloroflexi. However, in the Netherlands, Proteobacteria and Euryarchaeota (Archaea) were more abundant in conventional than organic systems, but vice versa for Acidobacteria and Planctomycetes (Lupatini et al. 2017). In both of these and many other trials (Tautges et al. 2016; Massaccesia et al. 2020), tillage was used in both systems; the organic and conventional systems differed only in soil nutrient supply and pest control methods. Such comparisons are justifiable for field trials that were established before the advent of no-till systems. However, no-till systems are widely used now in crop production on the Canadian prairies, enabled by use of herbicides and herbicide-resistant crops to control weeds. Therefore, tilled organic systems should be compared with no-till conventional systems. The objectives of this work were to compare tilled organic crop production rotations with no-till conventional rotations in soil microbial biomass, bacterial composition, and the activities of enzymes that mediate C, N, P, and S cycling. We also aimed to relate the composition of the soil microbiome to the enzyme activities.
Materials and methods
Experimental site, treatments, and design
Four-year rotations were established at Beaverlodge ((55.2° N, 119.4° W) in Alberta, Canada, from 2006 to 2009. The soil was a Dark Gray Luvisol (Hapludalf in Soil Taxonomy) with the following 0–15 cm depth characteristics: clay loam (27% sand, 39% clay, and 34% silt), pH (water) 7.9, and 5.1% OC. There were five treatments, consisting of two organic cropping treatments (Treatments 2 and 4) and three conventional cropping treatments (Treatments 1, 3, and 5):-
-
1.
Conventional, no-till: Pre-seeding glyphosate and in-crop herbicides controlled weeds. N and P fertilizers supplied the nutrients according to soil test results.
-
2.
Organic, tillage: No pre-seeding glyphosate, in-crop herbicides, or fertilizer were applied. Weeds were controlled by tillage, and N2-fixing legumes (without P fertilizer) supplied the nutrients.
-
3.
N2 fixing crops + fertilizer, no-till: Pre-seeding glyphosate and in-crop herbicides controlled weeds. N2-fixing legumes supplied the nutrients, which were supplemented by P fertilizer (to legumes) and N and P fertilizer (to non-legumes).
-
4.
Organic + compost N, tillage: No pre-seeding glyphosate or fertilizer was applied. Weeds were controlled by tillage, and compost was applied at the N-requirement rate to supply the nutrients.
-
5.
Compost P + fertilizer, no-till: Pre-seeding glyphosate and in-crop herbicides controlled weeds. Compost was applied at the P-requirement rate, and the extra N required was supplied by N fertilizer.
The crops and nutrient inputs in each treatment and year are listed in Table 1 and the compost chemical composition in Table 2. In Treatments 1 and 5, the crop rotation from 2006 to 2009 was field pea (Pisum sativum L.) – canola (Brassica napus L.) – barley (Hordeum vulgare L.) – oat (Avena sativa L.). In Treatments 2 to 4, canola was replaced with faba bean (Vicia faba L.) silage. The treatments were arranged in a randomized complete block design (RCBD) in plots 15.2 m long and 3.66 m wide, with four replicates.
Soil sampling
Soil samples were collected every year (2006–2009). The same plots were sampled once a year, but the crops grown in those plots varied each year according to the rotation. The samples were collected at the flag-leaf stage of cereals, flowering stage of canola, or flat-pod stage of legumes, usually in midsummer (July). Plants were excavated from four random 0.5-m lengths of row in each plot. Loose soil was shaken off the roots, and the remaining soil that had strongly adhered to the roots was carefully brushed off and kept as rhizosphere soil. Non-rhizosphere (bulk) soil (0–7.5 cm depth) was sampled with a core sampler from the middle of two adjacent crop rows at four locations per plot. The four bulk or rhizosphere soil samples from each plot were combined into one composite sample, passed through a 2-mm sieve and frozen at − 20 °C until analysis. Soil microbial biomass C was determined every year, but DNA sequencing and enzyme activity analyses were done in 2016. Soil storage at − 20 °C has minimal effects on soil microbial properties (Rubin et al. 2013; Cui et al. 2014).
Microbial biomass C (MBC)
Every year, soil MBC was measured using the substrate-induced respiration method, in which 300 mg of glucose was dissolved in 4.5 to 6.0 mL water and added to 50 g soil to bring it to 50% water-holding capacity. The exact amount of water added depended on the pre-determined water content and water-holding capacity of the soil. After stir-mixing, the soil was incubated in a 1 L jar for 3 h at 22 °C, and the amount of CO2 that accumulated in the head space was measured using gas chromatography.
Soil bacterial communities
The diversity and composition of the bacterial communities were characterized in the oat rhizosphere samples collected in 2009. DNA was extracted from the soil using the PowerSoil™ kit (Mo BIO Laboratories, Carsbad, CA). The 16S rRNA gene V4 variable region PCR primers 515/806 with barcode on the forward primer were used in a 28 cycle PCR (5 cycle used on PCR products) using the HotStarTaq Plus Master Mix Kit (Qiagen, USA) under the following conditions: 94 °C for 3 min, followed by 28 cycles of 94 °C for 30 s, 53 °C for 40 s, and 72 °C for 1 min, after which a final elongation step at 72 °C for 5 min was performed. After amplification, PCR products were checked in 2% agarose gel to determine the success of amplification and the relative intensity of bands. Multiple samples were pooled together (e.g., 100 samples) in equal proportions based on their molecular weight and DNA concentrations. The pooled samples were purified using calibrated AMPure XP beads. The pooled and purified PCR product was then used to prepare Illumina DNA library. Sequencing was performed at MR DNA (www.mrdnalab.com, Shallowater, TX, USA) on a MiSeq sequencer following the manufacturer’s instructions.
Bioinformatics
Sequence quality control, screening, and alignment processes completed using the MiSeq SOP in mothur (Kozich et al. 2013). In short, sequences were screened for length, ambiguous base calls, and homopolymer runs exceeding 8 bp. Sequences with < 200 bp, or that did not meet the other screening criteria were removed. Sequences that did not span the longest alignment region were also removed from the dataset and chimeras were removed from the samples using the sequence collection (UCHIME) as its own reference database (Edgar et al. 2011). Sequences were then aligned against the SILVA alignment database for 16S rRNA genes to define operational taxonomic units (OTUs) at 97% pairwise identity threshold (Kozich et al. 2013). All OTUs with a relative abundance > 0.1% were included in the statistical analysis. For calculation of the nonparametric species richness estimators including number of sequences, Good’s coverage, number of OTUs, Chao 1 index, Shannon index, and Simpson index, mothur software was also used. Diversity indices were calculated using rarified OTU data, calculated using the lowest number of sequences found in every sample. Sequences and OTUs were classified in mothur software against the Silva reference v128 database (Quast et al. 2013).
Soil enzyme activities
The soil microbial functioning was evaluated by quantifying the activities of β-glucosidase (C cycling), N-acetyl-β-glucosaminidase (NAG) (C and N cycling), acid phosphomonoesterase (P cycling), and arylsulphatase enzymes in oat rhizosphere samples collected in 2009. Microplate fluorimetric assays (Freeman et al. 1995; Deng et al. 2011) were used for β-glucosidase, NAG, and acid phosphomonoesterase, based on the detection of 4-methylumbelliferone (MUF) released by the enzymatic hydrolysis of MUF-labelled substrates incubated with soil at the optimal pH of each enzyme as described by Lupwayi et al. (2019). This method produced inconsistent data for arylsulphatase activity, and so a bench-scale assay was used to measure its activity by colorimetrically determining p-nitrophenol released by the enzyme after incubating 1 g soil with buffered (pH 6.0) p-nitrophenyl-β-D-glucoside (Dick et al. 1996).
Statistical analysis
The MBC data were analyzed by analysis of variance (ANOVA) as repeated-measures in the RCBD of the trial, where the repeated measure was year. Bacterial α-diversity (indices), bacterial relative abundance, and enzyme activity data were analyzed by ANOVA using the RCBD. In all cases, treatment differences were considered significant at 5% significance level, and means were separated by the least significant difference (LSD) method. Orthogonal contrast analyses were also conducted for (a) organic cropping systems (Treatments 2 and 4) vs conventional cropping systems (Treatments 1, 3, and 5), and (b) compost (Treatments 4 and 5) vs no-compost (Treatments 1–3). These differences were also considered significant at 5% significance level. The β-diversity of the bacteria was assessed by principal component analysis (PCA) ordination to classify treatments according to the relative abundances of the different bacterial classes. A correlation matrix of the relative abundance data was used in PCA, and, after classification, the bacterial classes that accounted for differences between treatments were identified by correlating principal component scores with the relative abundances of the bacterial classes. The soil microbial compositional properties were related to the functional properties by Pearson correlation analysis of the relative abundances of bacterial genera to enzyme activities.
Results
In 2006, the growth-period (May to August) monthly rainfall was above-normal (long-term average) in May and June, but 21% of normal rainfall in August (Table 3). The rainfall in June and July of 2007 and 2008 was below-normal. In 2009, June was dry (21% of normal rainfall), but July received above-normal (175%) rainfall.
From 2006 to 2009, the organic + compost treatment had higher soil MBC than other treatments except the compost + fertilizer treatment both in oat rhizosphere and bulk soil (Table 4). Contrast analysis confirmed that the treatments with compost had greater MBC than treatments without compost. Soil MBC was highest in 2009 when July (soil sampling time) had 175% of normal rainfall, and lowest in 2008 when July rainfall was 28% of normal rainfall. Therefore, rainfall, rather than the different crops grown, explained the yearly differences in soil MBC.
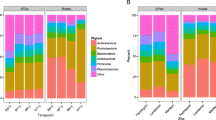
The rest of the results below are for oat rhizosphere in 2009, when the composition of the soil microbial communities and enzyme activities were characterized. There were 23 bacterial phyla and one archaeal phylum (Crenarchaeota) identified in the study. The predominant phyla were Proteobacteria (29.0% relative abundance, averaged over all treatments), Actinobacteria (24.3%), Acidobacteria (12.0%), Chloroflexi (8.2%), Gemmatimonadetes (8.1%), Bacteroidetes (5.0%), Verrucomicrobia (3.5%), Planctomycetes (3.2%), Crenarchaeota) (3.0%), and Firmicutes (1.3%). At class level, the relative abundances of the dominant (with at least 1% relative abundance) bacteria and archaea in all treatments are shown in Fig. 1. Analysis of variance showed that Gemmatimonadetes were most abundant in the Organic (without compost) treatment and least abundant in the compost + fertilizer treatment (Table 5). The relative abundance of γ-Proteobacteria was lower in the Organic (without compost) treatment than in all other treatments, and Bacilli (Firmicutes) were more abundant in the treatments with compost than in the other treatments. This latter result was confirmed by contrast analysis, which showed the same pattern for δ-Proteobacteria and γ-Proteobacteria, but the reverse for Solibacteres (Acidobacteria). For organic vs conventional cropping systems, Gemmatimonadetes, C0119 (Chloroflexi) and Thermomicrobia (Chloroflexi) were more abundant in organic than conventional cropping systems. Interestingly, the two contrasts were not significant at the same time, i.e., when the organic vs conventional cropping systems contrast was significant, the compost vs no-compost contrast was not, and vice versa. A possible reason is that one of the compost treatments was organic (organic + compost) while the other was conventional (compost + fertilizer).
Analysis of variance did not reveal any treatment effects on the α-diversity (at OTU level) of the soil bacteria, but contrast analysis showed that Shannon and Chao 1 indices were greater where compost had been applied (9.16 and 6139, respectively) than where no compost had been applied (9.00 and 5984, respectively). Principal component analysis (β-diversity) did not show clear separation of compost vs no-compost treatments, but PC 2 separated organic cropping systems from conventional cropping systems (Fig. 2a). The bacterial classes associated with organic cropping systems included Gemmatimonadetes, Thermomicrobia, and C0119 (Fig. 2b), in agreement with the analysis of variance and contrast analysis results above, and the classes associated with conventional cropping systems included α-Proteobacteria and β-Proteobacteria.
Oat rhizosphere bacterial community structures (β-diversity) in the cropping systems in 2009 (a), and the bacterial classes (full names in Fig. 2) that were associated with the different community structures (b). The ordination by principal component analysis (PCA) shows the percentages of data variance explained by PC1 and PC2
At genus level, the results (Table 6) were similar to those at class level (Table 5), i.e., either the abundances were greater in organic than conventional cropping systems or in compost than in no-compost treatments. There were two exceptions: the genus Janthinobacterium (β-Proteobacteria) was more abundant in conventional than in organic cropping systems (as PCA showed in Fig. 2), and Solibacillus spp. (class Bacilli, phylum Firmicutes) were more abundant in no-compost treatments than in compost treatments even though the opposite was true for Bacilli at class level (Table 5).
Analysis of variance did not detect treatment differences in the activities of the enzymes (Table 7). However, contrast analysis showed that acid phosphomonoesterase activity was greater in conventional than organic cropping systems. The relative abundances of Phycicoccus spp., Streptomyces spp. and Terracoccus spp. (which are all Actinobacteria) were negatively correlated with acid phosphomonoesterase activity, but the relative abundance of Solibacillus spp. was positively correlated with the activity of this enzyme (Table 8).
Discussion
Compost was probably the main input that affected the soil microbial communities in these cropping systems, whether organic or conventional. Microbial biomass C (Table 4), bacterial α-diversity, the relative abundances of the bacterial classes δ-Proteobacteria, γ-Proteobacteria, and Bacilli (phylum Firmicutes) were all greater in compost than no-compost treatments (Table 5). One exception was the genus Solibacillus spp. (class Bacilli, phylum Firmicutes), which was more abundant in no-compost treatments than in compost treatments. The increase in soil MBC and α-diversity with compost have been reported in other studies (Jacinthe et al. 2011; Lupwayi et al. 2018), but a decrease in α-diversity has also been reported (Tian et al. 2015). Proteobacteria and Firmicutes are both usually classified as copiotrophs, which are fast-growing bacteria found in C-rich and N-rich soils (Fierer et al. 2007; Ahn et al. 2016). Compost added organic C and N to the soil, and most soil microorganisms require organic C for energy and growth.
The differences between organic and conventional cropping systems in the composition of the soil microbiome seemed to reflect differences in soil nutrient availability. The bacterial classes Gemmatimonadetes, C0119 (phylum Chloroflexi), and Thermomicrobia (phylum Chloroflexi) were more abundant in organic than conventional cropping systems (Table 5), so were genera of the phylum Actinobacteria (Nocardia spp., Streptomyces spp., and Terracoccus spp.) (Table 6). These differences were also reflected in the β-diversity of the bacterial communities (Fig. 2). Gemmatimonadetes are oligotrophs (Li et al. 2019), which are slow-growing bacteria that are tolerant of low organic C and N soil conditions. Although Actinobacteria are classified as copiotrophs, they have been shown to exhibit characteristics of oligotrophs in their responses to organic manures in the Canadian prairies (Li et al. 2012; Lupwayi et al. 2017b). Chen et al. (2018) also reported greater relative abundances of Gemmatimonadetes, Chlorflexi, and Actinobacteria in organic cropping systems than in conventional systems. Therefore, the greater abundances of all these bacteria in organic than conventional cropping systems suggest that conventional cropping system had more available nutrients, especially N, than organic systems. No inorganic fertilizers were applied in organic cropping systems (Table 1), which explains the difference in the types of bacteria that were abundant there. Actinobacteria are some of the key bacteria in C cycling in breaking down cellulose in plant residues in the soil (Lewin et al. 2016), and their abundance in organic soils suggests their importance in soils that received only organic inputs. However, soil enzyme analyses showed no treatment effects on the activities of β-glucosidase (C cycling) or NAG (C and N cycling). One exception was the genus Janthinobacterium (β-Proteobacteria), which was more abundant in conventional than organic cropping system (Table 5), and the community structures also showed that β-Proteobacteria were associated with conventional cropping systems (Fig. 2b). β-Proteobacteria have been classified in different environments as copiotrophic (Fierer et al. 2007) or oligotrophic (Männistö et al. 2016). In bulk soil, Bakker et al. (2018) reported that more taxa of Proteobacteria and Acidobacteria were enriched in an organic system than those enriched in a conventional system. Our study on soil bacterial communities was on rhizosphere soil.
Of the soil enzymes studied, only acid phosphomonoesterase was affected by the experimental treatments of this study. The activity of this enzyme was greater in conventional than organic cropping systems. Many comparisons of organic vs conventional systems have shown greater acid phosphomonoesterase (and other enzymes) activity in the former than the latter systems (Niemi et al. 2008; Liang et al. 2014; Tautges et al. 2016). The reason is that inorganic P fertilizer, which was applied in conventional cropping systems in our study, increases soil available P that suppresses the activity or synthesis of acid phosphomonoesterase because the soil microbes do not need to acquire more P from organic sources (Colvan et al. 2001). Our seemingly contradictory results can probably be explained by compost applications. Manure/compost applied on the basis of crop N requirements adds more P to the soil than crops need, resulting in excessive available P accumulation in the soil (Eghball and Power 1999; Hao et al. 2008). It is recommended that manure/compost be applied according to crop P requirements when the buildup of soil P is a concern (Eghball and Power 1999). In this trial, compost was added according to crop N requirements in the organic treatment and according to crop P requirements in the conventional treatment. Because of that difference, Table 1 shows that in 2009, the compost applied in the organic treatment (15 t ha−1 in Treatment 4) was 2.4 × the amount in the conventional treatment (6.3 t ha−1 in Treatment 5). Therefore, the lower activity of acid phosphomonoesterase in the organic than conventional systems was most likely because it was suppressed by excessive soil P in the organic + compost treatment, and Table 7 confirms that this treatment had the lowest activity of this enzyme. Soil tillage in this treatment mixed the compost with the soil, which increased contact between the otherwise immobile soil P and the soil microbes. Therefore, the low acid phosphomonoesterase activity in the organic system was not a result of low soil available P, but high soil available P from compost. In an incubation experiment, Hazarika et al. (2021) also reported decreases in acid phosphomonoesterase activities with pig and poultry manure additions to an acid soil, but they attributed this effect to the liming effects of these manures.
Correlations between the relative abundances of three bacterial genera were negatively correlated with acid phosphomonoesterase activity mainly because their relative abundances were high, while the activity of this enzyme was low in organic cropping systems or where compost had been applied. The relative abundance of Solibacillus spp. was positively correlated with acid phosphomonoesterase enzyme mainly because this genus (class Bacilli, phylum Firmicutes) was more abundant in no-compost treatments than in compost treatments although the opposite was true for Bacilli at class level. Besides crop nutrition, biological crop protection is also important, especially in organic cropping systems where synthetic pesticides cannot be used. In Australian agricultural soils, the suppression of the pathogen Fusarium oxysporum was positively correlated with the relative abundances of Actinobacteria and Firmicutes, but negatively correlated with the relative abundance of Acidobacteria (Trivedi et al. 2017).
In the organic vs conventional system comparison in this study, the effects of individual field operations were compounded. For example, a no-till vs tillage comparison could not be made because both of the organic cropping system rotations were tilled and conventional system rotations were under no-till. That difference reflects the differences in weed control in the two systems. What we compared were differences in soil microbial properties between the two cropping systems as packages, i.e., the way that they are practiced, rather than individual field operations.
Summary and conclusion
The major input that affected soil microbial properties in both the tilled organic or no-till conventional cropping systems was compost. It increased soil MBC, bacterial α-diversity and the relative abundances of copiotrophic bacteria, presumably by adding more organic C to the soil than crop residues. The soil bacterial community β-diversity (community structure) was also altered because oligotrophic bacteria were associated more with organic cropping systems than conventional cropping systems. However, these differences did not translate into detectable differences in the activities of enzymes that mediate C, N, and S cycling in the soil. For P cycling, acid phosphomonoesterase activity was greater in conventional than in organic cropping systems. More compost was applied (and mixed with the soil with tillage) in the organic system than the conventional system, which presumably increased soil available P that suppressed the activity of this enzyme. The study has shown some soil microbial compositional and functional differences that may be important in nutrient cycling (and other biological processes) that occur in organic and conventional cropping systems.
Data availability
Upon request.
Code availability
Not applicable.
References
Ahn JH, Lee SA, Kim JM, Kim MS, Song J, Weon HY (2016) Dynamics of bacterial communities in rice field soils as affected by different long-term fertilization practices. J Microbiol 54:724–731
Alba OS, Syrovy LD, Duddu HSN, Shirtliffe SJ (2020) Increased seeding rate and multiple methods of mechanical weed control reduce weed mass in a poorly competitive organic crop. Field Crop Res 245:107648
Bakker MG, Looft T, Alt DP, Delate K, Cambardella CA (2018) Bulk soil bacterial community structure and function respond to long-term organic and conventional agricultural management. Can J Microbiol 64:901–914
Chen H, Xia Q, Yang T, Shi W (2018) Eighteen-year farming management moderately shapes the soil microbial community structure but promotes habitat-specific taxa. Front Microbiol 9:1766
Chen L, Li F, Ning Q, Li J, Zhang J, Ma D, Zhang C (2020) Organic amendment mitigates the negative impacts of mineral fertilization on bacterial communities in Shajiang black soil. Appl Soil Ecol 150:103457
Colvan SR, Syers JK, O’Donnel AG (2001) Effect of long-term fertilizer use on acid and alkaline phosphomonoesterase and phosphodiesterase activities in managed grassland. Biol Fertil Soils 34:258–263
Cui H, Wang C, Gu Z, Zhu H, Fu S, Yao Q (2014) Evaluation of soil storage methods for soil microbial community using genetic and metabolic finger printings. Eur J Soil Biol 63:55–63
Denbi DK, Shivani Sharma Toor AS, Kiranvil Brar Sodhi GPS, Garg AK (2018) Differences in soil organic carbon pools and biological activity between organic and conventionally managed rice-wheat fields. Organic Agric 8:1–14
Deng S, Kang H, Freeman C (2011) Microplate fluorometric assay for soil enzymes. In: Dick RP (ed) Methods in soil enzymology. Soil Sci Soc Am, Madison, pp 311–318
Dick RP, Breakwell DP, Turco RF (1996) Soil enzyme activities and biodiversity measurements as integrative microbiological indicators. In: Doran JW, Jones AJ (Eds) Methods for assessing soil quality. Soil Science Soc Am Special Publication Number 49. Soil Sci Soc Am Inc Madison, WI, pp247–271
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinform 27:2194–2200
Eghball B, Power JF (1999) Phosphorus- and nitrogen-based manure and compost applications: corn production and soil phosphorus. Soil Sci Soc Am J 63:895–901
Fernandez MR, Zentner RP, Schellenberg MP, Aladenola O, Leeson J, St. Luce M, McConkey BG, Cutforth H (2019) Soil fertility and quality response to reduced tillage and diversified cropping under organic management. Agron J 111:781–792
Fierer N, Bradford MA, Jackson RB (2007) Toward an ecological classification of soil bacteria. Ecol 88:1354–1364
Freeman C, Liska G, Ostle NJ, Jones SE, Lock MA (1995) The use of fluorogenic substrates for measuring enzyme activity in peatlands. Plant Soil 175:147–152
Gopinath KA, Supradip Saha Mina BL, Harit Pande Kundu S, Gupta HS (2008) Influence of organic amendments on growth, yield and quality of wheat and on soil properties during transition to organic production. Nutr Cycl Agroecosyst 82:51–60
Hao X, Godlinski F, Chang C (2008) Distribution of phosphorus forms in soil following long-term continuous and discontinuous cattle manure applications. Soil Sci Soc Am J 72:90–97
Hazarika S, Nabam A, Thakuria D, Kataki S, Krishnappa R (2021) Lime equivelence of organic manures and scope of their utilization as acid soil amendments. Arch Agron Soil Sci 67:660–674
Jacinthe PA, Shukla MA, Ikemura Y (2011) Carbon pools and soil biochemical properties in manure-based organic cropping systems of semi-arid New Mexico. Soil Use Manage 27:453–463
Kibet LC, Blanco-Canqui H, Jasa P (2016) Long-term tillage impacts on soil organic matter components and related properties on a Typic Argiudoll. Soil Tillage Res 155:78–84
Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD (2013) Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol 79:5112–5120
Lewin GR, Carlos C, Chevrette MG, Horn HA, McDonald BR, Stankey RJ, Fox BG, Currie CR (2016) Evolution and ecology of Actinobacteria and their bioenergy applications. Annux Rev Microbiol 70:235–254
Li R, Khafipour E, Krause DO, Entz MH, de Kievit TR, Fernando WGD (2012) Pyrosequencing reveals the influence of organic and conventional farming systems on bacterial communities. PLoS ONE 7(12):e51897
Li L, Larney FJ, Angers DA, Pearson DC, Blackshaw RE (2015) Surface soil quality attributes following 12 years of conventional and conservation management on irrigated rotations in southern Alberta. Soil Sci Soc Am J 79:930–942
Li H, Zhang Y, Yang S, Wang Z, Feng X, Liu H, Jiang Y (2019) Variations in soil bacterial taxonomic profiles and putative functions in response to straw incorporation combined with N fertilization during the maize growing season. Agric Ecosyst Environ 283
Liang ST, Grossman J, Shi W (2014) Soil microbial responses to winter legume cover crop management during organic transition. Eur J Soil Biol 65:15–22
Lupwayi NZ, Larney FJ, Blackshaw RE, Kanashiro DA, Pearson DC (2017a) Phospholipid fatty acid biomarkers show positive soil microbial community responses to conservation management of irrigated rotations in southern Alberta. Soil Tillage Res 168:1–10
Lupwayi NZ, May WE, Kanashiro DA, Petri RM (2017b) Soil bacterial community responses to black medic cover crop and fertilizer N under no-till. Appl Soil Ecol 124:95–103
Lupwayi NZ, Kanashiro DA, Eastman AH, Hao X (2018) Soil phospholipid fatty acid biomarkers and β-glucosidase activities after long-term manure and fertilizer N applications. Soil Sci Soc Am J 82:342–353
Lupwayi NZ, Zhang Y, Hao X, Thomas BW, Eastman AH, Schwinghamer TD (2019) Linking soil microbial biomass and enzyme activities to long-term manure applications and their nonlinear legacy. Pedobiol 74:34–42
Lupatini M, Korthals GW, de Hollander M, Jenssens TKS, Kuramae EE (2017) Soil microbiome is more heterogeneous in organic than in conventional farming system. Front Microbiol 7:2064
Männistö M, Ganzert L, Tiirola M, Häggblom MM, Stark S (2016) Do shifts in life strategies explain microbial community responses to increasing nitrogen in tundra soil? Soil Biol Biochem 96:216–228
Massaccesia L, Rondoni G, Tosti G, Conti E, Guiducci M, Agnellia B (2020) Soil functions are affected by transition from conventional to organic mulch-based cropping system. Appl Soil Ecol 253:103639
Niemi RM, Vepsalainen M, Wallenius K, Erkomaa K, Kukkonen S, Parojarvi A, Vestberg M (2008) Conventional versus organic cropping and peat amendment: impacts on soil microbiota and their activities. J Soil Biol 44:419–428
Nunes MR, Karlen DL, Veum KS, Moorman TB (2020) Biological soil health indicators respond to tillage intensity: a US meta-analysis. Geoderma 369:114335
Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO (2013) The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucl Acids Res 41(D1):D590–D596
Rubin BER, Gibbons SM, Kennedy S, Hampton-Marcell J, Owens S et al (2013) Investigating the impact of storage conditions on microbial community composition in soil samples. PLoS ONE 8(7):e70460
Statistics Canada (2017) 2016 census of agriculture. Statistics Canada, Ottawa
Tautges NE, Sullivan TS, Reardon CL, Burke IC (2016) Soil microbial diversity and activity linked to crop yield and quality in a dryland wheat production system. Appl Soil Ecol 108:258–268
Tian W, Wang L, Li Y, Zhuang K, Li G, Zhang J, Xiao X, Xi Y (2015) Responses of microbial activity, abundance, and community in wheat soil after three years of heavy fertilization with manure-based compost and inorganic nitrogen. Agric Ecosyst Environ 213:219–227
Trivedi P, Delgado-Baquerizo M, Trivedi C, Hamonts K, Anderson IC, Singh BK (2017) Keystone microbial taxa regulate the invasion of a fungal pathogen in agro-ecosystems. Soil Biol Biochem 111:10–14
Tully KL, McAskill C (2020) Promoting soil health in organically managed systems: a review. Org Agric 10:339–358
Acknowledgements
We are grateful to field staff at Beaverlodge Research Farm for the successful implementation of this field trial.
Funding
Funding was provided by Agriculture and Agri-Food, Canada.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lupwayi, N.Z., Grant, C.A., O’Donovan, J.T. et al. Soil microbial communities in tilled organic and no-till conventional crop production systems. Org. Agr. 11, 553–565 (2021). https://doi.org/10.1007/s13165-021-00360-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13165-021-00360-4