Abstract
Five strains of Streptomyces sp. (CAI-24, CAI-121, CAI-127, KAI-32, and KAI-90; demonstrated previously to have potential for control of Fusarium wilt disease in chickpea and plant growth promotion [PGP] in rice) were evaluated for their PGP capabilities in chickpea in the 2012–2013 and 2013–2014 post-rainy seasons. The plots inoculated with Streptomyces sp. significantly enhanced number of nodule, nodule weight, root weight, and shoot weight at 30 days after sowing (DAS) and number of pod, pod weight, leaf area, leaf weight, and stem weight at 60 DAS in both seasons over the un-inoculated control plots. At chickpea crop maturity, all of the Streptomyces strains significantly enhanced stover yield, grain yield, and total dry matter in both seasons over the un-inoculated control. In the rhizosphere, the Streptomyces strains also significantly enhanced soil biological and mineral nutrient activities including microbial biomass carbon, dehydrogenase activity, total nitrogen, available phosphorous, and organic carbon in both seasons over the un-inoculated control. All of the five strains were found superior in terms of nodule formation, root and shoot development, and crop productivity; however, KAI-xx had little edge over the other five strains. Scanning electron microscopy (SEM) analysis had revealed the success of colonization by the strains of Streptomyces sp. of the chickpea roots. Quantitative real-time PCR (qRT-PCR) analysis of selected PGP genes revealed overall upregulation of β-1,3-glucanase, indole-3-acetic acid, and siderophore genes in the Streptomyces species studied. This investigation further confirms the broad spectrum of PGP activities by the selected Streptomyces sp.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rhizospheric soil is usually rich in nutrients when compared to bulk soil, due to the accumulation of plant growth regulators, amino acids, sugars, vitamins, fatty acids, organic acids, nucleotides, phenols, and sterols, released from the roots by exudation, secretion, and deposition. This results in enrichment of microorganisms such as bacteria, fungus, algae, and protozoa. Rhizobacteria are categorized depending on their proximity to the roots as (i) bacteria colonizing soil near the roots (rhizosphere), (ii) bacteria colonizing on root surface (rhizoplane), (iii) bacteria colonizing in root tissue (endophytes) and inhabiting spaces between cortical cells, and (iv) bacteria colonizing inside cells, in specialized root structures such as nodules (Glick 1995). Bacteria that belong to any of these categories and promote plant growth through various mechanisms, such as nitrogen fixation, phosphate solubilization, iron chelation, phyto-hormone production, and suppression of plant pathogenic organisms are referred as plant growth-promoting (PGP) bacteria. The use of PGP bacteria for improving agricultural production, soil, and plant health has become one of the attractive strategies for developing sustainable agricultural systems in many parts of the world due to their eco-friendliness, low production cost, and reduced consumption of non-renewable resources. PGP bacteria in the rhizosphere has been reported to enhance root growth, shoot growth, plant hormone concentrations, nitrogen fixation, the solubilization of minerals, and the suppression of plant pathogens (Shaukat et al. 2006; Richardson et al. 2009). PGP bacteria including Bacillus, Pseudomonas, and Streptomyces were found effective in helping the plants not only to mobilize nutrients but also to control plant pathogens [Perner et al. 2006; Gopalakrishnan et al. 2011). Streptomyces promote plant growth either by producing indole-3-acetic acid (IAA) and siderophores, and/or by inhibiting soil-borne fungal pathogens on tomato, wheat, rice, bean, and pea (Aldesuquy et al. 1998; Trejo-Estrada et al. 1998; Tokala et al. 2002; Nassar et al. 2003; El-Tarabily 2008; Macagnan et al. 2008; Sadeghi et al. 2012; Gopalakrishnan et al. 2011, 2013, 2014).
Previously, we have reported five strains of Streptomyces sp. (CAI-24, CAI-121, CAI-127, KAI-32, and KAI-90) that were isolated from herbal vermicompost, to have the potential in the biocontrol of Fusarium wilt in chickpea, caused by Fusarium oxysporum f. sp. ciceri (Gopalakrishnan et al. 2011) and in PGP of rice (Gopalakrishnan et al. 2013). The main objectives of this study were to further evaluate the five Streptomyces strains for their PGP traits in chickpea under filed conditions, to demonstrate gene expression profiles by quantitative real-time PCR (qRT-PCR) analysis and to ensure colonizing ability in chickpea by scanning electron microscopy (SEM) analysis.
Materials and methods
PGP strains
Five strains of Streptomyces CAI-24 (NCBI accession: JN400112), CAI-121 (NCBI accession: JN400113), CAI-127 (NCBI accession: JN400114), KAI-32 (NCBI accession: JN400115), and KAI-90 (NCBI accession: JN400116), reported as potential for the biocontrol of Fusarium wilt in chickpea (Gopalakrishnan et al. 2011) and PGP in rice (Gopalakrishnan et al. 2013) were further studied.
Effect of Streptomyces strains for PGP potential on chickpea under field conditions
The field trials were carried out in two consecutive post-rainy seasons (2012−2013 and 2013–2014) at International Crops Research Institute for the Semi-Arid Tropics (ICRISAT), Patancheru (17° 30′ N; 78° 16′ E; altitude 549 m), Telangana, India. Soils at the trial site are classified as vertisols (fine montmorillonitic isohyperthermic typic pallustert) having 52 % clay, 26 % sand, and 22 % silt with an alkaline pH (7.6 − 8.4) and an organic carbon (OC) content of 0.4 − 0.6 %. The soil depth of the field used was ≥120 cm (maximum rooting depth by chickpea), and this soil retained 205 mm of plant-available water in a 120-cm soil profile. The field was kept fallow except for post-rainy season crop. The mineral content of the rhizosphere (top 15 cm) of soil include 23.6 mg kg−1 soil of available N, 8.7 mg kg−1 soil of available P, and 300 mg kg−1 soil of available K. The trial fields were prepared into broad beds and furrows with beds 1.2 m wide flanked by 0.3-m furrows in seasons, 2012−2013 and 2013–2014. At 3 days before sowing, surface application and incorporation of 18 kg N ha−1 and 20 kg P ha−1 as di-ammonium phosphate (DAP) were carried out in both seasons. The field trial was laid out in a randomized complete block design (RCBD) with three replicates and subplot sizes of 4 m × 3 ridges.
The five strains of Streptomyces (CAI-24, CAI-121, CAI-127, KAI-32, and KAI-90) were cultured individually on a starch casein broth (SCB) at 28 °C for 5 days. The field trial was conducted with a medium duration chickpea variety, ICCV 2, which normally yields 1.1−1.2 t ha−1. The seeds were treated with a strains of Streptomyces (containing 108 CFU ml−1) for 45–50 min and sown immediately by hand planting on 11 October 2012 in the first year and 1 November 2013 in the second year in rows 30 cm apart at a depth of 4–5 cm to achieve an estimated plant density of at least 26 plants m−2. Plants were inoculated with respective Streptomyces strains (1000 ml; 108 CFU mL−1) once every 15 days on the soil close to the plant until the flowering stage. Control plots were not inoculated with Streptomyces strains. No pesticide was sprayed during the cropping period, as no serious insect pests or phytopathogens attacks were observed. The plots were irrigated on the 21st and 49th days after sowing whereas weeding was done as and when required. The crop was harvested manually on 19 January 2013 in the first year and 8 Feb 2014 in the second year. During the two seasons, in both the 2012–13 and 20132014 seasons, at 30 days after sowing (DAS), the number of nodule, nodule weight, root weight, and shoot weight were noted and at 60 DAS, the plant height, number of pod, pod weight, leaf area, leaf weight, and stem weight were noted. At crop maturity, stover yield, grain yield, total dry matter (TDM), 1000-seed weight, pod weight, number of seed, and seed weight were noted in both seasons. Rhizosphere soil samples were collected from the top 15 cm depth of the soil profile at the crop maturity and analyzed for soil mineral nutrients (total nitrogen, available phosphorous, and organic carbon as described by Novozamsky et al. (1983), Olsen and Sommers (1982), and Nelson and Sommers (1982), respectively) and soil biological activity (microbial biomass carbon by the fumigation method and dehydrogenase activity by the triphenyl formazan production method as described by Anderson and Domsch (1989) and Casida (1977), respectively).
Scanning electron microscopy (SEM) analysis
The roots of chickpea were tested for colonization by Streptomyces sp. by SEM analysis (Bozzola and Russell 1998). In brief, the seeds of chickpea variety ICCV2 were surface sterilized first with 2.5 % sodium hypochlorite (for 5 min) followed by 70 % ethanol (for 5 min) and rinsed with sterilized water (eight times) before being allowed to sprout in a Petri dish overnight. The sprouted seeds were transferred into test Streptomyces strains (CAI-24, CAI-121, CAI-127, KAI-32, and KAI-90; grown in Bennett’s broth separately) for 45 min before being sown in the pots containing sterilized coarse sand (six seeds/plastic pot containing 2-kg sand). The Streptomyces strains (5 ml per seedling; 108 CFU ml−1) were applied 1 week after sowing in sand by soil drench method. The pots were incubated in a greenhouse for 2 weeks where temperature was set at 24 ± 2 °C. At the end of the 2 weeks incubation period, chickpea seedlings were removed from the sand pots and the roots were washed in phosphate buffer (0.1 M; pH 7.2). The tip of the roots were cut into 4–5 mm long pieces and fixed in 2.5 % glutaraldehyde in phosphate buffer for 24 h at 4 °C. At the end of incubation, the samples were washed with phosphate buffer, postfixed in 2 % osmium tetra oxide for 4 h and dehydrated using a graded series of ethanol. The dehydrated samples were dried with critical-point liquid carbon dioxide as a transition fluid and adhered onto aluminum specimen mounts with double-stick adhesive tape. The mounted root samples were coated with gold-palladium in an automated sputter coater (JEOL JFC-1600) and examined with a scanning electron microscope (JOEL-JSM 5600) as per the standardized procedure at RUSKA lab, College of Veterinary Science, Rajendranagar, Hyderabad, India. Observations of the presence of Streptomyces spores on root surfaces were recorded.
Gene expression profile studies
The five selected Streptomyces strains (CAI-24, CAI-121, CAI-127, KAI-32, and KAI-90) were grown in Bennett’s broth at 28 °C for 72 h. Total RNA was extracted from all of the Streptomyces strains using the “NucleoSpin® RNA Plant” kit (Macherey-Nagel). The quality and quantity of RNA was estimated by Nanodrop (Thermo Scientific) and RNA integrity was determined using a 2100 Bioanalyzer (Agilent).
Quantitative real-time polymerized chain reaction (qRT-PCR) was performed using the Applied Biosystems 7500 Real-Time PCR System with the SYBR green chemistry (Applied Biosystems, USA) as per the manufacturer’s instructions. Well-characterized genes relating to IAA production (F: GTCACCGGGATCTTCTTCAAC; R: GATGTCGGTGTTCTTGTCCAG), siderophore (F: ATCCTCAACACCCTGGTCTG; R: TCCTTGTACTGGTACGGGACTT) and were collected from the UniprotKB database (http://www.uniprot.org/uniprot) as described by Gopalakrishnan et al. 2014. Similarly, genes for β-1,3-glucanase (F: CCGAACACCACCTACTCCAC; R: CCAGGTTGAGGATCAGGAAG) were also selected for the study. Gene-specific primers for real-time PCR were designed using Primer3 software (Rosen and Skaletsky 2000). RNA polymerase principal sigma factor HrdB (SCO5820) (F: GGTCGAGGTCATCAACAAGC; R: CTCGATGAGGTCACCGAACT) was used as the endogenous control. qRT-PCR reactions were performed as described earlier (Gopalakrishnan et al. 2014). The data obtained were analyzed using the mean of the CT values of the three biological replicates that were normalized to the mean CT values of the endogenous gene. The expression ratios were calculated using the 2−∆∆Ct method. Relative transcription levels are presented graphically.
Statistical analysis
Data were analyzed by analysis of variance (ANOVA) and the GLM (general linear model) procedure in the software package SAS (SAS Inst. 2002–08, SAS V9.3), considering isolates and replication as fixed in RCBD. Isolate means were tested for significance and compared using Fisher’s protected least significant difference (LSD) test.
Results
Effect of Streptomyces strains for PGP potential on chickpea under field conditions
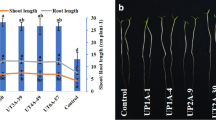
All the Streptomyces strains (CAI-24, CAI-121, CAI-127, KAI-32, and KAI-90) were seen to positively enhance various agronomic performances and, therefore, a mean of all the strains were presented. At 30 DAS, by a mean of the five strains, the number of nodules was enhanced by 70 % in 2012–2013 and 42 % in 2013–2014, nodule weight by 82 % in 2012–2013 and 29 % in 2013–2014, root weight by 6 % in 2012–2013 and 7 % in 2013–2014, and shoot weight by 21 % in 2012–2013 and 20 % in 2013–2014 in Streptomyces-treated plots over the un-inoculated control (Table 1). Similarly at 60 DAS, the plant height was enhanced by 3 % in 2012–2013 and 6 % in 2013–2014, number of pods by 51 and 31 %, pod weight by 85 and 23 %, leaf area by 9 and 18 %, leaf weight by 21 and 19 %, and stem weight by 32 % in 2012–2013 and 9 % in 2013–2014 in Streptomyces-treated plots over the un-inoculated control (Table 2). At crop maturity, the Streptomyces strains enhanced the stover yield by 39 % in 2012–2013 and 14 % in 2013–2014, grain yield by 12 and 11 %, total dry matter by 22 and 12 %, pod weight by 7 and 12 %, number of seed by 8 and 12 % and seed weight by 4 % in 2012–2013 and 10 % in 2013–2014 over the un-inoculated control (Table 3). In the rhizosphere soils (top 15-cm), at crop maturity, the Streptomyces-treated plots significantly enhanced the soil biological activities including the microbial biomass carbon by 55 % in 2012–2013 and 53 % in 2013–2014 and dehydrogenase activity by 17 and 19 % as well as soil mineral nutrient contents including total N by 5 and 3 %, available P by 37 and 20 %, and organic carbon by 9 % in 2012–2013 and 6 % in 2013–2014 over the un-inoculated control plots (Tables 4 and 5).
Scanning electron microscopy (SEM) analysis
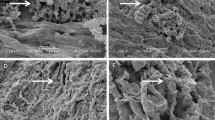
SEM analysis of chickpea roots revealed a remarkable degree of colonization by Streptomyces sp. Roots from inoculated plants exhibited significant surface colonization by Streptomyces sp. while those from un-inoculated plants did not. The sporulation of Streptomyces sp. on the surface cell layer of the roots of chickpea was clearly visible for all five strains. The hyphae of Streptomyces were also found to penetrate the surface cell layer of chickpea roots (Fig 1).
Gene expression profile studies
In the plant growth-promoting gene expression profiling studies, good quality RNA was isolated from all the Streptomyces strains (CAI-24, CAI-121, CAI-127, KAI-32, and KAI-90). qRT-PCR validation of IAA gene revealed high upregulation in the strains CAI-121 (10.0-fold) followed by CAI-127 (9.5-fold) while the gene siderophore was highly upregulated in CAI-121 (12.6-fold) followed by CAI-127 (12.4-fold), CAI-24 (6.2-fold), and KAI-32 (5.5-fold) and the gene β-1,3-glucanase showed up-regulation in KAI-90 (2.4-fold) followed by CAI-121 (2.1-fold) and CAI-24 (1.9-fold) (Fig. 2).
Discussion
The five Streptomyces spp. (CAI-24, CAI-121, CAI-127, KAI-32, and KAI-90), previously demonstrated to have a potential for control of wilt in chickpea (Gopalakrishnan et al. 2011) and PGP in sorghum and rice (Gopalakrishnan et al. 2013), were further evaluated in this investigation for their PGP in chickpea under field conditions. In the present study, at 30 DAS, the number of nodules and weight of nodules were found significantly higher in Streptomyces-treated plots over un-inoculated control plots. Though, Streptomyces spp. was not reported to have the nodulation capability but its colonization on the nodules and beneficial association with Rhizobium spp. were shown to increase growth, nodulation, nitrogen fixation and seed yield of pea and soybean (Tokala et al. 2002; Soe et al. 2010). The beneficial association of Streptomyces spp. with mycorrhiza is also well established. For instance, strains of Streptomyces and its metabolites provided a broad spectrum of antifungal activity that protected the mycorrhizal roots from fungal parasites and selected against mycorrhizal fungal competitors (Schrey et al. 2012). In the present investigation, all of the five Streptomyces strains were found to enhance yield and agronomic traits; however, KAI-90 enhanced most of the agronomic and yield parameters studied including stover yield, grain yield, and total dry matter, followed by KAI-32, CAI-24, CAI-127, and CAI-121, in descending order. The efficacy of Streptomyces on PGP in various agriculturally important crops was extensively reported (Tokala et al. 2002; Nassar et al. 2003; El-Tarabily 2008; Sadeghi et al. 2012; Gopalakrishnan et al. 2014). PGP agents having broad-spectrum traits offer effective novel strategies not only for the improvement of crop growth and yield but also for the control of insect pests and pathogens that attack crops. In addition to suppressing plant pathogens and insect pests by secreting secondary metabolites such as antibiotics, some PGP agents also elicit induced systemic resistance against a broad range of insects and pathogens (Jetiyanon and Kloepper 2002; Ryu et al. 2007). Although the Streptomyces used in this study excrete antimicrobial compounds, antagonism tests by the poisoned food technique indicate that none of the strains inhibit the growth of nodulating bacteria Mesorhizobium ciceri on yeast extract mannitol agar media (data not shown). Thus, the selected Streptomyces spp. appear to be compatible with Rhizobium.
In the present study, the highest magnitude of soil biological activities (including microbial biomass carbon and dehydrogenase activities) and mineral nutrient properties (including total N, available P, and organic carbon) were also found in KAI-90 followed by the other four isolates. Mandal et al. (2007) reported a close relationship between the soil microbial biomass and crop yields under both greenhouse as well as field conditions. Jannouraa et al. (2013) demonstrated close relationships between grain N and P concentrations and microbial biomass C, N, and P, thus suggesting the use of soil microbial biomass as an indicator of nutrient availability to plants. Microorganisms in soil played an important role in nutrient cycling and plant nutrition, reduced pathogen populations, increased soil organic matter, total carbon, cation exchange capacity, and lowered bulk density thus improving soil quality (Bulluck et al. 2002). Thus, it is concluded that the five Streptomyces spp. could be used in the organically managed agro-ecosystems.
Colonization of chickpea roots by Streptomyces strains at the right time and place is essential for enhanced PGP activity. Successful host-microbe interaction is essential which depends on the presence of a sufficient population of bacteria, as well as the rhizosphere competence, root-colonizing ability, and PGP ability of the bacteria (Lugtenberg and Dekkers 1999). The SEM analysis in addition to the data for grain and stover yield, roots, and other agronomical traits, the chemical and biological activities of the rhizosphere soil strongly suggest that the five Streptomyces sp. had multiplied and colonized the inoculated chickpea roots.
The mechanism by which the five Streptomyces sp. consistently enhanced agronomical and yield traits on sorghum, rice (from our previous study), and chickpea could be attributed to their enzymatic activities, such as the ability to produce siderophores, indole acetic acid, and β-1,3-glucanase activities (Gopalakrishnan et al. 2011, 2013). Siderophores form stable complexes with heavy metals such as Cu, Cd, In, U, Np, Al, Pb, Zn, and Ga increases the soluble metal concentration (Rajkumar et al. 2010) in the soil system; therefore, it helps to alleviate the various heavy metals stresses imposed on plants. Siderophores also act as solubilizing agents for iron from minerals under conditions of iron limitation (Indiragandhi et al. 2008). IAA-producing microorganisms are known to stimulate seed germination, initiate adventitious and lateral root formation and increase root length and surface area, thereby providing the host plant greater access to soil nutrients and water Ahemad and Kibret 2014). The cell wall of plant pathogens is composed of layers of β-1,3-glucan (as in the case of Fusarium oxysporum; the causal organisms of wilt in many crops) and lysis of this by β-1,3-glucanase-producing microorganism leads to the leakage of cell contents and the collapse of the pathogenic fungi (Singh et al. 1999). In the present investigation, validation of the IAA, siderophore, and β-1,3-glucanase genes confirmed the results of in vitro PGP attributes of the studied Streptomyces strains (Gopalakrishnan et al. 2011). Hence, it is concluded that the Streptomyces strains studied in this investigation contains multi-trait of PGP and, therefore, these can be exploited not only for PGP but also for biological control of plant pathogens.
Conclusion
It is concluded that the five Streptomyces sp. used in this study are apparently well adapted not only to the sorghum and rice rhizosphere, as reported earlier, but also to the chickpea rhizosphere, as demonstrated in the current investigation. Also, the five Streptomyces spp. contain a broad range of PGP traits and demonstrate multiple mechanisms of actions. Therefore, the five Streptomyces spp. used in this study are likely to be the potential candidates for the discovery of novel secondary metabolites and their usefulness in host plant resistance against a range of pathogens and insect pests that can assist in furthering the use of eco-friendly biopesticides and biofertilizers in integrated pest, disease, and nutrition management programs in the organically managed agro-ecosystems. However, there is a need to determine the effectiveness of these Streptomyces strains under different field conditions (multi-location trials) and to understand the nature of their interaction with other native soil microflora and microfauna with host plants and the environment.
References
Ahemad M, Kibret M (2014) Mechanisms and applications of plant growth promoting rhizobacteria: current perspective. J King Saud Univ 26:1–20
Aldesuquy HS, Mansour FA, Abo-Hamed SA (1998) Effect of the culture filtrates of Streptomyces on growth and productivity of wheat plants. Folia Microbiol 43:465–470
Anderson TH, Domsch KH (1989) Ratios of microbial biomass carbon to total organic carbon in arable soils. Soil Biol Biochem 21:471–479
Bozzola JJ, Russell LD (1998) In: Electron microscopy principals and techniques for biologists. 2nd edn. Jones and Barlett publishers, Sudbury, Massachusetts. pp. 19─24, 54─55, 63─67
Bulluck LR, Brosius M, Evanylo GK, Ristaino JB (2002) Organic and synthetic fertility amendments influence soil microbial, physical and chemical properties on organic and conventional farms. Appl Soil Ecol 19:147–160
Casida LE (1977) Microbial metabolic activity in soil as measured by dehydrogenase determinations. Appl Environ Microbiol 34:630–636
El-Tarabily KA (2008) Promotion of tomato (Lycopersicon esculentum Mill.) plant growth by rhizosphere competent 1-aminocyclopropane-1-carboxylic acid deaminase–producing Streptomycete actinomycetes. Plant Soil 308:161–174
Glick BR (1995) The enhancement of plant growth by free-living bacteria. Can J Microbiol 41:109–117
Gopalakrishnan S, Pande S, Sharma M, Humayun P, Kiran BK, Sandeep D, Vidya MS, Deepthi K, Rupela O (2011) Evaluation of actinomycete isolates obtained from herbal vermicompost for biological control of Fusarium wilt of chickpea. Crop Prot 30:1070–1078
Gopalakrishnan S, Srinivas V, Vidya MS, Rathore A (2013) Plant growth-promoting activities of Streptomyces sp. in sorghum and rice. Springer Plus 2(574):1–8
Gopalakrishnan S, Vadlamudi S, Bandikinda P, Sathya A, Vijayabharathi R, Rupela O, Kudapa B, Katta K, Varshney RK (2014) Evaluation of Streptomyces strains isolated from herbal vermicompost for their plant growth-promotion traits in rice. Microbiol Res 169:40–48
Indiragandhi P, Anandham R, Madhaiyan M, Sa TM, Indiragandhi P, Anandham R, Madhaiyan M, Sa TM (2008) Characterization of plant growth-promoting traits of bacteria isolated from larval guts of diamondback moth Plutella xylostella (Lepidoptera; Plutellidae). Curr Microbiol 56:327–333
Jannouraa R, Bruns C, Joergensena RG (2013) Organic fertilizer effects on pea yield, nutrient uptake, microbial root colonization and soil microbial biomass indices in organic farming systems. Eur J Agron 49:32–41
Jetiyanon K, Kloepper JW (2002) Mixtures of plant growth promoting rhizobacteria for induction of systemic resistance against multiple plant diseases. Biol Control 24:285–291
Lugtenberg BJJ, Dekkers LC (1999) What makes Pseudomonas bacteria rhizosphere competent? Environ Microbiol 1:9–13
Macagnan D, Romeiro RDA, Pomella AMV, deSouza JT (2008) Production of lytic enzymes and siderophores, and inhibition of germination of basidiospores of Moniliophthora (ex Crinipellis) perniciosa by phylloplane actinomycetes. Biol Control 47:309–314
Mandal A, Patra AK, Singh D, Swarup A, Masto RE (2007) Effect of long-term application of manure and fertilizer on biological and biochemical activities in soil during crop development stages. Bioresour Technol 98:3585–3592
Nassar AH, El-Tarabily KA, Sivasithamparam K (2003) Growth promotion of bean (Phaseolus vulgaris L.) by a polyamine producing isolate of Streptomyces griseoluteus. Plant Growth Reg 40:97–106
Nelson DW, Sommers LE (1982) Total organic carbon and organic matter’. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis, part 3, chemical and microbiological properties. SSSA, Madison, WI, pp 539–579
Novozamsky I, Houba VJG, Van ECKR, vanVark W (1983) A novel digestion technique for multiple element analysis. Commun Soil Sci Plant Anal 14:239–249
Olsen SR, Sommers LE (1982) Phosphorus. In Methods of soil analysis, Agron No 9, part 2, ‘chemical and microbial properties’, 2nd edition, Am Soc Agron Page AL (Ed), Madison WI, USA, pp.403 − 430
Perner H, Schwarz D, George E (2006) Effect of mycorrhizal inoculation and compost supply on growth and nutrient uptake of young leek plants grown on peat-based substrates. Hort Sci 41:628–632
Rajkumar M, Ae N, Prasad MNV, Freitas H (2010) Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol 28:142–149
Richardson AE, Barea JM, Mcneill AM, Combaret CP (2009) Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 321:305–339
Rosen S, Skaletsky HJ (2000) Primer 3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S (eds) Bioinformatics Methods and Protocols: Methods in Molecular Biology. Humana Press, Totowa, NJ, pp 365–386
Ryu CM, Murphy CF, Reddy MS, Kloepper JW (2007) A two strain mixture of rhizobacteria elicits induction of systemic resistance against Pseudomonas syringae and cucumber mosaic virus coupled to promotion of plant growth on Arabidopsis thaliana’. J Microbiol Biotechnol 17:280–286
Sadeghi A, Karimi E, Dahazi PA, Javid MG, Dalvand Y, Askari H (2012) Plant growth promoting activity of an auxin and siderophore producing isolate of Streptomyces under saline soil condition. World J Microbiol Biotechnol 28:1503–1509
Schrey SD, Erkenbrack E, Fruh E, Fengler S, Hommel K, Horlacher N, Schulz D, Ecke M, Kulik A, Fiedler H, Hampp R, Tarkka MT (2012) Production of fungal and bacterial growth modulating secondary metabolites is widespread among mycorrhiza-associated Streptomyces. BMC Microbiol 12:164
Shaukat K, Affrasayab S, Hasnain S (2006) Growth responses of Triticum aestivum to plant growth promoting rhizobacteria used as a biofertilizer. Res J Microbiol 1:330–338
Singh PP, Shin YC, Park CS, Chung YR (1999) Biological control of Fusarium wilt of cucumber by chitinolytic bacteria. Phytopathology 89:92–99
Soe KM, Bhromsiri A, Karladee D (2010) Effects of selected endophytic actinomycetes (Streptomyces sp,) and Bradyrhizobia from Myanmar on growth, nodulation, nitrogen fixation and yield of different soybean varieties. CMU J Nat Sci 9:95–109
Tokala RK, Strap JL, Jung CM, Crawford DL, Salove MH, Deobald LA, Bailey JF, Morra MJ (2002) Novel plant-microbe rhizosphere interaction involving Streptomyces lydicus WYEC108 and the pea plant (Pisum sativum). Appl Environ Microbiol 68:2161–2171
Trejo-Estrada SR, Paszczynski A, Crawford DL (1998) Antibiotics and enzymes produced by the biocontrol agent Streptomyces violaceusniger YCED-9. J Ind Microbiol Biot 21:81–90
Acknowledgements
This work has been undertaken as part of the CGIAR Research Program on Grain Legumes. ICRISAT is a member of CGIAR Consortium. We thank Dr. M Lakshman, Associate Professor, Ruska Lab, College of Veterinary Science, Rajendranagar, Hyderabad, for SEM analysis and all of the staff of the biocontrol unit of ICRISAT including M/s PVS Prasad, P Manohar, B Nagappa, D Barath, A Jabbar, and S Rohini for their significant contribution in the laboratory and field studies.
Conflict of interest
All of the authors declare that they have no financial/commercial conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gopalakrishnan, S., Srinivas, V., Alekhya, G. et al. Evaluation of Streptomyces sp. obtained from herbal vermicompost for broad spectrum of plant growth-promoting activities in chickpea. Org. Agr. 5, 123–133 (2015). https://doi.org/10.1007/s13165-015-0099-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13165-015-0099-1