Abstract
The Mississippi River Delta (MRD) is one of the largest wetlands in the world and is experiencing widespread dieback of its most prevalent plant species, Phragmites australis. The potential for revegetation of dieback sites was assessed by planting three genetically distinct lineages of P. australis at seven sites in the MRD that varied in water depth. These lineages include Delta, the most prevalent in the MRD, EU, a highly invasive lineage, and Gulf, a common lineage of the Gulf Coast and southern United States. Three additional wetland species were planted at a subset of these sites. Plantings were monitored for 32 months during which time, we surveyed plantings for survival, growth and infestation by a scale insect that has been implicated in P. australis dieback. Survivorship differed considerably among plant types and by 6 months, Delta, Gulf and California bulrush (Schoenoplectus californicus) had twice the survivorship of the other plant types. By the end of the study, Delta, Gulf and bulrush were the only plant types alive, with Delta persisting in more than twice as many sites and having > 6 times more area of coverage as Gulf and bulrush. For all P. australis lineages, water depth was negatively related to survivorship. Finally, although scale insects achieved twice the abundance on Delta than the other lineages, we found no evidence that scales were inhibiting the growth and survival of P. australis. Despite potential negative legacy effects of dieback, Delta can be used revegetate shallow-water sites within a year following dieback.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Globally, the Mississippi River has the seventh largest discharge of freshwater, nutrients and sediments in the world (Allison et al. 2012; Milliman and Meade 1983). Currently, about two-thirds of the river is drained via the Holocene-age deltaic plain known as the Mississippi River Deltaic Plain (MRD) (Saucier 1994). This coastal wetland is amongst the most productive ecosystems in the world, providing invaluable economic and ecological services including flood control, habitat for wildlife, maintenance of water quality, support of natural resource-based industries and recreational and commercial fisheries (Day et al. 2004, 1997). Despite its economic value, the MRD has experienced amongst the highest rates of coastal wetland loss worldwide (Allison and Meselhe 2010; Barras et al. 2004; Day et al. 2000).
An important contributor of wetland loss in coastal Louisiana has been vegetation dieback (Alber et al. 2008; Suir et al. 2022; Webb and Mendelssohn 1996). In 2000, over 10,000 ha of Spartina alterniflora Loisel (= Sporobolus alterniflora; Poaceae) dominated salt marshes experienced a sudden and acute dieback event (termed “brown marsh”). More recently, the dominant emergent vegetation in the Lower MRD, a perennial marsh grass, Phragmites australis (Cav.) Trin. ex Steud has experienced extensive dieback (Knight et al. 2018; Ramsey and Rangoonwala 2017; Suir et al. 2018; Suir et al. 2022). Large-scale dieback events have been reported from other regions of the world including P. australis throughout Europe (Brix 1999; van der Putten 1997) and in China (Li et al. 2013), seepweed (Suaeda salsa L.) in China (He et al. 2017), and mangrove forests in Australia, South Africa and the Caribbean (Breen and Hill 1969; Duke et al. 2017; Lovelock et al. 2017; Rossi et al. 2020). Vegetation dieback events are often precursors to land loss because they result in the conversion of marshes into bare mudflats or open water (Brix 1999; Turner 1990; van der Putten 1997).
In the MRD, P. australis inhabits freshwater and brackish wetland systems and grows in dense monospecific stands. Symptoms of P. australis dieback include retreat from deep waters, clumped distribution of stems, stunted stem and root growth, premature senescence, and increased occurrences of dead or decaying rhizomes (Armstrong et al. 1996; Brix 1999; van der Putten 1997). The cause of dieback in P. australis is complex and currently under investigation. However, one important contributing factor appears to be a nonnative scale insect, Nipponaclerda biwakoensis (Kuwana) (Hemiptera: Aclerdidae), whose discovery coincided with the appearance of widespread P. australis dieback in the MRD (Cronin et al. 2020; Knight et al. 2020, 2018). The scales reach mean densities in the summer of 150 per meter of stem and reduce P. australis growth and biomass (Knight et al. 2020). Moreover, scale insect density across stands of P. australis in the MRD is negatively correlated with the normalized difference vegetation index (NDVI); a measure of standing plant biomass (I. A. Knight, G. Suir and R. Diaz, unpublished data). Abiotic factors associated with climate change and human activities such as extended flooding, elevated soil salinity or nutrient pollution have also been linked to P. australis dieback (Cronin et al. 2020; Knight et al. 2020; Lee et al. 2023).
An additional factor complicating our understanding of dieback in the MRD is that there are three genetic and ecologically distinct lineages of P. australis that differ in their susceptibility to dieback. The dominant lineage in the MRD is Delta (haplotype M1; Hauber et al. 2011), accounting for > 95% of P. australis coverage (Hauber et al. 2011; Knight et al. 2018). It is of North African and Mediterranean origin (Hauber et al. 2011; Lambertini et al. 2012). Another lineage found in the MRD is of Eurasian origin (haplotype M; hereafter, the EU lineage) and is highly invasive of coastal and wetland marshes throughout the continental United States and southern provinces of Canada (Saltonstall 2002). The EU lineage did not occur in the MRD until the 1990s when it was first noted in the Lower MRD (Hauber et al. 2011). Stands of the EU lineage are often found embedded within or contiguous with much larger Delta stands (Cronin et al. 2020; Knight et al. 2018). Finally, the Gulf lineage (haplotype I, Land-type or subspecies P. australis berlandieri) is widely distributed along the southernmost United States from Florida to California and south of the border into Central and South America (Colin and Eguiarte 2016; Hauber et al. 2011; Meyerson et al. 2010; Saltonstall 2002). The Gulf lineage inhabits more upland and less saline areas of the marsh (Achenbach and Brix 2014; Cronin et al. 2020). Based on field observations and experimental studies, the Delta lineage is most impacted by scale insects (i.e., it has a greater proportional reduction in biomass in the presence of scales) and the Gulf lineage is most impacted by flooding and increased salinity (Achenbach and Brix 2014; Cronin et al. 2020; Knight et al. 2020). The EU lineage is more tolerant to these stressors and is less prone to exhibiting symptoms of dieback than the Delta or Gulf lineages (Cronin et al. 2020; Knight et al. 2020).
Restoration has become a key conservation strategy to combat coastal wetland degradation, promote biological diversity and enhance biogeochemical functions (Nyman et al. 2006; Ogburn and Alber 2006; Silliman et al. 2015; Zhou et al. 2003). With the acceleration of coastal habitat loss due to climate change and anthropogenic activities, planting habitat-forming species has been widely adopted for wetland plant species (Brix et al. 2014; Edwards et al. 2005; Howard et al. 2008; Rolletschek et al. 1999; Silliman et al. 2015; Wilsey et al. 1992). However, in dieback events, habitat restoration through natural revegetation or replanting efforts may be hindered by the presence of adverse edaphic, hydrological or biotic stressors that were the cause or consequence of dieback (Crawford and Stone 2015). For example, dieback soils in wetland marshes are known to have toxic levels of sulfides, owing to anaerobic decomposition of accumulated organic material (Armstrong et al. 1996; Brix 1999; van der Putten 1997; Webb and Mendelssohn 1996), that could inhibit revegetation.
Given the unprecedented and substantial loss of P. australis in the Lower MRD (Suir et al. 2022), we conducted an in situ restoration field trial to assess the potential for revegetation of recent P. australis dieback sites. At seven dieback sites varying in water depth, we planted three lineages of P. australis (Delta, EU and Gulf). Although P. australis is our primary focus, at some of the sites, we also planted up to three other common wetland plant species: Schoenoplectus californicus (C.A. Meyer) Palla (California bulrush), Spartina patens (Aiton) Muhl. (saltmeadow cordgrass) and Zizaniopsis miliacea (Michx.) Döll. & Asch. (giant cutgrass). Plantings took place in 2018 and were monitored (through ground surveys and drone imagery analysis) over the next 32 months to assess the growth and survival of each plant lineage and species. For P. australis, we also monitored scale insect density as these insects may play a role in inhibiting successful re-establishment. We predicted that (1) because these sites were formerly occupied by P. australis, P. australis lineages would be better capable of tolerating the environmental conditions (e.g., relatively high water levels, periodic salt intrusion) and have higher survival and growth than the other plant species; (2) the EU lineage of P. australis would outperform the other lineages of P. australis because it is more tolerant of scale insects and dieback conditions (Cronin et al. 2020; Knight et al. 2020; Lee et al. 2023); (3) shallow-water sites would have higher initial survivorship of plantings because wetland transplants are often limited by inundation (Howard and Rafferty 2006; Konisky and Burdick 2004; Lenssen et al. 1999, 1998); and (4) scale insects would be more abundant on the Delta and Gulf than EU lineages, potentially limiting restoration using these former lineages (Cronin et al. 2020; Knight et al. 2020). Based on our findings, we discuss recommendations for restoration of dieback sites in the MRD.
Methods
Study Area
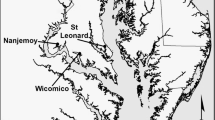
The study was conducted in the Lower MRD, Plaquemines Parish, Louisiana, USA along two of three main channels stemming from the Head of Passes: Pass-a-Loutre Wildlife Management Area (WMA) and South Pass Campground. Dieback of P. australis has been widespread within these areas since 2016 (Suir et al. 2018, 2022). Pass-a-Loutre WMA and South Pass are composed of coastal freshwater marshes (average salinity < 1 ppt) and river channels and accompanying banks, natural bayous and manmade canals with surrounding beach and barrier island habitats. We chose seven sites for our plantings, five within Pass-a-Loutre and two within South Pass (Fig. 1 and Table 1). All sites were coastal freshwater marsh habitats with mean salinity < 1 ppt for each site (range: 0.15 – 3.0 ppt; using a YSI Professional Plus multimeter [YSI Inc., Yellow Springs, OH, USA]). We purposely chose sites that reflected the range of water depths observed where P. australis grows in these two passes. Water depth, determined using a meter stick and recorded at each survey period during the first two years of the study (Table 2), ranged from 0 cm (exposed mudflat) to 85 cm on average among sites. All seven sites had a previous history of P. australis dieback (within the past 1–2 years since trials were initiated) and were unvegetated at the start of the study; either as open water or bare mudflat.
Sources and propagation of plants
In June of 2018, approximately four months prior to transplantation, plant material was acquired from various sources, propagated and maintained in an outdoor plot at a private nursery by staff at Stream Wetland Services, L.L.C. (Lake Charles, Louisiana; https://streamwetlands.com/). For the EU and Delta lineages of P. australis, rhizome material was excavated from healthy stands along the north bank of Pass-A-Loutre WMA. Because of lower survivorship of Delta during propagation, a second collection of Delta was made one month later from Rockefeller Refuge in southwestern Louisiana. Gulf rhizomes were also collected from Rockefeller Refuge. For S. californicus, (Louisiana ecotype) and Zizaniopsis miliaea (Louisiana ecotype), Stream Wetland Services sourced the plant materials through their nursery. Lastly, S. patens (Gulf Coast ecotype) was obtained from the USDA Natural Resources Conservation Services, Golden Meadow Plant Materials Center (Golden Meadow, Louisiana) as 5 cm diameter plugs. Coordinates for the location of source populations is provided in Table S1. Rhizomes of all plant species were grown outdoors for approximately four months in peat-based garden soil in trade-gallon pots (2.8 l).
Plants for use in transplantation had to meet specific growth criteria: a minimum number of live and actively growing stems with a minimum stem height (see Table S1). For the three lineages of P. australis, the criteria were the same. For the other plant species, owing to their different growth forms, different heights and numbers of live stems were required (Table S1). Within ten days prior to planting at the seven sites, stems were pruned to a maximum height of 122 cm to facilitate transportation and planting.
Experimental setup
On October 18–19, 2018, four months after initial planting, personnel from Stream Wetland Services transported the potted plants and plugs to our sites in Pass-a-Loutre and South Pass. At each of the seven sites, we created separate plots for each species and P. australis lineage (Figure S1). Plots were separated by 4.6 m and aligned in parallel to each other. Plantings within a plot were laid out in a gridded pattern consisting of four columns and 13 rows (only two plantings in row 13) and a total of 50 plantings (Fig. 2, Figure S1). The first row was oriented closest to the interior marsh side and the 13th row was closest to the channel, bayou, or open water. The overall area of each plot was 83.6 m2 (18.3 m × 4.6 m). At all seven sites, plots for each of the three P. australis lineages were established. Owing to the size of some dieback sites and limited plant material, the other three plant species were not represented at every site (see Table S2). For S. californicus, we had sufficient material and space to establish two plots at Chenier Crevasse and Willow Tree Site. Finally, although it was not the ideal situation, for logistical reasons, the arrangement of species/lineage plots was the same at every site (e.g., EU P. australis was always planted in the middle). Overall, a total of 400 plants per P. australis lineage, 400 of S. californicus, and 100 each of S. patens and Z. miliacea were transplanted for the restoration field trials for a grand total of 1,800 plants (Table S2).
Field surveys
We conducted field surveys of the plants within each restoration plot and within the nearest-naturally occurring P. australis patch (hereafter within-plot and nearest-patch) at periodic intervals over a 32-month period. The first survey was conducted approximately two months post-planting on 17 December 2018. Subsequent surveys were conducted on 9 April 2019 (6-months), 29–30 July 2019 (9-months), 23 September 2019 (11 months), 15–16 June 2020 (20-months), 10–11 August 2020 (22 months), and 2 June 2021 (32 months). Data collection differed among sites and is described below (see also Table 2).
Plant Survival (2, 6 and 9 months)
At each site and for each plant species and lineage, we recorded whether each planting was alive or dead. Plants were marked as “alive” if green plant tissue was visible. Plant survival was also converted to a proportion alive (total # alive/total planted). At two edges and center of each plot per site, we measured water depth and salinity (ppt; using a YSI Professional Plus multimeter). Finally, aerial photographs using a drone (DJI Phantom 3 Professional with a built-in 4 K-color camera; DJI Corporation, Shenzhen, Guangdong, China) were taken at each site from a height of 15 m above ground level (for example, see Fig. 2).
P. australis scale density and plant condition (11 months)
This survey was conducted in September (2019), a time period when scale insects are usually near peak abundance (Knight et al. 2020). During this survey, we focused only on the P. australis plantings and the factors that influenced scale insect density (number per m of stem) per planting. At each site and for each P. australis lineage, we randomly selected five plantings (using the RAND function in Microsoft Excel 365; Microsoft Corp, Redmund, Washington, USA). For each planting, we inserted a 0.25 m2 ring over its center and counted the number of live stems within that ring. We converted this count to the number of stems per m2. Water depth was measured at the perimeter of each growing planting. Three live stems were haphazardly selected, clipped at the soil surface and transported to the laboratory. In the laboratory, stem heights were measured, number of live scales per stem counted and the mean number of scales per meter of stem (scale density) for each planting was estimated.
Using similar methods (see also Knight et al. 2020), we also estimated scale density from the nearest natural P. australis stand (in all cases, the Delta lineage). Live stem density was measured from five randomly chosen locations within the stand (number of live stems per 0.25 m2 circular quadrat). Scale incidence (presence/absence) per stem was assessed from visually inspecting 30 stems. Ten scale-infested stems were then clipped at the soil surface and returned to the laboratory where we measured stem heights and live scales per stem. The mean number of scale per meter of stem was computed as the number of live scale per stem stem height in m × proportion of stems infested with scales.
Plant Area Coverage (20–22 and 32 months)
By twenty months after the start of this restoration trial, many plantings had died, and others coalesced making it impossible to accurately assess survival of individual plantings. For the remainder of the survey dates, we measured area of coverage for plant species/lineages that were still present. We used the DJI Phantom 3 drone and the Drone Deploy mission planning software (Drone Deploy, San Francisco, CA, USA) to create linear transects from 15 m above ground level with 80% frontal and 75% lateral overlap to ensure optimal speed, direction, and alignment of the images. The aerial images were processed by Pix4D Cloud (Pix4D, Lausanne, Switzerland) to generate 2D orthomosiac and 3D maps. Maps were imported into ArcGIS Pro 3.0 (ESRI 2022). An outline of the perimeter of each growing planting was traced in ArcGIS Pro, and the area computed. Total area per plant species or lineage was computed as the sum for all plantings for that species or lineage. Weather conditions prevented us from obtaining drone images from all sites during the 20-month survey. The remaining sites were surveyed two months later. We refer to this as the 20–22-month survey.
Data Analysis
Plant Survival
Plant survival was measured at 2, 6 and 9 months. Although these are repeated measures on the same plants, and therefore non independent, we opted to treat each date as a separate analysis so that survival differences within a time period could be more easily understood. At each date, we conducted a plant-level analysis that focused on whether species or lineage type influenced each planting’s status: alive (= 1) or dead (= 0). We used a generalized linear mixed model (GLMM) with plant status (live, dead) as our response variable and species/lineage as a fixed factor. To account for nonindependence within sites and for differences among sites (e.g., water depth), site was included in the model as a random effect. Although there is the potential for microhabitat differences to exist between plots (the grouping of plants from the same species or lineage into 4 columns × 6–13 rows; see Figure S1), we could not account for this in our GLMM because each plot represents a species/lineage. Consequently, including plot as a random effect to account for spatial effects was not possible. However, we note here that the boundaries for each site were chosen so that there was minimal microhabitat variation within the site. A separate analysis for each survey period was conducted using R version 4.2.2 (R Core Team 2022) and package glmer with family = binomial and a logit link function. Overdispersion was deemed not to be an issue using package blmeco and dispersion_glmer function. Differences in log-odds ratios between species/lineage pairs were evaluated with Tukey’s method and package emmeans. Goodness-of-fit was calculated using the Delta method in package MuMin which reports the marginal R2 (variance explained by the model’s fixed effects) and the conditional R2, (the combined variance explained by the entire model with fixed and random effects) (Nakagawa and Schielzeth 2013).
We conducted a second set of analyses at the plot level to assess the effects of species/lineage, mean water depth and the species/lineage × water depth interaction on the proportion of plantings surviving at each survey period. Proportion alive was approximately normally distributed and required no transformation. At the 2- and 6-month surveys, water depths were well distributed across a range from 0 to 85 cm and were treated as a continuous variable. However, at the 9-month survey, water depths were either low (0–15 cm) or high (51–65 cm) and so for this survey, we treated water depth as a categorical variable. Separate GLMM analyses were conducted for each survey, with site included as a random effect. Analyses were conducted in R using the lme4 package in R. Diagnostics for the GLMM were assessed using residual plots and quantile–quantile plots (for normality of residuals). The presence of outliers was assessed by examining studentized residuals. Finally, we used the aov function in R to generate traditional ANOVA tables from the GLMM analyses.
P. australis growth, water depth and scale density
We conducted separate GLMMs to determine whether two measures of P. australis growth, mean stem height and stem density (numbers/m2) were related to P. australis lineage, water depth, scale density (mean number of live scales per meter of stem) within each planting and all possible two-way interactions. No three-way interactions were considered. As in the analysis of plant survival, site was included in the model as a random effect. To normalize data distributions, scale densities and stem densities were ln transformed. We used Akaike’s information criteria corrected for finite sample size (AICc) to select the most informative model (Burnham and Anderson 2010). Candidate models were constructed using all possible combinations of predictor variables and two-way interactions. There were three restrictions to the possible combinations of variables. (1) The basic mixed-effects framework was retained in all models to account for the nonindependence within sites, (2) Interaction terms could only be present in the model if their main effects were also present in the model, and (3) only two-way interactions were considered. Candidate models were ranked by AICc from lowest to highest value and AICcs with a Δi value (= AICci—AICcmin) of ≤ 2 were considered to have substantial support (Burnham and Anderson 2010). AICc weights (wi) were reported which indicate the weight of evidence (as a proportion) in favor of model i being the best model given the set of candidate models. Model selection was performed in R using package AICcmodavg. We also computed the Pearson’s product moment correlation between our two response variables using package cor in R.
We note that in the analyses above, we cannot ascribe cause and effect to the relationship between scale density and plant growth metrics. Because we were also interested what influences scale abundance among plants, we conducted another analysis in which scale density was the response variable. P. australis lineage, mean stem height, stem density, water depth and all two-way interactions were included in the full model. Because scale density within plantings may be related to background scale densities, we also included in the model the mean scale density of the nearest neighboring P. australis stand (ln-transformed). Site was again included as a random effect and model selection was used to choose the model that best explained the data.
Plant area coverage at 20–22 and 32 months
By 20 months, area covered by each lineage or species was quite variable, with some sites having complete mortality of a lineage or species and others exhibiting strong evidence of clonal growth. For each site, we summed the areas of coverage (m2) for all surviving out-planted material for each lineage and species. Among all sites, the summed areas per lineage/species were neither normally distributed nor were the variances among lineages/species homogeneous (i.e., zeros were common, some lineages/species were absent from all site and others were limited to one or a couple of sites). Considering this issue, we simplified the analysis to reporting means and nonparametric 95% CIs for each lineage and species. Confidence intervals were computed via bootstrapping using the R package boot and boot.ci function with 1,000 bootstrapped estimates and basic bootstrap method. Means with non-overlapping 95% CIs were deemed significantly different.
Results
Site conditions and fates
Sites varied considerably in water depth but not salinity over the 32 months of this study. Four of the sites (Boundary Line Ponds 1 and 2, Cheniere Crevasse and Willow Tree Site) averaged 0.51 cm deep over the course of the study, while the other three sites averaged ca. 1/3 as much water (Fig. 3). Salinities registered within the margin of error of our YSI meter as pure freshwater at all survey dates (< 0.3 ppt). Table 2 shows the status of our plantings (fraction of sites with at least one live planting).
Mean water depth ± se among survey periods for each site. At each site, water depth was the average from three points taken at both edges and the middle of the site. The site mean ± se was determined from data collected at 2, 6, 9, 20 and 22 months. Site codes are Boundary Line Pond Site 1 (BLP1), Boundary Line Pond Site 2 (BLP2), Chenier Crevasse (CC), Chenier Pass Splay (CPS), Rattlesnake Flat (RF), South Pass Campground (SPC) and Willow Tree Site (WTS)
Plant survival at 2, 6 and 9 months
The transplantation of three lineages of P. australis and three additional wetland species was successful – among plots, 87.5 ± 3.1% (mean ± se, n = 31) of the plantings survived to two months (Fig. 4). The three lineages of P. australis had a mean survivorship of > 78%. At the individual plant scale, there were significant differences between species pairs in the odds of being alive at two months, but the lineage/species effect explained only 4.2% of the variance in the logistic-regression model (based on the marginal R2 value, R2m; Table S3A). Differences in survival of plantings became stronger with increasing time since the initiation of the study. At six months, based on logistic regression analysis (Table S3B), the Delta and Gulf lineages and S. californicus had significantly higher survival – twice the mean survival – of the EU lineage, Z. miliacea and S. patens (Fig. 4). At nine months, S. californicus had highest survival among all 332 species/lineages (67%), 2.6 times higher than all other lineages/species combined (Fig. 4, Table S3B). Within P. australis, Delta and Gulf had similar survival (35%); 1.9 times greater than for EU. By nine months, S. patens was absent from all plots. Overall, at two, six and nine months, the proportion of variation in the likelihood of being alive that was explained by species/lineage was 16%, 24% and 23%, respectively (Table S3A).
The proportion of plantings alive at two-, six- and nine-months following insertion into the Mississippi River Delta. Means ± se are reported. Within a survey period, different letters denote significant log-odds ratios for the pairwise comparison (Tukey’s method, P ≤ 0.05). There was no S. patens present in the 9 month survey
Through the first two survey periods, mean water depth (at the time of the survey) or its interaction with species/lineage had no significant effect on the proportion of plantings that were alive; whether we focused on all lineages and plant species combined or just the P. australis lineages (Table S4). However, at the nine-month survey, there was both a significant species/lineage and species/lineage × water depth interaction for all species/lineages combined (at this point, the only species other than P. australis that had a minimum of three sites with survivorship data was S. californicus) (Table S4A). Schoenoplectus californicus had a higher mean proportion surviving than all three P. australis lineages (Tukey’s pairwise comparison tests, P < 0.05; Fig. 5). Interestingly, the significant interaction term arises from the contrasting responses to water depth from S. californicus and the three P. australis lineages. Schoenoplectus californicus had higher proportional survival than all three P. australis lineages at high-water depth (Tukey’s tests, P ≤ 0.03 for all comparisons) but there were no differences in among species/lineages at low water depth. When only P. australis lineages were considered, survival was three times higher (marginal means ± SE: 0.48 ± 0.09 versus 0.16 ± 0.08) at low than high water level; (P = 0.051, Table S4B).
The relationship between water depth (cm) and the mean proportion of plantings alive at nine months ± SE. Water depth was converted to a categorical variable: Low (0–15 cm) and High (51–65 mm). Based on a GLMM analysis, there was a significant Species/Lineage effect (F3,14.245 = 6.77, P = 0.005) and Species/Lineage × Water depth interaction (F3,14.245 = 3.55, P = 0.042) (Table S4). Based on Tukey’s pairwise comparison tests, S. californicus at low water levels had significantly higher survival than all P. australis lineages at the same depth. No other significant differences were detected
P. australis growth and scale density at 11 months
Our P. australis growth measures, ln mean stem height and ln stem density per planting were significantly positively correlated (r = 0.41, n = 72, P < 0.001) but there was enough unexplained variance between these two metrics that we chose to treat them as separate measures of growth in our subsequent analyses. For stem density, the AICc-best model included only the intercept (Table S5). There was only one other supported model, which included a single fixed factor (scale density), and it had half as much support as the best model (based on AICc weights (Table S5). This model suggested that scale density increased with increasing stem density (t = 4.14, df = 71, P = 0.01, R2m = 0.18).
In comparison, the AICc-best model for P. australis mean stem height included lineage, water depth, scale density and lineage × scale density interaction. This model had an AICc weight of 0.86 and there were no other supported models among the candidate list (Table S5). The proportion of variation in mean stem height explained by the fixed effects in the model (R2m) was 0.58 (Table 3). The Delta lineage averaged 188.5 ± 7.8 cm tall, 44% taller on average than the EU or Gulf lineages (Fig. 6A). There was no evidence that scale density was negatively associated with stem height. In fact, taller EU and Gulf plants were associated with more scales, although the relationship was only significant for EU (t = 6.14, df = 20, P = 0.02, R2m = 0.56; Fig. 6A). The Delta lineage exhibited no relationship between scale density and stem height. Finally, stems were generally taller as water depth increased but the relationship was weak (r = 0.12, n = 72, P = 0.32; after accounting for all other factors in the GLMM).
At 11 months, the relationship between (A) scale density (scales per meter of stem), P. australis lineage and mean stem height and (B) stem density (stems per m2), P. australis lineage and scale density. In the left panels, lines are fit by least-squares regression using raw data. For both figures, density is plotted on a logarithmic (base 10) scale. Bar graphs in the right panels depict marginal means ± se, after controlling for all other factors in the AICc-best model (see Table 3). In (A), the AICc-best model included a scale density × lineage interaction, whereas in (B), it included a stem density × lineage interaction. In both cases, the slope of the relationship is significant only for the EU lineage
During the first summer following the initiation of the restoration trials, scale densities in nearest-neighbor P. australis stands (all Delta lineage) were high but variable, ranging from 162 per m of stem in Chenier Pass Splay to 802 per m of stem in Rattlesnake Flats (mean ± SE among sites: 453.3 ± 109.1, n = 7). In all but two sites, South Pass Campground and Willow Tree Site, our transplanted P. australis had evidence of scale insects on their stems. We excluded these two sites from the following analyses. The AICc-best model to explain scale density on transplanted P. australis was one that included P. australis lineage, ln stem density and the lineage × ln stem density interaction (Table 3, Table S5). Surprisingly, we could find no relationship between scale density in neighboring stands and scale density on transplants. This model had an AICc weight of 0.87 and was 29 times more likely than the next best model to explain the data. Fixed effects in the model explained 22% of the variation in scale density. The Delta lineage had the highest mean density of scale insects, averaging 4.2 times higher than scale densities on EU and Gulf (Fig. 6B). The lineages also differed in their responses to stem density of the plantings (lineage × ln stem density interaction). Based on separate GLMMs for each P. australis lineage, the only significant relationship was a positive one between EU stems/m2 and scale density (t = 2.54, df = 20.95, P = 0.019, R2m = 0.22).
Plant area cover
At 20–22 months, Chenier Crevasse had to be dropped from our surveys because it was no longer accessible by boat, owing to extremely low water levels. Based on our survey at this time, there were no surviving S. patens and Z. miliacea was present at only one of two sites (total area: 8.3 m2; Table 2). Schoenoplectus californicus was present in four of six sites and had the highest mean area of coverage per site (110.0 m2; Fig. 7). At Willow tree site, it existed in two plots (see Methods, Figure S1) with a total area of 355.7 m2. Among the P. australis lineages, Delta, EU and Gulf were present in four, one and three sites, respectively (Table 2). Area of coverage for Delta averaged 48.7 m2 and was 31.4 times greater than for EU and 9.7 times greater than for Gulf (Fig. 7). Based on bootstrapped 95% confidence intervals, only the areas of coverage for Delta and EU were significantly different.
Mean total area of coverage (m2) per site for each of the P. australis lineages (Delta, EU and Gulf) and S. californicus. (A) 20–22 months post planting (June – August, 2020) and (B) 32 months post planting (August, 2021). Means and 95% bootstrapped CIs are reported. The y-axis is on a logarithmic (base 10) scale. Stars represent the summed area covered for each lineage/species at each site. Those symbols at the bottoms of bars represent zero area of coverage. Other plant species were excluded because they had ≤ 2 sites with plantings that survived. There were no EU plants alive at any of the sites at the 32-month survey
Between 20–22 and 32 months, the last remaining planting of EU (Chenier Pass Splay) disappeared. Also, Gulf disappeared from one site (Chenier Pass Splay) and S. californicus disappeared from two sites (Chenier Crevasse, Rattlesnake Flat). Interestingly, at all remaining sites with S. californicus, area of coverage shrunk to an average of only 7.9 m2; a 93% reduction from 20–22 months (Fig. 7). Delta increased by 36% during that time and had the highest mean area of coverage at 66.3 m2; 6.1 times higher than Gulf (10.9 m2) and 8.4 times higher than S. californicus. Based on 95% CIs, Delta had significantly higher coverage than both Gulf and S. californicus (Fig. 7).
Discussion
Almost seven years since the first reports of widespread and pervasive dieback of Phragmites in the MRD (Bumby and Farrer 2022; Cronin et al. 2020; Knight et al. 2020, 2018; Suir et al. 2018, 2022), there has been relatively little sign of recovery of the previously impenetrable and tall stands of P. australis (unpublished data). Ours is the first study to investigate the potential for restoration of dieback areas in the MRD. Moreover, this study is one of just a few to consider restoration trials with different lineages or genotypes of P. australis (Howard et al. 2008; but see e.g., Kuehl and Zemlin 2000). Our main findings were that 1) P. australis outperformed the other three plant species in terms of survival and area of coverage at 32 months; 2) contrary to expectations, the EU lineage performed worst among the three P. australis lineages, persisting only in none of the seven sites within which it was planted; 3) in general, for all P. australis lineages, survival during the first year following planting was greatest in the shallow-water sites; and finally 4) although scale insects quickly colonized the planted P. australis, we found no evidence in the short term (11 months) that they hindered plant establishment. Encouragingly, replanting dieback sites with Delta (the original vegetation in these sites) had the most promise and, despite legacy effects of dieback, replanting could be successful within a year after the dieback event.
Plant species/lineage performance
Spartina patens is generally found in brackish habitats (Bertness 1991; Hester et al. 1996, 2001), whereas S. californicus and Z. miliacea occupy freshwater habitats and co-occur with P. australis in the area where our study was conducted (Chabreck 1970; Howard and Rafferty 2006; Li et al. 2018). At two months post planting, all species/lineages had high survivorship (mean of 88%), despite considerable variability in water depths. We attribute this to our selection process for transplantation – plantings were chosen that had a minimum height of 61 cm (Table S1) whereas water depths were, in most cases, below this level (see Fig. 3). However, by six months post-planting, S. patens disappeared from all sites (Table 2).
By nine months post-planting, survivorship of plantings from all species and sites was just 32%. One possible explanation for this generally low performance is legacy effects from the dieback. In studies of P. australis in Europe, eutrophic conditions, coupled with elevated sulfide concentrations and organic matter accumulation were evident following dieback events (Brix 1999; van der Putten 1997). Similar results were found in areas of S. alterniflora dieback in coastal Louisiana (Alber et al. 2008; Crawford and Stone 2015; Elmer et al. 2013) and for the recent P. australis dieback in the MRD (Lee et al. 2023). There is also the possibility that dieback was brought about by soil-borne pathogens that may persist in the soil for some time after a dieback event (Elmer et al. 2013). Specialist soil pathogens may inhibit ree-stablishment of pre-dieback vegetation (e.g., P. australis in the MRD), whereas generalist pathogens may prevent successful establishment of new plant species. Interestingly, lineages of P. australis have distinctly different microbial communities (Bowen et al. 2017; Bumby and Farrer 2022) such that dieback-associated pathogens could be lineage specific. At this point, we do not know how long the legacy effects persist, although Lee et al. (2023) found that the chemistry of dieback soils differed markedly from healthy (non-dieback) soils 1–2 years following a dieback event and that these differences translated into reduced growth rates for all three lineages of P. australis. Although there have been other attempts to restore marsh habitats following dieback events (Bakker et al. 2018; Kuehl and Zemlin 2000; Ogburn and Alber 2006; Ostendorp et al. 1995; Tang et al. 2006), research is needed to understand how soil legacies may affect restoration success and how long those legacy effects persist.
Another explanation for low plant survival was variation in water depth among restoration sites. We purposely selected sites that varied in water depth and by nine months post-planting, all three P. australis lineages had a proportion surviving that was three times higher in sites where water depth was low (≤ 15 cm) as compared to sites where water depth was high (≥ 51 cm) (Fig. 5). Schoenoplectus californicus survival was unaffected by water depth. By nine months, we did not have enough live Z. miliaea or S. patens to explore water depth effects on their survival. However, the much shorter stature of S. patens in comparison to the other species could explain why it did so poorly and died out in the sites within which it was planted. We conclude that our prediction three, that deeper-water sites would have lower restoration potential, appears to be upheld, at least for all three lineages of P. australis. Even though our focal plants thrive in wetland environments, biomass and survival can be negatively affected by prolonged waterlogging (Latham et al. 1994; Li et al. 2018; McKee et al. 1989; Mendelssohn and Mckee 1988; Sloey et al. 2015; Snedden et al. 2015; Weisner 1996). For example, P. australis is regarded as tolerant of high-water levels because of its ability to aerate flooded tissues by transporting oxygen through the aerenchyma, creating an extensive network of internal airspaces (Armstrong and Armstrong 1991; Eller et al. 2017; Jackson and Armstrong 1999). Yet, prolonged flooding and deeper waters are known to negatively affect P. australis (Cronin et al. 2020; Hellings and Gallagher 1992). Consequently, P. australis usually first establishes upslope and then, through rhizomatous spread, expands into deeper water (Chambers et al. 2003; Kuehl and Zemlin 2000).
Although we expected S. patens to be eventually outcompeted by other freshwater species, particularly P. australis (Bertness 1991; Silliman and Bertness 2004; Yuan et al. 2013), it disappeared well before other potential competing plant species expanded into its grid plots. Consequently, other unknown abiotic or biotic factors (including water depth) were likely involved in the disappearance of S. patens. Similarly, Z. miliacea was gone by 20 months from both sites in which it was planted. This species has been shown to do best in shallow water (Latham et al. 1994; Li et al. 2018) which may have limited its success in these sites.
Our results supported our first prediction that P. australis would have higher survival and greater clonal growth than the other species used in this study. However, this conclusion was only true for the Delta plantings which, by the end of the study, were still present in four of six sites. Phragmites australis, as with the other plant species used in this study, spread through the production of rhizomes. Compared to other wetland plant species, P. australis has a very large root/rhizome system that occupies a greater soil volume (Lissner and Schierup 1997a) which has several advantages: greater ability to compete for belowground nutrients (Burdick and Konisky 2003), the ability to penetrate into deep, permeable soil layers that contain lower salinity water (Burdick et al. 2001) and resists wave action and storm surges (Coleman et al. 2022; Coops and van der Velde 1996).
In comparison to Delta, plantings of S. californicus survived in only two of six sites and the area of coverage of this species averaged 8.4 times less than that of Delta. At first glance, our findings are at odds with the study by Howard et al. (2020) which found similarly high survivorship of S. californicus and P. australis in a restored marsh in Louisiana. However, there are two main differences between our studies that make comparisons unwarranted. First, P. australis in the Howard et al. (2020) study was determined to be primarily of European origin but the genetic methods used (see Saltonstall 2002) were not capable of distinguishing between Delta and EU. Also, up to 1/3 of the plantings in Howard et al. (2020) reportedly could have been the Gulf lineage. As our current study and past work on P. australis clearly demonstrate, lineages of P. australis are functionally very different (Cronin et al. 2020; Knight et al. 2020). Second, high rates of survival among species in Howard et al. (2020) could be due to the fact that fencing was erected around plantings to prevent herbivory from nutria (Myocastor coypus), an important and common non-native inhabitant of Louisiana coastal marshes (Sasser et al. 2018). Nutria are abundant in the MRD and we cannot rule out that they played a role in damaging and killing our experimental plants.
Surprisingly, we must reject our second prediction, that the EU lineage would outperform the other P. australis lineages in our restoration trials. In fact, EU performed worst among the three lineages; surviving to 32 months in none of six sites in comparison to four of six and two of six for Delta and Gulf, respectively (Table 2). The one site in which EU persisted to 20–22 months, Cheniere Pass Splay, was one of our shallowest sites (Table 1) that receives fresh water and sediments from an adjacent channel. Regarding area of coverage, the differences among lineages were more apparent. Numerous studies have reported that the EU lineage – the cryptic and highly invasive haplotype M (Saltonstall 2002) – exhibits higher rates of clonal growth (i.e., rhizome spread, tiller production, above and belowground biomass production), greater plasticity in response to different environmental conditions, and more efficient nutrient assimilation than the North American native lineage of P. australis (Bhattarai et al. 2017; Eller et al. 2017; Holdredge et al. 2010; League et al. 2006; Price et al. 2014; Vasquez et al. 2005). Comparisons between EU and other P. australis lineages are less common (but see Allen 2016; Cronin et al. 2020; Eller et al. 2014; Knight et al. 2020). Most notably, we previously found that in both field surveys and common-garden experiments, the EU lineage was more resistant to scale insects (i.e., supported lower densities) and appeared to be impervious to the dieback syndrome exhibited by co-occurring Delta (Cronin et al. 2020; Knight et al. 2020, 2018). One possible reason why Delta performed better than EU is that Delta is more tolerant of inundation (a water depth of 40 cm) than EU or Gulf (Cronin et al. 2020). In that study, Gulf was least tolerant of inundation and this lineage is generally found at higher elevations, usually on roadsides and river embankments on the margins of the MRD (Cronin et al. 2020). Consequently, we were surprised that it did better than EU in our restoration trials.
Relationship between scale density and plant performance
The roseau cane scale, N. biwakoensis, is an important pest of P. australis in its native habitat in eastern Asia (Brix et al. 2014; Kaneko 2004; Schneider et al. 2022) and has been implicated in the dieback of P. australis in the MRD (Cronin et al. 2020; Knight et al. 2020). Even at modest densities (20 adult females per stem), the scale can depress P. australis growth (Cronin et al. 2020) and therefore can impose a constraint on restoration success. The potential for herbivores to thwart restoration efforts has been reported for P. australis (Bakker et al. 2018; Temmink et al. 2022) and other wetland systems (e.g., Llewellyn and Shaffer 1993; Qian et al. 2021; Wasson et al. 2021).
In this study, we found that the scale insects can quickly colonize P. australis plantings in less than a year, spreading up to 40 m to our most isolated plantings. Active dispersal by this species occurs via first-instar crawlers; which probably limits its movement to a meter or less. However, passive dispersal by winds, water currents and avian vectors is possible and is probably responsible for most of the colonization of our experimental plantings. As has been reported among naturally occurring stands of P. australis in the MRD (Bumby and Farrer 2022; Knight et al. 2020), plantings of the Delta lineage had more than four times the density of scale insects as the other two lineages. Interestingly, scale density was positively related to stem density for the EU lineage. With passive dispersal, it would make sense that more dense patches would “capture” more colonists (Hambäck and Englund 2005). Alternatively, colonists may simply have higher growth rates on more vigorously growing patches of plants (plant vigor hypothesis; Price 1991). For the other two lineages, plant density was unrelated to scale density. We cannot easily explain this result, although a temporal separation between when scale colonization occurred and when we measured stem density at 11 months could have resulted in a disconnect or even reversal in the relationship between scale abundance and stem density. Unfortunately, the dispersal and colonization behavior of N. biwakoensis, as with most scale insects, is poorly understood (but see e.g., Gullan and Kosztarab 1997; Magsig-Castillo et al. 2010).
We have no evidence to suggest that N. biwakoensis negatively impacted survivorship of our plantings of P. australis. Neither stem density nor mean stem heights of the plantings exhibited a negative relationship with scale density. In fact, we found a positive association between stem height and scale density for the EU lineage. Again, this finding could be due to taller plants being better traps for passive dispersers than shorter plants or that scales do better on vigorously growing stems. However, at 11 months, it may have been too early to expect to find measurable negative effects of scales on planting growth. We did not measure scale insect density on subsequent survey dates because of the rapidly declining survivorship of EU and Gulf. However, given that the Delta lineage has greatest susceptibility to scale attack (Cronin et al. 2020; Knight et al. 2020), and was most prevalent at the end of the study, it seems unlikely that the scales played a determining role in which lineage was best in these restoration trials. It remains to be seen if the scales eventually cause dieback of our surviving Delta plantings.
Implications for Restoration
The fate of the Lower MRD depends on the stabilization of soil elevation by emergent vegetation which is under threat by eutrophication, salinity intrusion, elevated atmospheric CO2 concentrations, increasing intensity and frequency of hurricanes and storms and flooding events (Cahoon et al. 2011; Turner 1990). The Mississippi River Delta is of particular concern given its high rates of sea level rise (8.5–9.5 mm year−1), high land subsidence rates that can exceed 1 cm/yr−1 and coastal wetland degradation (Day et al. 1993; González and Tornqvist 2006; Shea and Karen 1990). As such, it is no surprise that the ongoing P. australis dieback in the Lower MRD is attributed to complex interactions of multiple abiotic and biotic stressors, making identification of the causal agent(s) difficult (Cronin et al. 2020; Knight et al. 2020). While natural recolonization following dieback may occur, the rate of growth may be insufficient to revegetate vast regions of the MRD before soil erosion makes that impossible. Moreover, some areas of dieback, have been colonized by less desirable invasive and shallow-rooting or floating aquatic plants; e.g., water hyacinth (Pontederia crassipes (Mart.) Solms or elephant ear (Colocasia esculenta (L.) Schott) (Cronin et al. 2020).
Restoration using vegetation plantings is often designed to promote vertical accretion for the maintenance or building of marsh elevation (Bricker-Urso et al. 1989; Chmura and Hung 2004; Hatton et al. 1983; Nyman et al. 1993). Vertical accretion via plant growth involves mechanisms that promote the accumulation of organic matter, expansion of belowground root structures and sediment trapping via surface litter (Craft et al. 1993; Nyman et al. 2006; Wolaver et al. 1988). Phragmites australis is adept at accreting sediments (Kiviat 2013; Rooth and Stevenson 2000; Rooth et al. 2003). Additionally, the typically dense clonal stands of P. australis are connected through their extensive belowground rhizome network which facilitate nutrient acquisition and can mitigate against some, but obviously not all, potential biological and physiological stress (Lissner and Schierup 1997b).
Although much is known about the ability of the EU lineage of P. australis to invade and spread under a wide variety of marsh conditions (Chambers et al. 1999; Clevering 1999; Saltonstall 2002), relatively little is known about the Delta lineage whose distribution is limited to the MRD and surrounding areas (Achenbach and Brix 2014; Cronin et al. 2020; but see e.g., Hauber et al. 2011). Our study clearly suggests that the Delta lineage would be the best choice for revegetating areas of dieback. Revegetation can begin shortly after the dieback event, despite some modest negative legacy effects mediated through changes in soil chemistry (Lee et al. 2023). Planting would need to take place in relatively shallow water, preferably under 0.5 m in depth. Revegetation of deeper-water areas will likely have to take place through growth and expansion of the planted material from shallow- to deeper-water areas (e.g., down a channel embankment).
Data availability
Data and drone images are available upon reasonable request from the corresponding author.
Code availability
Not applicable.
References
Achenbach L, Brix H (2014) Can differences in salinity tolerance explain the distribution of four genetically distinct lineages of Phragmitesaustralis in the Mississippi River Delta? Hydrobiologia 737:5–23
Alber M, Swenson EM, Adamowicz SC et al (2008) Salt marsh dieback: An overview of recent events in the US. Estuarine Coastal and Shelf Science 80:1–11
Allen WJ (2016) Biological invasions: Biogeography and multitrophic interactions. Louisiana State University, Baton Rouge, Louisiana, USA
Allison MA, Meselhe EA (2010) The use of large water and sediment diversions in the lower Mississippi River (Louisiana) for coastal restoration. Journal of Hydrolog 387:346–360
Allison MA, Demas CR, Ebersole BA et al (2012) A water and sediment budget for the lower Mississippi-Atchafalaya River in flood years 2008–2010: Implications for sediment discharge to the oceans and coastal restoration in Louisiana. Journal of Hydrolog 432–433:84–97
Armstrong J, Armstrong W (1991) A convective through-flow of gases in Phragmitesaustralis (Cav.) Trin. ex Steud. Aquatic Botany 39:75–88
Armstrong J, Armstrong W, van der Putten WH (1996) Phragmites die–back: bud and root death, blockages within the aeration and vascular systems and the possible role of phytotoxins. New Phytologist 133:399–414
Bakker ES, Veen CGF, Ter Heerdt GJN, et al. (2018) High grazing pressure of geese threatens conservation and restoration of reed belts. Frontiers in Plant Science 9. https://doi.org/10.3389/fpls.2018.01649
Barras J, Beville S, Britsch D et al (2004) Historical and projected coastal Louisiana land changes: 1978–2050. U.S. Geological Survey, Washington D. C.
Bertness MD (1991) Zonation of Spartina patens and Spartinaalterniflora in a New England salt marsh. Ecology 72:138–148
Bhattarai GP, Meyerson LA, Anderson J et al (2017) Biogeography of a plant invasion: genetic variation and plasticity in latitudinal clines for traits related to herbivory. Ecological Monographs 87:57–75
Bowen JL, Kearns PJ, Byrnes JEK et al (2017) Lineage overwhelms environmental conditions in determining rhizosphere bacterial community structure in a cosmopolitan invasive plant. Nature Communications 8:433
Breen CM, Hill BJ (1969) A mass mortality of mangroves in the Kosi Estuary. Transactions of the Royal Society of South Africa 38:285–303
Bricker-Urso S, Nixon SW, Cochran JK et al (1989) Accretion rates and sediment accumulation in Rhode Island salt marshes. Estuaries 12:300–317
Brix H (1999) The European research project on reed die-back and progression (EUREED). Limnologica 29:5–10
Brix H, Ye S, Laws EA et al (2014) Large-scale management of common reed, Phragmitesaustralis, for paper production: A case study from the Liaohe Delta, China. Ecological Engineering 73:760–769
Bumby C, Farrer EC (2022) Nipponaclerdabiwakoensis infestation of Phragmitesaustralis in the Mississippi River Delta, USA: Do fungal microbiomes play a role? Wetlands 42:15
Burdick D, Konisky RA (2003) Determinants of expansion for Phragmitesaustralis, common reed, in natural and impacted coastal marshes. Estuaries 26:407–416
Burdick DM, Buchsbaum R, Holt E (2001) Variation in soil salinity associated with expansion of Phragmites australis in salt marshes. Environmental and Experimental Botany 46:247–261
Burnham KP, Anderson DR (2010) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer-Verlag, New York, New York
Cahoon DR, White DA, Lynch JC (2011) Sediment infilling and wetland formation dynamics in an active crevasse splay of the Mississippi River delta. Geomorphology 131:57–68
Chabreck RH (1970) Marsh zones and vegetative types in the louisiana coastal marshes PhD Dissertation. Department of Botany. Louisiana State University, Baton Rouge, Louisiana, USA
Chambers RM, Meyerson LA, Saltonstall K (1999) Expansion of Phragmitesaustralis into tidal wetlands of North America. Aquatic Botany 64:261–273
Chambers RM, Osgood DT, Bart DJ et al (2003) Phragmitesaustralis invasion and expansion in tidal wetlands: Interactions among salinity, sulfide, and hydrology. Estuaries 26:398–406
Chmura GL, Hung GA (2004) Controls on salt marsh accretion: A test in salt marshes of Eastern Canada. Estuaries 27:70–81
Clevering OA (1999) The effects of litter on growth and plasticity of Phragmitesaustralis clones originating from infertile, fertile or eutrophicated habitats. Aquatic Botany 64:35–50
Coleman DJ, Cassalho F, Miesse TW et al (2022) The role of invasive Phragmites australis in wave attenuation in the Eastern United States. Estuaries and Coasts 46(2):404–416. https://doi.org/10.1007/s12237-022-01138-x
Colin R, Eguiarte LE (2016) Phylogeographic analyses and genetic structure illustrate the complex evolutionary history of Phragmitesaustralis in Mexico. American Journal of Botany 103:876–887
Coops H, van der Velde G (1996) Effects of waves on helophyte stands: mechanical characteristics of stems of Phragmitesaustralis and Scirpuslacustris. Aquatic Botany 53:175–185
Craft CB, Seneca ED, Broome SW (1993) Vertical accretion in microtidal regularly and irregularly flooded estuarine marshes. Estuarine, Coastal and Shelf Science 37:371–386
Crawford JT, Stone AG (2015) Relationships between soil composition and Spartinaalterniflora dieback in an Atlantic salt marsh. Wetlands 35:13–20
Cronin JT, Johnston J, Diaz R (2020) Multiple potential stressors and dieback of Phragmitesaustralis in the Mississippi River Delta, USA: implications for restoration. Wetlands 40:2247–2261
Day JW, Conner WH, Costanza R et al (1993) Impacts of sea level rise on coastal systems with special emphasis on the Mississippi River deltaic plain. In: Warrick RA, Barrow EM, Wigley TML (eds) Climate and sea-level change: Observations, projections and implications. Cambridge University Press, New York, New York, USA
Day JW, Martin JF, Cardoch L et al (1997) System functioning as a basis for sustainable management of deltaic ecosystems. Coastal Management 25:115–153
Day JW, Britsch LD, Hawes SR et al (2000) Pattern and process of land loss in the Mississippi Delta: A Spatial and temporal analysis of wetland habitat change. Estuaries 23:425–438
Day JW, Ko J-Y, Rybczyk J et al (2004) The use of wetlands in the Mississippi Delta for wastewater assimilation: a review. Ocean & Coastal Management 47:671–691
Duke NC, Kovacs JM, Griffiths AD et al (2017) Large-scale dieback of mangroves in Australia’s Gulf of Carpentaria: a severe ecosystem response, coincidental with an unusually extreme weather event. Marine and Freshwater Research 68:1816–1829
Edwards KR, Travis SE, Proffitt CE (2005) Genetic effects of a large-scale Spartinaalterniflora (smooth cordgrass) dieback and recovery in the northern Gulf of Mexico. Estuaries 28:204–214
Eller F, Lambertini C, Nguyen LX et al (2014) Increased invasive potential of non-native Phragmitesaustralis: elevated CO2 and temperature alleviate salinity effects on photosynthesis and growth. Global Change Biology 20:531–543
Eller FJ, Sorrell BK, Lambertini C et al (2017) The cosmopolitan model species Phragmitesaustralis: Ecophysiology and responses to global change. Frontiers in Plant Science. https://doi.org/10.3389/fpls.2017.01833
Elmer WH, Useman S, Schneider RW et al (2013) Sudden Vegetation dieback in Atlantic and Gulf Coast salt marshes. Plant Disease 97:436–445
ESRI (2022) ArcGIS Pro: Release 3.0. Environmental Systems Research Institute, Redlands, CA
González JL, Tornqvist TE (2006) Coastal Louisiana in crisis: Subsidence or sea level rise? Eos, Transactions American Geophysical Union 87:493–498
Gullan PJ, Kosztarab M (1997) Adaptations in scale insects. Annual Review of Entomology 42:23–50
Hambäck PA, Englund G (2005) Patch area, population density and the scaling of migration rates: the resource concentration hypothesis revisited. Ecology Letters 8:1057–1065
Hatton RS, DeLaune RD, Patrick WH Jr (1983) Sedimentation, accretion, and subsidence in marshes of Barataria Basin, Louisiana. Limnology and Oceanography 28:494–502
Hauber DP, Saltonstall K, White DA et al (2011) Genetic variation in the common reed, Phragmitesaustralis, in the Mississippi River Delta marshes: evidence for multiple introductions. Estuaries and Coasts 34:851–862
He Q, Silliman BR, Liu Z et al (2017) Natural enemies govern ecosystem resilience in the face of extreme droughts. Ecology Letters 20:194–201
Hellings SE, Gallagher JL (1992) The effects of salinity and flooding on Phragmitesaustralis. Journal of Applied Ecology 29:41–49
Hester MW, Mendelssohn IA, Mckee KL (1996) Intraspecific variation in salt tolerance and morphology in the coastal grass Spartina patens (Poaceae). American Journal of Botany 83:1521–1527
Hester MW, Mendelssohn IA, Mckee KL (2001) Species and population variation to salinity stress in Panicumhemitomon, Spartina patens, and Spartinaalterniflora: Morphological and physiological constraints. Environmental and Experimental Botany 46:277–297
Holdredge C, Bertness MD, von Wettberg E et al (2010) Nutrient enrichment enhances hidden differences in phenotype to drive a cryptic plant invasion. Oikos 119:1776–1784
Howard RJ, Rafferty PS (2006) Clonal variation in response to salinity and flooding stress in four marsh macrophytes of the northern Gulf of Mexico. USA Environmental and Experimental Botany 56:301–313
Howard RJ, Travis SE, Sikes BA (2008) Rapid growth of a Eurasian haplotype of Phragmitesaustralis in a restored brackish marsh in Louisiana, USA. Biological Invasions 10:369–379
Howard RJ, Rafferty PS, Johnson DJ (2020) Plant community establishment in a coastal marsh restored using sediment additions. Wetlands 40:877–892
Jackson MB, Armstrong W (1999) Formation of aerenchyma and the processes of plant ventilation in relation to soil flooding and submergence. Plant Biology 1:274–287
Kaneko S (2004) Within-plant vertical distributions of the scale insect Nipponaclerdabiwakoensis and its five parasitoids that exhibit frequent successful multiparasitism on the common reed. Entomological Science 7:331–339
Kiviat E (2013) Ecosystem services of Phragmites in North America with emphasis on habitat functions. AoB Plants 5:plt008. https://doi.org/10.1093/aobpla/plt008
Knight IA, Wilson BE, Gill M et al (2018) Invasion of Nipponaclerdabiwakoensis (Hemiptera: Aclerdidae) and Phragmitesaustralis die-back in southern Louisiana, USA. Biological Invasions 20:2739–2744
Knight IA, Cronin JT, Gill M et al (2020) The role of plant phenotype, salinity, and infestation by the Roseau Cane Scale in the die-back of Phragmitesaustralis in the Mississippi River Delta, Louisiana, USA. Wetlands 40:1327–1337
Konisky RA, Burdick DM (2004) Effects of stressors on invasive and halophytic plants of New England salt marshes: a framework for predicting response to tidal restoration. Wetlands 24:434–447
Kuehl H, Zemlin R (2000) Increasing the efficiency of reed plantations on stressed lake and river shores by using special clones of Phragmites australis. Wetlands Ecology and Management. [print] 8:415–424
Lambertini C, Mendelssohn IA, Gustafsson MHG et al (2012) Tracing the origin of Gulf Coast Phragmites (Poaceae): a story of long-distance dispersal and hybridization. American Journal of Botany 99:538–551
Latham PJ, Pearlstine LG, Kitchens WM (1994) Species association changes across a gradient of freshwater, oligohaline, and mesohaline tidal marshes along the lower Savannah River. Wetlands 14:174–183
League MT, Colbert EP, Seliskar DM et al (2006) Rhizome growth dynamics of native and exotic haplotypes of Phragmitesaustralis (Common Reed). Estuaries and Coasts 29:269–276
Lee H, Diaz R, Cronin JT (2023) Dieback and dredge soils of Phragmites australis in the Mississippi River Delta negatively impact plant biomass. Scientific Reports In review 737:5
Lenssen JPM, Ten DGE, Blom CWPM (1998) The effect of flooding on the recruitment of reed marsh and tall forb plant species. Plant Ecol 139:13–23
Lenssen J, Menting F, van der Putten W et al (1999) Control of plant species richness and zonation of functional groups along a freshwater flooding gradient. Oikos 86:523–534
Li H, Shao JJ, Qiu SY et al (2013) Native Phragmites dieback reduced its dominance in the salt marshes invaded by exotic Spartina in the Yangtze River estuary, China. Ecological Engineering 57:236–241
Li S, Hopkinson CS, Schubauer-Berigan JP et al (2018) Climate drivers of Zizaniopsismiliacea biomass in a Georgia, U.S.A. tidal fresh marsh. Limnology and Oceanography 63:2266–2276
Lissner J, Schierup H-H (1997a) Effects of salinity on the growth of Phragmites australis. Aquatic Botany 55:247–260
Llewellyn DW, Shaffer GP (1993) Marsh restoration in the presence of intense herbivory: The role of Justicialanceolata (Chapm.) small. Wetlands 13:176–184
Lovelock CE, Feller IC, Reef R et al (2017) Mangrove dieback during fluctuating sea levels. Scientific Reports 7:1680
Magsig-Castillo J, Morse JG, Walker GP et al (2010) Phoretic dispersal of armored scale crawlers (Hemiptera: Diaspididae). Journal of Economic Entomology 103:1172–1179
McKee KL, Mendelssohn IA, Burdick DM (1989) Effect of long-term flooding on root metabolic response in five freshwater marsh plant species. Canadian Journal of Botany 67:3446–3452
Mendelssohn IA, Mckee KL (1988) Spartinaalterniflora dieback in Louisiana - time-course investigation of soil waterlogging effects. Journal of Ecology 76:509–521
Meyerson LA, Lambert AM, Saltonstall K (2010) A tale of three lineages: expansion of common reed (Phragmitesaustralis) in the U.S. southwest and Gulf Coast. Invasive Plant Science and Management 3:515–520
Milliman JD, Meade RH (1983) World-wide delivery of river sediment to the oceans. The Journal of Geology 91:1–21
Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecology and Evolution 4:133–142
Nyman JA, DeLaune RD, Roberts HH et al (1993) Relationship between vegetation and soil formation in a rapidly submerging coastal marsh. Marine Ecology Progress Series 96:269–279
Nyman JA, Walters RJ, Delaune RD et al (2006) Marsh vertical accretion via vegetative growth. Estuarine, Coastal and Shelf Science 69:370–380
Ogburn MB, Alber M (2006) An investigation of salt marsh dieback in Georgia using field transplants. Estuaries and Coasts 29:54–62
Ostendorp W, Iseli C, Krauss M et al (1995) Lake shore deterioration, reed management and bank restoration in some Central European lakes. Ecological Engineering 5:51–75
Price PW (1991) The plant vigor hypothesis and herbivore attack. Oikos 62:244–251
Price AL, Fant JB, Larkin DJ (2014) Ecology of native vs. Introduced Phragmitesaustralis (Common Reed) in Chicago-area wetlands. Wetlands 34:369–377
Qian W, Chen J, Zhang Q et al (2021) Top-down control of foundation species recovery during coastal wetland restoration. Science of the Total Environment 769:144854
Ramsey EW III, Rangoonwala A (2017) Mapping the change of Phragmites australis live biomass in the lower Mississippi River Delta marshes. Open-File Report, Reston, VA
Rolletschek H, Rolletschek A, Kuhl H et al (1999) Clone specific differences in a Phragmitesaustralis stand. II. Seasonal development of morphological and physiological characteristics at the natural site and after transplantation. Aquatic Botany 64:247–260
Rooth JE, Stevenson JC (2000) Sediment deposition patterns in Phragmitesaustralis communities: implications for coastal areas threatened by rising sea level. Wetlands Ecology and Management 8:173–183
Rooth JE, Stevenson JC, Cornwell JC (2003) Increased sediment accretion rates following invasion by Phragmitesaustralis: The role of litter. Estuaries 26:475–483
Rossi RE, Archer SK, Giri C et al (2020) The role of multiple stressors in a dwarf red mangrove (Rhizophora mangle) dieback. Estuarine, Coastal and Shelf Science 237:106660
Saltonstall K (2002) Cryptic invasion by a non-native genotype of the common reed, Phragmitesaustralis, into North America. Proceedings of the National Academy of Sciences of the United States of America 99:2445–2449
Sasser CE, Holm GO, Evers-Hebert E et al (2018) The Nutria in Louisiana: A current and historical perspective. In: Day JW, Erdman JA (eds) Mississippi Delta Restoration: Pathways to a sustainable future. Springer International Publishing, Cham, pp 39–60
Saucier RT (1994) Geomorphology and quaternary geologic history of the Lower Mississippi Valley, vol 1. U.S Army Corps of Engineers, Mississippi River Commission, Vicksburg, Mississippi
Schneider SA, Broadley HJ, Andersen JC et al (2022) An invasive population of Roseau Cane Scale in the Mississippi River Delta, USA originated from northeastern China. Biological Invasions 24:2735–2755
Shea P, Karen ER (1990) Relative sea-level rise in Louisiana and the Gulf of Mexico: 1908–1988. Journal of Coastal Research 6:323–342
Silliman BR, Bertness MD (2004) Shoreline development drives invasion of Phragmitesaustralis and the loss of plant diversity on New England salt marshes. Conservation Biology 18:1424–1434
Silliman BR, Schrack E, He Q et al (2015) Facilitation shifts paradigms and can amplify coastal restoration efforts. Proceedings of the National Academy of Sciences 112:14295–14300
Sloey TM, Willis JM, Hester MW (2015) Hydrologic and edaphic constraints on Schoenoplectusacutus, Schoenoplectuscalifornicus, and Typhalatifolia in tidal marsh restoration. Restoration Ecology 23:430–438
Snedden GA, Cretini K, Patton B (2015) Inundation and salinity impacts to above- and belowground productivity in Spartina patens and Spartinaalterniflora in the Mississippi River deltaic plain: Implications for using river diversions as restoration tools. Ecological Engineering 81:133–139
Suir GM, Saltus CL, Reif MK (2018) Geospatial assessments of Phragmites australis die-off in south Louisiana: Preliminary findings. APCR Program Ed. US Army Corps of Engineers, Baton Rouge LA
Suir GM, Saltus CL, Reif MK (2022) Remote sensing-based structural and functional assessments of Phragmitesaustralis diebacks in the Mississippi River Delta. Ecological Indicators 135:108549
Tang N, Cui B-s, Zhao X-S (2006) The restoration of reed (Phragmites australis) wetland in the Yellow River Delta. Act Ecologica Sinica 26:2616–2624
Team RC (2022) R: A language and environment for statistical computing. R Foundation for Statistical Computing, version 4.2.2, Vienna, Austria
Temmink RJM, van den Akker M, van Leeuwen CHA et al (2022) Herbivore exclusion and active planting stimulate reed marsh development on a newly constructed archipelago. Ecological Engineering 175:106474
Turner RE (1990) Landscape development and coastal wetland losses in the Northern Gulf of Mexico. American Zoologist 30:89–105
van der Putten W (1997) Die-back of Phragmitesaustralis in European wetlands: An overview of the European research programme on reed die-back and progression (1993–1994). Aquatic Botany 59:263–275
Vasquez EA, Glenn EP, Brown JJ et al (2005) Salt tolerance underlies the cryptic invasion of North American salt marshes by an introduced haplotype of the common reed Phragmitesaustralis (Poaceae). Marine Ecology Progress Series 298:1–8
Wasson K, Tanner KE, Woofolk A et al (2021) Top-down and sideways: Herbivory and cross-ecosystem connectivity shape restoration success at the salt marsh-upland ecotone. Plos One 16:e0247374
Webb EC, Mendelssohn IA (1996) Factors affecting vegetation dieback of an oligohaline marsh in coastal Louisiana: Field manipulation of salinity and submergence. American Journal of Botany 83:1429–1434
Weisner SEB (1996) Effects of an organic sediment on performance of young Phragmitesaustralis clones at different water depth treatments. Hydrobiologia 330:189–194
Wilsey BJ, McKee KL, Mendelssohn IA (1992) Effects of increased elevation and macro- and micronutrient additions on Spartinaalterniflora transplant success in salt-marsh dieback areas in Louisiana. Environmental Management 16:505–511
Wolaver TG, Dame RF, Spurrier J et al (1988) Sediment exchange between a euhaline salt marsh in South Carolina and the adjacent tidal creek. Journal of Coastal Research 4:17–26
Yuan Y, Wang K, Li D et al (2013) Interspecific interactions between Phragmitesaustralis and Spartinaalterniflora along a tidal gradient in the Dongtan Wetland, Eastern China. Plos One 8:e53843–e53843
Zhou C-F, Qin P, Xie M (2003) Vegetating coastal areas of east China: species selection, seedling cloning and transplantation. Ecological Engineering 20:275–286
Acknowledgements
We thank Todd Baker, Vaughan McDonald and Trebor Victoriano from the Louisiana Department of Wildlife and Fisheries for technical and logistical support. Cindy Steyer from the U.S.D.A National Resources Conservation Service was also instrumental in incorporating this research into the Coastwide Vegetation Planning Project. We also thank the following people for their assistance with field work and scale counts: Leslie Aviles, Matthew Berry, Joey Breaux, Wendy Gibson, Nate Harms, Ian Knight, Herie Lee, Giovana Matos, Tshililo Mphephu.
Funding
This project was funded by the LSU AgCenter, Louisiana Department of Wildlife and Fisheries, U.S.D.A. APHIS (award number LAB94095 to R.D, J.A.N. and J.T.C.) and the U.S.D.A (LA-39 CWVP Year 7A; Coastwide Vegetative Planting Project; https://lacoast.gov/new/Projects/Info.aspx?num=LA-39).
Author information
Authors and Affiliations
Contributions
JT Cronin, JA Nyman and R Diaz conceived of and designed this study. JT Cronin conducted the statistical analyses and H Lee and J Johnston conducted the drone-image analysis and developed the GIS for this study. IA Knight was instrumental in the setup of the experiment. Finally, JT Cronin and H Lee wrote the manuscript and all coauthors participated in the editing of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethical approval and consent to participate.
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, H., Diaz, R., Johnston, J. et al. Vegetation restoration following dieback of Phragmites australis in the Mississippi River Delta, USA. Wetlands 43, 98 (2023). https://doi.org/10.1007/s13157-023-01746-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13157-023-01746-8