Abstract
Urban developments in estuarine areas affect benthic organisms, notably the macrobenthos, by physically modifying the territory and generating waste. The polychaete assemblages and the environmental parameters in the estuarine area of Sungai Terengganu were examined based on nine stations along a salinity gradient from oligohaline to polyhaline. Samples were collected using a Smith McIntyre grab and yielded 1886 individuals, from 43 species grouped into 24 families. The fauna was mainly dominated by Composetia sp. (39.48%) and Cossura sp. (31.87%); the former in oligohaline areas and the latter in the polyhaline areas. The densities of both species had significant positive correlations with salinity, temperature, and silt, clay, and gravel contents. This study will benchmark the polychaete community distribution in this tropical estuary, which could be similar to environments along the east coast of Peninsular Malaysia (South China Sea).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The highly dynamic and fragile estuarine environment plays a vital role as one of the most productive ecosystems in the world due to the combined influence of tides, and seawater and freshwater mixing (Scanes et al. 2017), with the physical, chemical, and biological fluctuations giving rise to highly functional and fertile systems. Estuaries are governed by complex interactions of wind, irradiance, rainfall, water level, and freshwater runoff. Thus, the associated biome may change significantly over time and space (Montagna et al. 2018). Changes may occur along gradients in salinity, sediment characteristics, water turbidity, nutrient composition, dissolved gases, and trace metals, affecting the organisms' abundance, diversity, and productivity (Wolanski et al. 2012). Estuaries are not only ecotones, but also provide habitats for a wide range of organisms, such as benthic invertebrates, mangroves, seagrass, and migratory and coastal birds (Barletta et al. 2017). Some inhabitants are permanent, while others are temporary (e.g., come in and out with the tides and during specific life stages). This gives rise to a highly diversified ecosystem, with complex and interconnected trophic relationships supporting many top predators, including humans (NOAA 2021).
Estuaries are frequent in temperate and tropical climates, and Malaysia is no exception (Scanes et al. 2020). Its tropical coasts harbor lagoon, mangrove, and river estuaries, which differ in the degree of closure and the amount of circulating freshwater and marine inflows (Scanes et al. 2017). Particularly, river estuaries in Malaysia are continuously evolving to cater to the local community's demands. Riverbanks are converted into residential settlements, while the estuaries receive modern harbors, fish farming, recreation and tourism activities, and waste disposal facilities (Boerema and Meire 2017).
The Sungai Terengganu estuary (sungai = river), located on the east coast of Peninsular Malaysia, is a center of development in Kuala Terengganu, the capital of Terengganu. With a surface area of approximately 46,000 km2 and 65 km in length, its basin is made up of two main tributaries, the Sungai Terengganu and the Sungai Nerus (Lee et al. 2017), with the river mouth opening to the South China Sea. It is highly urbanized and has been extensively affected by urban development, marine fish landing, tourism, navigation channels, and other socio-economic activities (Suratman et al. 2018). Its socio-economic importance led the Terengganu state government to construct a semi-enclosed breakwater at the river mouth in 2008. With an opening of 200 m wide and a water depth of 8-10 m (Mohd-Salim et al. 2018), it shelters the Terengganu estuary from offshore waves, providing an environment quiet inner while deepening the area available for navigational purposes (Lee et al. 2017). These artificial barriers successfully reduced the wave energy impacting the coast, thus altering sedimentation rates, organic matter, and nutrient loadings, leading to drastic modifications of the associated benthic assemblages (Airoldi et al. 2005; Martin et al. 2005).
Nutrients carried by rivers contribute to high estuarine productivity (Fatin Adlina et al. 2019), which allows for maintaining a high abundance and diversity of organisms, including primary producers, such as salt marshes, grasses, algae, and phytoplankton. This attracts secondary consumers such as annelids, mollusks, arthropods, fishes, and birds to these areas (van Niekerk et al. 2020). Food is thus one of the main resources widely available in estuaries, with the smaller invertebrate species, including the soft-bodied polychaetes, being among the most appreciated prey for catadromous fish (Fontoura et al. 2019) and coastal birds (Rush et al. 2010).
Polychaetes are among the most abundant and diverse organisms in brackish and marine environments, particularly benthic communities (Martins and Barros 2022). These segmented, soft-bodied organisms are reported worldwide, with over 23,774 accepted extant species (Read and Fauchald 2022). Besides burrowing mainly in the mud and sand, they also inhabit under rocks, among coral crevices, rotten vegetation, algal holdfast, surf grassroots, and coconut husks (Díaz-Castañeda and Reish 2009; Glasby et al. 2021; Rouse et al. 2022). Polychaetes of body lengths from less than one millimeter and up to more than three meters (Al-Omari 2011; Górska et al. 2019) may act as bioturbators and ecosystem engineers while being an essential food web link between organisms with different feeding guilds (Campanyà-Llovet et al. 2017; Bergström et al. 2019; Kim et al. 2021). Moreover, they are typically considered as excellent bioindicators (Giangrande et al. 2005).
Most polychaete studies in Malaysia were conducted on the west coast of Peninsular Malaysia (Nakao et al. 1989a; Ahmad et al. 2011; Gholizadeh et al. 2012; Idris and Arshad 2013; Guan et al. 2014; Rosli et al. 2016), followed by the east coast (Nakao et al. 1989b; Ibrahim et al. 2017, 2019) with very few studies focusing on Sabah and Sarawak (Rosli et al. 2018). Considering the scarce information on polychaetes from the east coast of Peninsular Malaysia and their relationship with anthropogenic organic enrichment in estuarine environments, we are here describing the spatial distribution and diversity of their assemblages in relation to the parameters driving the environment in the Sungai Terengganu estuary.
Materials and Methods
Sampling
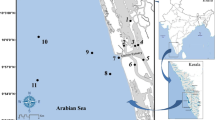
Sampling was carried out from June 2016 to July 2017 at nine stations (Fig. 1) from oligohaline (0.5–5.0 ppt) and polyhaline (18.0–30.0 ppt) zones (as defined by Montagna et al. 2013), along 7.14 km of the Sungai Terengganu estuary, at intervals of 0.30 to 1.70 km, and affected by different anthropogenic activities (Table 1).
Water temperature, pH, salinity, and dissolved oxygen (DO) were recorded in situ in triplicate readings using a Hydrolab Quanta Multiprobe. Three replicate sediment samples were collected at each station using a Smith McIntyre grab with a surface area of 0.05 m2. Approximately 150 g of sediments from each replicate were separated and kept in plastic containers for further analyses of grain size and total organic matter (TOM) in the laboratory. The remaining sediments were wet-sieved through a 500 μ mesh sieve, and the retained polychaetes were extracted and preserved following Rouse et al. (2022) and brought back to the laboratory for identification.
Laboratory Analyses
The identification of polychaetes to genus level was based on Glasby et al. (2000), Barrosa and Paiva (2007), Sendall and Salazar-Vallejo (2013), Zhadan (2015), and Jeong et al. (2017) and therefore has been used to avoid misidentifications during further monitoring (Masucci et al. 2021). All genera were checked for validity against the World Polychaeta Database (Read and Fauchald 2022). Specimens were stained using methylene blue solution to highlight the key identification character before observation using stereoscopic and compound light microscopes. The number of individuals per species was counted. The density was then expressed as individuals per square meter (ind/m2) and used to estimate the Shannon diversity index, H’ (Shannon 1948), and the Pielou’s evenness index, J’ (Pielou 1975) of the assemblages.
The 150 g sediments from the plastic containers were dried in an oven at 60 °C for 72 h following Bachok et al. (2006). Then, TOM was determined using 5 g of the oven-dried sediments from each replicate by loss-on-ignition method (Sultan and Shazili 2010). Particle size distribution was determined by both dry-sieve (particles ≥ 63 μm) and laser diffraction (particles < 63 μm) methods (Buller and McManus 1979; Bachok et al. 2006) and expressed as percentages of gravel, sand, silt, and clay, while the logarithmic method of moments was used to obtain the mean of sorting and skewness using GRADISTAT.
Data and Statistical Analyses
Water quality, sediment characteristics, polychaete density, diversity, and evenness were log10 (x + 1) transformed and analyzed by the Shapiro–Wilk test to check for normal distributions. Based on the non-normally distributed data (< 0.05), the mean comparisons of the abiotic parameters (water quality and sediment characteristics) and biotic parameters (polychaete’ density, diversity, and evenness) among the nine sampling stations were analyzed by the non-parametric Kruskal–Wallis H Test.
The structure of the polychaete assemblages was assessed by hierarchical cluster analysis (CA) based on square root-transformed densities. The resemblance matrix was based on Bray–Curtis similarity, while the similarity profile routine (SIMPROF) was applied to identify the most significant similarity percentage between stations.
The principal component analysis (PCA) was used to assess the relationship between the water quality parameters (temperature, pH, salinity, and DO) and sediment characteristics (percentages of gravel, sand, silt, clay, and TOM) based on square-root transformed data and a Euclidean distance.
The relationships between abiotic and biotic parameters were assessed by the Spearman correlation coefficient based on a 95% confidence limit (p = 0.05).
Mean comparison and Spearman correlation analyses were performed using the IBM Statistical Package for Social Science (SPSS) version 20 (Arbuckle 2012), while CA and PCA were conducted using Plymouth Routine in Multivariate Ecological Research (PRIMER) Version 6 (Clarke and Warwick 2001).
Results
Abiotic Parameters
The temperature ranged from 27.69 ℃ ± 1.05 ℃ (station 2) to 29.62 ℃ ± 0.81 ℃ (station 9), the pH from 6.21 ± 0.90 (station 3) to 7.22 ± 0.65 (station 8), the salinity from 1.25 ppt ± 5.05 ppt (station 2) to 30.62 ppt ± 3.19 ppt (station 9), and the DO from 4.16 mg/L ± 1.73 mg/L (station 9) to 4.98 mg/L ± 0.99 mg/L (station 4). Temperature, pH, and salinity significantly increased (p < 0.05) from oligohaline to polyhaline stations, while DO did not significantly differ (p > 0.05) among the stations (Fig. 2; supplementary material).
The sediments in stations 1–5 were dominated by sand (50.02% ± 29.03% to 70.37% ± 19.45%) and gravel (7.92% ± 6.98% to 37.78% ± 14.68%), while in stations 6–9, the sediments consisted of silt (66.84% ± 33.30% to 83.00% ± 5.52%) and clay (8.90% ± 4.23% to 13.11% ± 3.83%) (Fig. 3). TOM ranged between 1.77% ± 1.19% (station 3) and 15.01% ± 7.26% (station 9), being significantly lower (p < 0.05) in the oligohaline than in the polyhaline stations (supplementary material).
The sediments in all stations were poorly sorted, which indicates high variability of grain size. In addition, skewness measurement showed all stations had fine skewed distribution except for station 5, which had very fine skewed distribution (Table 2).
Biotic Parameters
A total of 1886 individuals of polychaetes from 24 families and 43 species have been recorded in the Sungai Terengganu estuary (Table 3). The density ranged from 666.67 ind/m2 ± 10.07 ind/m2 (station 1) to 2186.67 ind/m2 ± 12.74 ind/m2 (station 8) and was significantly lower (p < 0.05) in the oligohaline than in polyhaline stations (supplementary material).
The most dominant species were Composetia sp. (752; 39.87%) in stations 1–5 and Cossura sp. (595; 31.55%) in stations 6–9 (Table 3; Fig. 4). The most dominant families were Nereididae (42.21%), Cossuridae (31.87%) and Capitellidae (12.68%) (Fig. 5), followed by Poecilochaetidae, Sternaspidae, Pilargidae, Spionidae, and Paraonidae (3.2% to 1.16%), while Maldanidae, Onuphidae, and Paralacydoniidae were represented by less than 1.0%.
H’ ranged from 0.176 ± 0.034 (station 3; oligohaline) to 1.865 ± 0.190 (station 8; polyhaline) and J’ from 0.335 ± 0.036 (station 4; polyhaline) to 0.652 ± 0.055 (station 2; oligohaline) (Fig. 6). H’ was significantly higher and J’ significantly lower in the estuarine river mouth (polyhaline) than in the landward (oligohaline) stations (supplementary material).
Structure of the Assemblages
Two groups of stations were obtained at 33% of similarity in the CA (Fig. 7), including stations 1–4 (Group 1) and stations 5–9 (Group 2). Group 1 grouped stations homogeneously dominated by Composetia sp., whereas those at Cluster 2 were dominated by Cossura sp.
Relationships Between Abiotic Parameters and Polychaete Assemblages
The first two axes of the PCA accounted for 93.4% of the cumulative variation in abiotic parameters, with PC1 explaining the highest percentage (i.e., 81.8%) resulting from an approximately equal-weighted combination of all parameters, except DO (mg/L). Conversely, DO and salinity were the most influencing for PC2 (Table 4). Stations 1–3 and 4–5, grouped along PC1, were associated with gravel and sand, respectively, meanwhile, stations 6–9 were correlated with silt, clay, TOM, temperature, pH, and salinity (Fig. 8).
Density and H’ were positively correlated with temperature, pH, and TOM (Table 5), with the former being also positively correlated with salinity, and the latter being positively correlated with silt and clay and negatively with gravel (Table 5). No significant correlations were found between J’ and these abiotic parameters.
The high densities of Cossura sp. occurred in association with the highest temperature, salinity, and silt and clay contents, as well as with the lowest gravel contents (Fig. 9A), while Composetia sp. showed exactly an opposite trend (Fig. 9B).
Discussion
The polychaete assemblages found in our study differs from the previous study in Sungai Terengganu by Nakao et al. (1989b) in the absence of Ampharetidae, Phyllodocidae, and Oweniidae, which are now replaced by representatives of 24 different families, of which Nereididae, Cossuridae, Capitellidae, Poecilochaetidae, Sternaspidae, Pilargidae, Spionidae, and Paraonidae showed > 1% in abundance. The increasing taxonomic complexity is probably related to the higher sampling frequency and covered area, and also differences in sampling methods (Rosli et al. 2018). Our study included nine stations along the whole estuary, versus the five downstream ones in Nakao et al. (1989b). Additionally, the reference materials were restricted and the involvement of polychaete taxonomists were limited in 1989 which, together with the absence of voucher specimens, raises some doubts on the validity of the identification.
In general, the oligohaline area is dominated by Composetia sp. (except in stations 4–5), while Cossura sp. dominates the polyhaline area (stations 6–9), which can be explained by these stations having particular specific characteristics in terms of salinity, sediment texture, and organic matter content (Rehitha et al. 2019). The capability of an organism to be adapted to altered physical environments and the resulting abiotic interactions are directly related to its capacity to survive, with the persistence of the environmental changes having direct and indirect impacts on biota endurance. Composetia sp., for example, has broad tolerance to environmental changes, which allows it to be dominant in most studied stations. Its co-occurrence with Cossura sp. appears to be possible thanks to their differences in feeding habits (Checon et al. 2017), as Composetia sp. is an omnivore, and Cossura sp. is a surface deposit feeder (Jumars et al. 2015). So, they are exploiting different compartments allowing them to avoid competing for the same food sources.
The polyhaline area was able to support higher polychaete densities than the oligohaline, as found in the Eastern Brazil Marine Ecoregion, Brazil (Bissoli and Bernardino 2018) and the Chesapeake Bay, Virginia USA (Seitz et al. 2011). In addition to salinity, sediment characteristics also influence the polychaete density and distribution (Rosli et al. 2018). The highest number of species and density were associated with the highest silty-clay and TOM contents at stations 6–9 (i.e., within the area semi-enclosed by the breakwater), which coincided with the finding of finer sediment, possibly caused by the lowering in water energy (Masucci et al. 2020). In contrast, the lowest number of taxa occurred in station 3, with the highest percentage of gravel and the lowest of sand, silt, and TOM. Moreover, the granulometry (and, thus, the associated assemblages) at this station was certainly affected by the infrastructure construction and sand mining nearby. Sediment organic content tends to decrease with the increasing grain size (Evans et al. 1990), with coarser sediments (i.e., sand and gravel) having a loose structure, difficulting an efficient organic matter retention (Soto et al. 2017). Conversely, silty-clayish sediments show a higher absorptive capacity and greater surface area, which explains their positive relationship with organic matter. This higher capacity of organic retention provides a vital food source for soft-bottom species (Soto et al. 2017), which favoured the presence of a high density of polychaetes (Rosli et al. 2018) in terms of biomass, and species (Dikaeva and Frolova 2020), and the Sungai Terengganu estuary is not an exception.
The spatial distribution of Cossura sp. was significantly influenced by the ratio of silt and clay vs. gravel, as reported for Cossura coasta Kitamori, 1960 (Varghese and Miranda 2014; Feebarani et al. 2016). The species of Cossura are deposit feeders being often abundant in silty areas (Musale et al. 2015), likely because fine sediments tend to have more available organic matter (Jayaraj et al. 2008). Therefore, we hypothesize that our species of Cossura could be better adapted to feed in muddy rather than in silty bottoms. Cossura sp. also dominates in stations 8–9. As some species of this genus have been reported as being an opportunistic and well adapted to survive in stressful environments, its presence suggests that the area might be organically polluted by urban runoff or sewage, as it occurs in the Wulan Delta estuary, Indonesia (Fadlillah et al. 2017). Polychaete diversity and abundance seem to be higher in modified than in non-modified estuaries, with organic enrichment contributing to an increased nutrient level and thus also polychaete diversity (Zan et al. 2015). Composetia sp. was dominant in the stations with high gravel content, although it also occurred (with much lower densities) in stations with high silt and clay contents. This suggests a wide trophic range, with a preference for macrophagic over filter or deposit feeding, allowing it to better survive in these conditions. The dominance of Composetia sp. also explains the lowest J' recorded at station 3, while at the nearby station 2, the equivalent representation of all species present gives rise to a much higher J'. Therefore, our data agree with the common trend of coarse sediments being dominated by carnivores and omnivores, and fine sediments being mainly dominated by deposit feeders (Abrogueña et al. 2021).
Besides silty-clay and TOM contents, higher polychaete density and H’ were favored in the area with higher pH (polyhaline, downstream), as reported by van der Linden et al. (2017). Diversity was higher at the river mouth (polyhaline) than landward (oligohaline), as in the Uppanar and Vellar estuaries, India (Ajmal-Khan et al. 2014). By affecting the tolerance to physiological stress (Carrier-Belleau et al. 2021), salinity often combines with depth, sediment grain size, and organic content to explain the spatial patterns of polychaete diversity in estuaries (Holzhauer et al. 2019). However, the overall H’ in the Sungai Terengganu estuary was very low (i.e., < 2.0), as it seems to be common in estuarine ecosystems, and being often in relation to high abundances of some macro-invertebrates taxa, as well as to the high levels of environmental variability (Xue et al. 2019). In India, a low polychaete diversity was related to anthropogenic pollutants in Thane creek (Quadros et al. 2009) and to disturbances by waterway dredging in the navigation channel of Mumbai Port (Sukumaran and Devi 2009). In the Sungai Terengganu estuary, we hypothesize that the low polychaete diversity could likely be attributed to urbanization and sand mining activities.
Conclusion
The present study documented 43 polychaete taxa along the Sungai Terengganu estuary. Stations 1–5 (landward), dominated by Composetia sp., mainly had sand and gravels, while stations 6–9 (estuarine river mouth), dominated by Cossura sp., mainly had silt and clay sediments. The organic matter content was significantly lower in the oligohaline (1–3) than in the polyhaline (4–9) stations. Density and diversity were significantly lower in the oligohaline than in the polyhaline stations, and were positively correlated with temperature, pH, and total organic matter. We are thus concluding that, in the Sungai Terengganu estuary, the polychaete assemblages are influenced by complex interactions of water quality parameters and sediment characteristics, most likely including the influence of artificial constructions and urban waste pollution.
Data Availability
The datasets used and analysed during the current study are available from the authors upon reasonable request.
Code Availability
Not applicable.
References
Abrogueña JBR, Joydas TV, Pappathy M, Cali NA, Alcaria J, Shoeb M (2021) Structure and composition of the macrobenthic community associated to shallow mangrove-seagrass habitat along the southern Red Sea coast, Saudi Arabia. Egyptian Journal of Aquatic Research 47(1):61–66
Ahmad O, Fang TP, Yahya K (2011) Distribution of intertidal organisms in the shores of Teluk Aling, Pulau Pinang, Malaysia. Publications of the Seto Marine Biological Laboratory 41:51–61
Airoldi L, Abbiati M, Beck MW, Hawkins SJ, Jonsson PR, Martin D, Moschella PS, Sundelöf A, Thompson RC, Åberg P (2005) An ecological perspective on the deployment and design of low-crested and other hard coastal defence structures. Coastal Engineering 52(10–11):1073–1087
Ajmal-Khan S, Manokaran S, Lyla PS (2014) Assessment of ecological quality of Vellar and Uppanar estuaries, southeast coast of India, using benthos. Indian Journal of Geo-Marine Sciences 43:1–7
Al-Omari NHA (2011) A guide to polychaetes (Annelida) in Qatar marine sediments. Qatar University Environmental Studies Center, Doha
Arbuckle JL (2012) IBM SPSS Amos 21 user’s guide. IBM Corporation, Chicago
Bachok Z, Mfilinge PL, Tsuchiya M, Meziane T (2006) Food sources of coexisting suspension-feeding bivalves as indicated by fatty acid biomarkers, subjected to the bivalves abundance on a tidal flat. Journal of Sustainability Science and Management 1:92–111
Barletta M, Lima ARA, Costa MF, Dantas DV (2017) Estuarine ecoclines and the associated fauna: ecological information as the basis for ecosystem conservation. In: Finkl CW, Makowski C (eds) Coastal wetlands: alteration and remediation. Springer, Cham, pp 479–512
Barrosa R, Paiva PC (2007) Amphinomidae (Annelida: Polychaeta) from Rocas Atoll, Northeastern Brazil. Arquivos Do Museu Nacional, Rio de Janeiro 65(3):357–362
Bergström P, Hällmark N, Larsson KJ, Lindegarth (2019) Biodeposits from Mytilus edulis: a potentially high-quality food source for the polychaete, Hediste diversicolor. Aquaculture International 27(1):89–104
Bissoli LB, Bernardino AF (2018) Benthic macrofaunal structure and secondary production in tropical estuaries on the Eastern Marine Ecoregion of Brazil. PeerJ 6:e4441
Boerema A, Meire P (2017) Management for estuarine ecosystem services: A review. Ecological Engineering 98:172–182
Buller AT, McManus J (1979) Sediment sampling and analysis. In: Dyer KR (ed) Estuarine, hydrography and sedimentation. Cambridge University Press, Cambridge, pp 87–130
Campanyà-Llovet N, Snelgrove PVR, Parrish CC (2017) Rethinking the importance of food quality in marine benthic food webs. Progress in Oceanography 156:240–251
Carrier-Belleau C, Drolet D, McKindsey CW, Archambault P (2021) Environmental stressors, complex interactions and marine benthic communities’ responses. Scientific Reports 11:4194
Checon HH, Pardo EV, Amaral ACZ (2017) Breadth and composition of polychaete diets and the importance of diatoms to species and trophic guilds. Helgoland Marine Research 70(1):1–11
Clarke KR, Warwick RM (2001) Change in marine communities: an approach to statistical analysis and interpretation, 2nd edn. PRIMER-E, Ltd., Plymouth Marine Laboratory, Plymouth
Díaz-Castañeda V, Reish D (2009) Polychaetes in environmental studies. Annelids as model systems in the biological sciences. John Wiley & Sons, pp 205–227
Dikaeva D, Frolova E (2020) Distribution of polychaeta communities in the western and northern part of the Barents Sea. International Applied Research Conference 2020:44–50
Evans KM, Gill RA, Robotham PWJ (1990) The PAH and organic content of sediment particle size fractions. International Journal of Environmental Pollution 5(1):13–31
Fadlillah LN, Sunarto WM, Marfai MA (2017) The impact of human activities in the Wulan Delta Estuary, Indonesia. IOP Conference Series: Earth and Environmental Science 148:012032
Fatin Adlina MN, Suratman S, Lee DQ (2019) Distribution and behaviour of nutrients in Besut river estuary, Terengganu, Malaysia. Malaysian Journal of Analytical Sciences 23(6):1077–1089
Feebarani J, Joydas TV, Damodaran R, Borja A (2016) Benthic quality assessment in a naturally and human-stressed tropical estuary. Ecological Indicators 67:380–390
Fontoura NF, Schulz UH, Alves TP, Silveira TCL, Pereira JJ, Antonetti DA (2019) How far upstream: A review of estuary-fresh water fish movements in a large neotropical basin. Frontiers in Marine Science 6:1–12
Gholizadeh M, Yahaya K, Talib A, Ahmad O (2012) Distributions of macrobenthic polychaetes families in relation to environmental parameters in North West Penang, Malaysia. International Journal of Environmental, Ecological, Geological and Mining Engineering 6(12):145
Giangrande A, Licciano M, Musco L (2005) Polychaetes as environmental indicators revisited. Marine Pollution Bulletin 50(11):1153–1162
Glasby CJ, Hutchings P, Fauchald C, Paxton H, Rouse GW, Russell CW, Wilson RS (2000) Class Polychaeta. In: Beesley PL, Ross GJB, Glasby CJ (eds) Polychaetes & allies the southern synthesis. Fauna of Australia. CSIRO Publishing, Melbourne, pp 1–297
Glasby CJ, Erséus C, Martin P (2021) Annelids in extreme aquatic environments: Diversity, adaptations and evolution. Diversity 13(2):1–23
Górska B, Gromisz S, Włodarska-Kowalczuk M (2019) Size assessment in polychaete wor–s - application of morphometric correlations for common North Atlantic taxa. Limnology and Oceanography, Methods 17(4):254–265
Guan WS, Mazlan AG, Masni MA, Zaidi CC (2014) The polychaeta (Annelida) communities of the Merambong and Tanjung Adang shoals, Malaysia and its relationship with the environmental variables. Malayan Nature Journal 66:168–183
Holzhauer H, Borsje BW, van Dalfsen JA, Mijnberg KM, Hulscher SMH, Herman PMJ (2019) Benthic species distribution linked to morphological features of a barred coast. Journal of Marine Science and Engineering 8(16):1–23
Ibrahim NF, Ibrahim YS, Sato M (2017) Taxonomic study of polychaete community in Setiu Wetlands, Terengganu. In: Mohamad F, Ibrahim YS, Baharuddin N, Abd Rahman AA, Borkhanuddin MH (eds) Invertebrates of Setiu Wetlands. Penerbit UMT, pp 113–1120
Ibrahim NF, Ibrahim YS, Sato M (2019) New record of an estuarine polychaete, Neanthes glandicincta (Annelida, Nereididae) on the eastern coast of Peninsular Malaysia. ZooKeys 831:81–94. https://doi.org/10.3897/zookeys.831.28588
Idris I, Arshad A (2013) Checklist of polychaetous Annelids in Malaysia with redescription of two commercially exploited species. Asian Journal of Animal and Veterinary Advances 8(3):409–436
Jayaraj KA, Josia J, Kumar PKD (2008) Infaunal macrobenthic community of soft bottom sediment in a tropical shelf. Journal of Coastal Research 24(3):708–718
Jeong MK, Wi JH, Suh HL (2017) A new species of Leiochrides from the Korean subtidal waters with notes on the taxonomic status of the genus Pseudomastus (Annelida, Capitellidae). ZooKeys 685:91–103
Jumars PA, Morgan KM, Lindsay SM (2015) Diet of worms emended: an update of polychaete feeding guilds. Supplemental Material: Annual Review of Marine Science 7:497–520
Kim SL, Lee HG, Yu OH (2021) Correlation between rocky reefs and surrounding benthic habitats: Distribution and diversity patterns of polychaetes in the macrobenthic community in the East Sea of South Korea. Journal of Sea Research 174:102083
Lee HL, Tangang F, Gisen JI, Suratman S (2017) Prediction of salinity intrusion in the sheltered estuary of Terengganu River in Malaysia using 1-D empirical intrusion model. Acta Oceanologica Sinica 36(5):57–66
Martin D, Bertasi F, Colangelo MA, de Vries M, Frost M, Hawkins SJ, ... Ceccherelli VU (2005) Ecological impact of coastal defense structures on sediment and mobile fauna: evaluating and forecasting consequences of unavoidable modifications of native habitats. Coastal engineering, 52(10–11): 1027–1051
Martins AD, Barros F (2022) Ecological functions of polychaetes along estuarine gradients. Front Mar Sci 9(780318):1–14
Masucci GD, Acierno A, Reimer JD (2020) Eroding diversity away: Impacts of a tetrapod breakwater on a subtropical coral reef. Aquatic Conservation: Marine and Freshwater Ecosystems 30(2):290–302
Masucci GD, Biondi P, Reimer JD (2021) Impacts of coastal armouring on rubble mobile cryptofauna at shallow coral reefs in Okinawa, Japan. Plankton and Benthos Research 16(4):237–248
Mohd-Salim J, Radzi MA, Mohd-Razali S, Cooke FM (2018) Coastal landscapes of Peninsular Malaysia: The changes and implications for their resilience and ecosystem services. In: Loures L (ed) Landscape reclamation-rising from what’s left. IntechOpen, London, pp 1–17
Montagna P, Palmer P, Pollack J (2013) Hydrological changes and estuarine dynamics, vol 8. Springer, New York
Montagna PA, Hu X, Palmer TA, Wetz M (2018) Effect of hydrological variability on the biogeochemistry of estuaries across a regional climatic gradient. Limnology and Oceanography 63(6):2465–2478
Musale AS, Desai DV, Sawant SS, Venkat K, Anil AC (2015) Distribution and abundance of benthic macroorganisms in and around Visakhapatnam Harbour on the east coast of India. Journal of the Marine Biological Association of the United Kingdom 95(2):215–231
Nakao S, Hirotaka N, Abdul-Satar M (1989a) Macrobenthos and sedimentary environments in a Malaysian intertidal mudflat of the cockle bed. Bulletin of Fisheries Sciences, Hokkaido University 40(4):203–213
Nakao S, Shazili NA, Umar S (1989b) Benthic communities in the areas under and around the fish-culture rafts at the Kuala Terengganu River estuary, Malaysia. Bulletin of Fisheries Sciences, Hokkaido University 40(3):154–158
National Oceanic and Atmospheric Administration (NOAA) (2021) Estuary education Part 1: estuary ecology. https://coast.noaa.gov/estuaries/. Accessed 7 June 2021
Pielou EC (1975) Ecological diversity. Wiley, New York
Quadros G, Sukumaran S, Athalye RP (2009) Impact of the changing ecology on intertidal polychaetes in an anthropogenically stressed tropical creek, India. Aquatic Ecology 43:977–985
Read G, Fauchald K (Ed.) (2022). World polychaeta database. Accessed at https://www.marinespecies.org/polychaeta on 2022–05–06
Rehitha TV, Madhu NV, Vineetha G, Vipindas PV, Resmi P, Revichandran C (2019) Spatio-temporal variability in macrobenthic communities and trophic structure of a tropical estuary and its adjacent coastal waters. Environmental Monitoring and Assessment 191(6):1–18
Rosli NS, Yahya N, Arifin I, Bachok Z (2016) Diversity of polychaeta (Annelida) in the Continental Shelf of Southern South China Sea. Middle-East Journal of Scientific Research 24(6):2086–2096
Rosli NS, Yahya N, Idris I, Bachok Z (2018) Polychaetous annelid community structure in relation to soft bottom sediment characteristics in Continental Shelf of the Southern South China Sea. Journal of Sustainability Science and Management 10(1):37–45
Rouse G, Pleijel F, Tilic E (2022) Annelida. Oxford University Press, United Kingdom
Rush SA, Olin JA, Fisk AT, Woodrey MS, Cooper RJ (2010) Trophic relationships of a marsh bird differ between gulf coast estuaries. Estuaries and Coasts 33(4):963–970
Scanes P, Ferguson A, Potts J (2017) Estuary form and function: implications for palaeoecological studies. Applications of paleoenvironmental techniques in estuarine studies. Springer, Dordrecht, pp 9–44
Scanes E, Scanes PR, Ross PM (2020) Climate change rapidly warms and acidifies Australian estuaries. Nature Communications 11(1):1–11
Seitz RD, Knick KE, Westphal M (2011) Diet selectivity of juvenile blue crabs (Callinectes sapidus) in Chesapeake Bay. Integrative and Comparative Biology (ICB) 51(4):598–607
Sendall K, Salazar-Vallejo SI (2013) Revision of Sternaspis Otto, 1821 (Polychaeta, Sternaspidae). ZooKeys 286:1–74
Shannon CE (1948) A mathematical theory of communication. The Bell System Technical Journal 27:379–423
Soto E, Quiroga E, Ganga B, Alarcón G (2017) Influence of organic matter inputs and grain size on soft-bottom macrobenthic biodiversity in the upwelling ecosystem of central Chile. Marine Biodiversity 47(2):433–450
Sukumaran S, Devi KS (2009) Polychaete diversity and its relevance in the rapid environmental assessment of Mumbai Port. Current Science 97(10):1439–1444
Sultan K, Shazili NA (2010) Geochemical baselines of major, minor and trace elements in the tropical sediments of the Terengganu River basin, Malaysia. International Journal of Sediment Research 25(4):340–354
Suratman S, Aziz AA, Tahir NM, Lee LH (2018) Distribution and behavior of nitrogen compounds in the surface water of Sungai Terengganu Estuary, Southern Waters of South China Sea, Malaysia. Sains Malaysiana 47(4):651–659
van der Linden P, Marchini A, Smith CJ, Dolbeth M, Simone LRL, Marques JC, Molozzi J, Medeiros CR, Patrício J (2017) Functional changes in polychaete and molluscs communities in two tropical estuaries. Estuarine, Coastal and Shelf Science 187:62–73
van Niekerk L, Adams JB, James NC, Lamberth SJ, MacKay CF, Turpie JK, Rajkaran A, Weerts SP, Whitfield AK (2020) An estuary ecosystem classification that encompasses biogeography and a high diversity of types in support of protection and management. African Journal of Aquatic Science 45(1–2):199–216
Varghese SJ, Miranda MTP (2014) Macrobenthic polychaete distribution and abundance along the Arthunkal Coast of Kerala, South West Coast of India: Relationships to environmental variables. Journal of Aquatic Biology and Fisheries 2(2001):696–702
Wolanski E, Andutta F, Delhez E (2012) Estuarine hydrology. In: Bengtsson L, Herschy RW, Fairbridge RW (eds) Encyclopedia of lakes and reservoirs. Encyclopedia of earth sciences series. Springer, Dordrecht, pp 238–249
Xue J, Yang J, Wang Q, Aranson RB, Wu J (2019) Community structure of benthic macroinvertebrates in reclaimed and natural tidal flats of the Yangtze River estuary. Aquaculture and Fisheries 4(5):205–213
Zan X, Zhang C, Xu B, Xue Y, Ren Y (2015) Distribution of polychaete assemblage in relation to natural environmental variation and anthropogenic stress. Journal of Ocean University of China 14:749–758
Zhadan A (2015) Cossuridae (Annelida: Polychaeta: Sedentaria) from Australian and adjacent waters: The first faunistic survey. Records of the Australian Museum 67(1):1–24
Acknowledgements
The authors would like to thank the crew of research boats Discovery 1 and Discovery 2, Centre of Research and Field Services (CRAFS), laboratory staff at the Faculty of Science and Marine Environment and Faculty of Fisheries and Food Science, Universiti Malaysia Terengganu, for their technical support during sampling and analysis. Sincere appreciation also goes to a team of final year and postgraduate students who helped during the fieldwork.
Funding
The funding for this project was provided by Universiti Malaysia Terengganu under the ‘Tabung Penyelidik Muda’ research grant (Vot No. 68007/2016/80) awarded to Izwandy Idris.
Author information
Authors and Affiliations
Contributions
NSA designed the research, performed data collection and analysis, supervised by II and ZB. MAH and NFI prepared and revised the original drafts of the manuscript, performed data analysis, and edited by II. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
No ethics approvals were required for this research.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests Statement
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alias, N.S., Abd Hamid, M., Ibrahim, N.F. et al. Polychaete Assemblages in the Sungai Terengganu Estuary (East Coast of Peninsular Malaysia): Spatial Distribution Patterns. Wetlands 42, 111 (2022). https://doi.org/10.1007/s13157-022-01631-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13157-022-01631-w