Abstract
Despite increasing pressures on freshwater resources worldwide, and the threatened status of most freshwater turtles, there is still limited knowledge of habitat use and niche partitioning in Afrotropical freshwater turtle communities. In this study, we describe habitat associations, community diversity, and temporal patterns of occurrence of freshwater turtle species in the Dahomey Gap ecoregion of Ghana (West Africa). We gathered data from 13 sites in central Ghana and along the Sene Arm of Lake Volta in the Digya National Park (Bono East Region). We employed opportunistic short-term surveys (at seven sites) together with longer-term (six-months duration) standardized evaluations of turtle presence and numbers in different habitats (at six sites). Overall, a total of 210 turtle individuals of four species (Trionyx triunguis, Cyclanorbis senegalensis, Pelomedusa sp. and Pelusios castaneus) were recorded; precise capture sites and habitat type were recorded for 139 individuals, but the 71 individuals observed in marketplaces were not considered in our analyses. At a local scale, we observed three sympatric species in various study sites. In each of these sites, the dominant species was either C. senegalensis or Pelomedusa sp., with the latter species being more abundant in temporary waterbodies and C. senegalensis more numerous in permanent ones. A Multiple Correspondence Analysis suggested that, in permanent waterbodies all species were associated with similar physical habitat variables. In a Canonical Correspondence Analysis, we showed that the density of herbaceous emergent vegetation was more important for P. castaneus than for C. senegalensis. Comparisons of diversity metrics between our study sites and previous studies revealed that turtle community composition was similar across savannah sites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Freshwater turtles depend on aquatic and terrestrial habitats for different parts of their life cycle such as predator avoidance, feeding, courtship and mating, basking and nesting activities (Ficetola et al. 2004; Steen and Gibbs 2004). A complex network and interplay of biotic and abiotic factors control the structure of turtle communities (e.g., Bodie et al. 2000; Aresco 2009; Kessler et al. 2020; Luiselli et al. 2020). When species cooccur in a single habitat, each species has been shown to select habitat features that best fit their life strategies (Lindeman 1999; Riedle et al. 2016). Consequently, turtle species may show affinity for certain aquatic environmental conditions such as water level, water quality, flow characteristics, substrate type and vegetation, but also choose associated terrestrial features for basking and nesting (Ficetola et al. 2004; Vignoli et al. 2015; Luiselli et al. 2020). A turtle community is also influenced by the characteristics of terrestrial habitats surrounding the water bodies (Marchand and Litvaitis 2004; Luiselli et al. 2020). Thus, the selection of contrasting bank characteristics by sympatric species may be due to the need to minimize interspecific competition (e.g., Cadi and Joly 2003, 2004; Luiselli 2008; Petrozzi et al. 2021).
Previous freshwater turtle research in West Africa has focused on taxonomy and distribution of species (e.g., Hoogmoed 1980; Bour and Maran 2003; Bombi et al. 2011; Segniagbeto et al. 2014 and references therein). More recently, much effort has been placed on studying what habitat characteristics, including the effects of habitat pollution and degradation, affect turtle community ecology (e.g., Luiselli et al. 2000, 2004), and niche partitioning and interspecific competition within turtle assemblages in rainforest areas (e.g., Luiselli et al. 2006a, b; Petrozzi et al. 2021). By contrast, only one turtle community study is available from the forest-savanna habitats in the Dahomey Gap (Luiselli et al. 2020). The Dahomey Gap is a region of Ghana, Togo and Benin where extends to the coast, and separates the Upper Guinean forests (from Guinea to Western Ghana) from the Lower Guinean forests (Nigeria and Cameroon) (Weber 2001). This region is one of the most ecologically interesting areas in West Africa because savannahs divide forest habitat causing the separation and genetic divergence of many organisms, increasing species diversity (Dinerstein et al. 2017). Despite its biological importance, turtle communities have been only studied in Benin (see Luiselli et al. 2020), and no studies are available for the other countries within the Dahomey Gap savannah biome (i.e., Ghana, Burkina Faso, Togo, Mali, Niger, Senegal, part of Nigeria and part of Ivory Coast).
In this study, we evaluated diversity metrics, temporal trapping success and physical habitat characteristics of freshwater turtles in distinct sites in Guinean savannah waterbodies in Ghana. Overall, the data presented in this study were opportunistically collected or recorded during a relatively short time period, and as a consequence sample sizes are relatively low. Given that there are no available data on the turtle community ecology in Ghana, and that turtles are facing an unprecedented global risk of extinction (Stanford et al. 2020), we present this information to fill a research void. More specifically, we ask the following questions:
-
(1)
How many species occur sympatrically in the savannahs of Ghana?
-
(2)
What are the main habitat features preferred by each turtle species in the community?
-
(3)
Are the turtle communities of the various sites relatively similar across the whole Dahomey Gap biome? Or is there site-specific variation?
Materials and Methods
Taxonomic Note
The taxonomy of African turtles has been substantially revised during the last decade (e.g., Branch 2008). For practical reasons, we follow the taxonomic nomenclature of the Turtle Taxonomy Working Group (2017). For Pelomedusa we used the taxon attribute “sp.” since the criteria for distinguishing P. olivacea, P. subrufa or to P. variabilis are still being debated.
Study Sites and Protocol
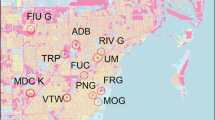
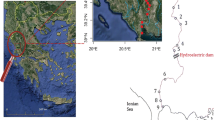
Data presented herein are based on (i) short-term data collected opportunistically from distinct sites across Ghana (Fig. 1), and (ii) a longer-term (6-months) standardized field survey in the Lake Volta region (Fig. 2). Although there is some geographical variation, the climate in all study sites is tropical, with a dry season from October to April and a wet season from May to September. In all study areas, especially in Lake Volta, inland fisheries are well-established (Braimah 1995). Fisheries in inland waters have impacted aquatic biodiversity, including turtles (Braimah 1995).
Short term surveys
Overall, seven distinct study areas were surveyed by short-term sampling, six in the south-central part of Ghana (Akuapim South, Akuapim North, Akenkenso, Amansie East, Amansuri wetlands, Tano River) and one in the Lovi River, Mole National Park in the north of the country (Fig. 1). In Akuapim South, Akuapim North, Akenkenso, and Amansie East field surveys were carried out during February-March and November-December 2003 as part of a larger project on tortoises throughout West Africa (Luiselli 2006; Luiselli et al. 2008). In Amansuri wetlands, Tano River surveys were carried out during 2017 and 2018, and in Mole National park in 2011–2012 and 2017–2018. At two localities (Akenkensio and Amansie East) we surveyed temporary ponds and seasonal waterbodies, whereas in all other sites we surveyed permanent waterbodies (Table 1).
Field research at each site lasted 7–13 days. At each site, we caught turtles using hand nets and traps. All turtles trapped/captured in the field were identified to species and sexed, measured for carapace and plastron length and then released unharmed in the capture site. Each trap, constructed using fine mesh was approximately 120 to 180 cm in length and had a hoop diameter of about 91 cm. In most cases, we placed the same number of traps (n = 30) baited with fish at each site and each day. The fine mesh size prevented the legs of the turtles from becoming entangled. The top of the traps remained above water to allow entrapped turtles to obtain air. All traps were checked daily. No captured turtles died during our study. Fisher catches and specimens for sale in local markets were also examined. The number of turtle individuals obtained by fishers versus those that were trapped, for each study site, is given in Table 1. Information on the capture sites of the various market specimens was obtained from interviews with the traders/fishers, but habitat data were not considered for the analyses for specimens that were not directly captured by us or observed when just caught by local fisherfolk. Thus, we excluded from the statistical analyses all the turtle individuals (n = 71) that were recorded in markets and were not attributable to any specific locality with precision.
Longer term surveys
We also surveyed another six distinct localities using standardized trapping between January and June 2017, along a 20-km stretch of the Sene Arm of Lake Volta, in the Digya National Park (Fig. 2). This National Park is the oldest protected area in Ghana and second largest in the country, covering an area of 3,743km2. It is bordered to the North, East and South by Lake Volta and drains up to 70 % of the total landmass of Ghana. In each of these six localities, we set 10 locally constructed hoop traps (Fig. 3A) at each sampling site and monitored them for a month before switching onto a different river stretch. Traps were permanently set at each trap station for an entire month, and checked daily. Traps were visited and rebaited daily with canned sardines for the first month, and with fresh fish for all subsequent months. All captured turtles were processed as reported above.
Types of traps used for capturing turtles at the survey stations in Ghana, and a typical habitat where turtles were captured in the Volta Lake area. (A) Setup of a hoop trap in an inlet along Sene Arm of the Volta Lake. (B) Local traps used for both fish and turtles by the fishers’ communities. (C) Section of Sene Arm of the Volta Lake dominated by herbaceous emergent vegetation
For each trap station the dominant bank vegetation along the shoreline was recorded by eye and categorized into: (i) herbaceous vegetation, (ii) shrubs, and (iii) trees. Common herbaceous vegetation included Vossia cuspidata and Polygonum senegalense. A common shoreline shrub was Mimosa pigra while Khaya spp. and Diospyros mespiliformis werecommon woody emergent found in the study area. Following Riedle et al. (2016), emergent vegetation cover of aquatic habitats was estimated using a 25 m radius circular plot around each trap site and percentage cover categorised into three classes: absent (> 90 % of the water surrounding the trap site was free of emergent vegetation at time of trapping), scarce (50–90 % of the water surface free of emergent vegetation), and abundant (< 50 % of the water surface free of emergent vegetation). Density of woody emergent vegetation was determined by estimating the number of woody plants within a 25 m radius surrounding each trap site. The estimated number of woody emergents was categorised into three classes; absent (< 25 emergent woody plants around the trap site at time of trapping), scarce (25–50 emergent woody plants), and abundant (> 50 emergent woody plants).
Shoreline and benthic substrate type was determined by collecting two samples from each site and by subjectively assessing the degree of roughness and the relative amount of plant matter by rolling it between two fingers (Riedle et al. 2016). Substrate samples were classified into: (i) organic (> 50 % of woody debris, leaf litter and other plant matter) or (ii) inorganic (> 50 % of stone, sand and clay) (Riedle et al. 2016).
Statistical Analyses
We evaluated whether our sampling effort captured the true species richness and diversity within each study site by (i) building a rarefaction curve for species discoveries at each site (and generating the 95 % confidence intervals of the estimate after 9999 bootstraps); and (ii) calculating Chao-1 index. This latter index represents the theoretical number of species that can be expected on the basis of the sampling regime. In addition, the following univariate community diversity metrics were calculated for each site: (i) Species richness (total number of species recorded at each site); (ii) Dominance (D); (iii) Simpson index (S) S = 1 – D; (iv) Shannon entropy index (H’; Shannon and Weaver 1963) and (v) Evenness (e), calculated by Buzan and Gibson’s formula (Magurran 1988). The combined use of these indices is useful to understand which areas are ecologically better suited for turtles, where the conservation value of each site increases with decreasing values of D and increasing values of S, H’ and E (Magurran 1988). For each diversity metric, we also generated upper and lower 95 % confidence intervals by performing a bootstrap analysis with 9999 random samples, each with the same total number of individuals as in each original sample generated (Harper 1999).
Comparisons of mean univariate diversity metrics values between our study areas and another study in comparable habitats in Benin (Luiselli et al. 2020) were made by one-way Analysis of Variance (ANOVA). Chi-squared (χ2) tests were used to compare the frequencies of turtles across the various types of substrate/habitats and in relation to the relative availability of each substratum/habitat type in the field (used as null model). Using monthly records as samples, we performed a Multiple Correspondence Analysis (MCA) on the occurrence of turtle species and individual habitat variables collected in the Lake Volta study area, using the package FactoMine R for windows 3.2.2 (R core Team 2015). For the site/species matrix, we used a Canonical Correspondence Analysis (Legendre and Legendre 1998) to assess species occurrence in relation to habitat variables.
All statistical analyses were performed in PAleontological STatistics (PAST Ver. 4.0). For all statistical tests, alpha was set at 5 %, and normality and homoscedasticity of variables were assessed by Shapiro-Wilk W test (P > 0.05) prior to using any nonparametric analysis.
Results
Overall, we recorded a total of 210 turtle individuals belonging to four species; 139 were attributed to precise capture sites with habitat recorded, whereas 71 individuals were observed in marketplaces, for which their capture locality remained ambiguous. These were 23 Pelomedusa sp., 20 Pelusios castaneus, 18 Cyclanorbis senegalensis, and 10 Trionyx triunguis.
Short-term surveys
During these surveys we captured/examined 120 turtles with confirmed locality of capture, belonging to four species (Table 1): Pelomedusidae – Pelomedusa sp. (n = 40), Pelusios castaneus (n = 23); Trionychidae – Cyclanorbis senegalensis (n = 42), Trionyx triunguis (n = 15). Two other species, potentially present in the area according to historical records, Cyclanorbis elegans and Pelusios cupulatta, were never observed. Overall, 18.3 % of the recorded individuals (n = 120) were observed in the possession of local fisherfolk, the rest captured in traps or directly observed in the wild. The percentage of individuals provided by local fisherfolk varied substantially across localities (range: 0-36.7 %) and among species; 12.5 % in Pelomedusa sp., 17.4 % in P. castaneus, 21.4 % in C. senegalensis and 26.7 % in T. triunguis. Overall, the percentage number of turtle individuals provided by fisherfolks was not significantly different between Trionychidae (22.8 %, total n = 57) and Pelomedusidae (14.3 %, n = 63) (χ2 = 0.3, df = 1, P = 0.584).
Diversity profiles differed between sites (Fig. 4a), with Akenkenso being characterized by a more simplified community composition than the other three sites. Saturation curves showed that species diversity was adequately sampled in five study sites except Tano River and Mole National Park; the latter two because of low sample sizes (Fig. 4b). In all sites with adequate sample sizes, the total number of sympatric species was the same, i.e., 3 (Table 2). However, only one species was recorded in two sites of smaller inadequate sample size (Table 1). Pelomedusa sp. was the most abundant species in the two sites where temporary waterbodies were surveyed, whereas C. senegalensis appeared to be the most common species in all sites with permanent waterbodies (Table 1). No Pelomedusa individual was ever caught in permanent waterbodies (Table 1).
Dominance indices at our study areas did not differ from those observed in similar habitats in Benin (data in Luiselli et al. 2020): ANOVA F = 2.172, P = 0.473; the same was true for Simpson index (F = 1.043, P = 0.955), Shannon index (F = 1.577, P = 0.387), and Evenness index (F = 1.77, P = 0.237).
Lake Volta surveys
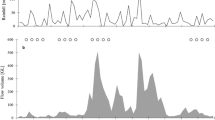
Despite the longer duration of this study, only a total of 19 individuals were recorded belonging to three species (7 Trionyx triunguis, 9 Cyclanorbis senegalensis and 3 Pelusios castaneus). The small sample size of P. castaneus did not allow us to discern seasonality patterns. By contrast, trapping success of T. triunguis was highest during the wet season months of April to June, with at least one individual of C. senegalensis captured in each survey month (Fig. 5A). We found no preference for terrestrial substrates (Fig. 5B) for C. senegalensis and P. castaneus (in both cases, P > 0.5 at χ2 test), but T. triunguis clearly favoured organic substratum (P < 0.05 at χ2 test). There was no clear difference among species in terms of dominant bank vegetation (Fig. 5C). Trionyx triunguis individuals differed significantly from the other species (P < 0.05 at χ2 test) in terms of their choice of sites with more emergent aquatic vegetation (Fig. 6A and B); this species preferred sites with abundant wood and herbaceous vegetation, whereas the two other species had no special preference for any type of aquatic vegetation. Results of the Canonical Correspondence Analysis indicated that C. senegalensis and T. triunguis were more similar in their choice of habitat than P. castaneus (Fig. 7). Cyclanorbis senegalensis and T. triunguis presence was more defined by the density of woody emergent and substrates. Positive values for woody emergents was linked to the presence of T. triunguis but not for C. senegalensis. By contrast, the existence of P. castaneus was related to negative values in the measured habitat variables except for herbaceous vegetation, the latter positively correlated with increased density of this species.
Aquatic vegetation characteristics of the capture sites for freshwater turtles at Sene Arm of the Volta Lake, Ghana. (A) emergent woody vegetation (absent, scarce or abundant); (B) emergent herbaceous vegetation (absent, scarce or abundant). For turtles, numbers would indicate the raw numbers of collected individuals
Freshwater turtles association with (i) sampling period and broad scale categories of habitat variables (graphic A), and (ii) with fine scale habitat variables in the Sene Arm of the Volta Lake (graphic B). Code: EsHerbEmerg = estimated herbaceous emergent, Es Woody Emerg = estimated woody emergent, TerSub = terrestrial substrate, AquSub = aquatic substrate, TerVeg = Terrestrial vegetation, 1–6 = January to June respectively. Symbols: H d = herbaceous density (absent, scarce or abundant), w d = woody emergent density (absent, scarce or abundant), a s = aquatic substrate type (organic or inorganic), t s = terrestrial substrate type (organic or inorganic)
Discussion
In most of our study areas because data was collected opportunistically or recorded during a relatively short time period, our sample sizes were relatively small. However, our saturation curve analysis and the Chao-1 estimate showed that for sites where we could obtain an adequate sample size, turtle species richness was low (three taxa). This result mirrors data for waterbodies in the Guinean savannahs in Benin, where also only three species (maximum 4) occurred sympatrically (Luiselli et al. 2020). In the savannah waterbodies of both Benin and Ghana, the same pair of species (C. senegalensis and P. castaneus) occurred in every available habitat, together with either Pelomedusa sp. (in temporary waterbodies) or T. triunguis (in perennial waterbodies). Pelomedusa sp. and T. triunguis were always parapatric in our study areas; it is unlikely that these species partition their niche since they differ in body size (T. triunguis being much larger), morphology and ecology (Branch 2008), and hence the potential for interspecific competition is likely to be low. However, it also possible that T. triunguis predates on juveniles of Pelomedusa or that the juveniles of T. triunguis compete with Pelomedusa adults as both species are fundamentally carnivorous (see Luiselli et al. 2011). Unfortunately, there are no data available in the literature to confirm these hypotheses.
Univariate diversity indices also were comparable among our study areas in Ghana and those in Benin (Luiselli et al. 2020). This points to the turtule community composition in the Dahomey Gap savannahs of West Africa being similar, both qualitatively and quantitatively.
Despite the relatively long duration of our field study in the Lake Volta area, we caught a very few numbers of turtles compared to previous studies in West African savannahs (Luiselli et al. 2020). This could have been caused by differences in population abundance across sites, sampling success being affected by the trapping method (Tesche and Hodges 2015), or by the time of the year when trapping was carried out. The ideal period for catching freshwater turtles at our Lake Volta study area in Ghana was the dry season. This may be linked to the lowering of water levels, which increases turtle density within aquatic habitats and thus the probability of their detection. In temporary ponds aestivation during dry season may occur, thus lowering turtle numbers (Luiselli et al. 2000), but this was not the case in our study since the field research in the Lake Volta region was undertaken in a permanent water body. Trapping success differed slightly for the three species, suggesting that preferences for waterbodies may differ between species (see also Luiselli et al. 2000). While T. triunguis occurs mostly in large rivers, C. senegalensis prefers smaller streams and seasonal ponds and marshes (Gramentz 2008). Unfortunately, no other study has analysed seasonal variation of trapping success in African savannah turtles.
In our study, C. senegalensis and T. triunguis preferred inorganic substrate in benthic and terrestrial zones (Fig. 8). Other turtles species are known to prefer organic substrates to aestivate and nesting (e.g., Marchand and Litvaitis 2004); these substrates also provide comparatively better foraging opportunities as they house a higher density of potential prey items (Marchand and Litvaitis 2004). The preference of the two trionychid species for high densities of woody emergent vegetation may also be linked to prey availability. Turtles of the Trionychidae family are largely carnivorous and feed mainly on amphibians, snails and fishes (Akani et al. 2001; Luiselli et al. 2004; Gramentz 2008), prey items that, at our study areas, are usually more abundant in spots characterized by high woody emergent density (our unpublished observations). The differences in habitat preferences between the two trionychids (C. senegalensis and T. triunguis) may also be due to resource partitioning to minimize interspecific competition (Pritchard 2001; Luiselli 2008; Akani et al. 2018). The same mechanism could be playing a role in the case of Pelomedusa sp. versus P. castaneus, given that these taxa are ecologically, morphologically and phylogenetically very similar. Despite their similarity, the latter two species differed remarkably in the number of trapped individuals during surveys in Ghana and Nigeria (Luiselli et al. 2000). In addition, signs of interspecific competition among sympatric populations of these two taxa were evidenced during field surveys carried out in the savannahs of Benin (Luiselli et al. 2020). Further investigations are needed to clarify interactions among these sympatric turtles.
Our study did not reveal the presence of the Critically Endangered C. elegans, possibly totally extirpated from Ghana and from the whole of West Africa (Luiselli et al. 2021). Cyclanorbis elegans, was historically known to occur in the Guinean savannah rivers of Ghana and one of the most threatened species in the world (Gramentz 2008; Baker et al. 2015). This species has been recently rediscovered in South Sudan (Demaya et al. 2019a, b), but no individual has been observed in Ghana (and in the rest of West Africa) for several decades (Stanford et al. 2018; IUCN 2020; Luiselli et al. 2021). Trionyx triunguis populations are threatened in West Africa (Segniagbeto et al. 2014; IUCN 2020), and the fact that we captured 15 different individuals of this species, from four distinct localities, suggests that this species is still relatively widespread in Ghana. We recommend that Ghana may become a country of first choice for promoting the conservation and management of T. triunguis in the West African Region.
During our surveys in the Lake Volta area, we captured a lower number of turtle individuals than that obtained in similar habitats and in a lower number of days in the adjacent Benin (Luiselli et al. 2020). This evidence would suggest that the turtle communities are heavily depleted in this part of Ghana. The rarest turtle of Africa (Baker et al. 2015), C. elegans, may have gone extinct locally due to overfishing. Also the West African endemic Pelusios cupulatta was not observed during our surveys, but it is possible that this was due to unsatisfying field effort at the single locality where this species may be present (Tano River). This species was fairly common in similar habitats in Cote d’Ivoire, where it occurs sympatrically with P. castaneus (Petrozzi et al. 2021).
Overall, despite the intensive field sampling regime and timeline, we collected only very few turtles, implying a true need for additional fisheries-independent surveys to understand better the conservation status of the freshwater turtles. It would be interesting to explore additional sites, in more remote areas, to see whether the pattern of turtle depletion that we observed at our study areas may be valid more generally in the country. Despite of the limited empirical observations presented in this study we suggest that turtle populations in Ghana are more depleted than those in the neighbouring countries (for instance, Benin and Nigeria; see Luiselli et al. 2006b).
Data Availability
All data are presented in the paper.
Code Availability
Not applicable.
References
Akani GC, Capizzi D, Luiselli L (2001) Diet of the softshell turtle, Trionyx triunguis, in an Afrotropical forested region. Chelonian Conservation Biology 4:200–201
Akani GC, Eniang EA, Amadi N, Dendi D, Hema EM, Diagne T … Chirio L (2018) Macrohabitat and microhabitat usage by two softshell turtles (Trionyx triunguis and Cyclanorbis senegalensis) in West and Central Africa. Herpetological Conservation Biology 13:642–651
Aresco MJ (2009) Environmental correlates of the abundances of three species of freshwater turtles in lakes of northern Florida. Copeia 2009(3):545–555
Baker PJ, Diagne T, Luiselli L (2015) Cyclanorbis elegans (Gray 1869) – Nubian Flapshell Turtle. In: Rhodin AGJ, Pritchard PCH, van Dijk PP, Saumure RA, Buhlmann KA, Iverson JB, Mittermeier RA (eds) Conservation biology of freshwater turtles and tortoises: a compilation project of the IUCN/SSC Tortoise and Freshwater Turtle Specialist Group. Chelonian Research Monographs 5(8):089.1–7, https://doi.org/10.3854/crm.5.089.elegans.v1.2015, http://www.iucn-tftsg.org/cbftt/
Bodie JR, Semlitsch RD, Renken RB (2000) Diversity and structure of turtle assemblages: associations with wetland characters across a floodplain landscape. Ecography 23:444–456
Bombi P, Luiselli L, D’Amen M (2011) When the method for mapping species matters: defining priority areas for conservation of African freshwater turtles. Diversity and Distributions 17(4):581–592
Bour R, Maran J (2003) Une nouvelle espèce de Pelusios de Cote d’Ivoire (Reptilia, Chelonii, Pelomedusidae). Manouria 6(21):24–43
Braimah LI (1995) Recent developments in the fisheries of Volta Lake (Ghana) 18p. In: Crul RCM, Roest FC (eds) Current status of fisheries and fish stocks of the four largest African reservoirs: Kainji, Kariba, Nasser/Nubia and Volta. FAO, CIFA Technical Papers 30, 142 p
Branch B (2008) Tortoises, terrapins and turtles of Africa. New Holland Publishing, Cape Town
Cadi A, Joly P (2003) Competition for basking sites between the endangered European pond turtle (Emys orbicularis galloitalica) and the introduced red-eared slider (Trachemys scripta elegans). Canadian Journal of Zoology 81:1392–1398
Cadi A, Joly P (2004) Impact of the introduction of the read-eared slider (Trachemys scripta elegans) on survival rates of the European pond turtle (Emys orbicularis). Biodiversity and Conservation 13:1511–2518
Demaya GS, Benansio JS, Lado TF, Diagne T, Dendi D, Luiselli L (2019a) Rediscovery of the Nubian flapshell turtle (Cyclanorbis elegans) in South Sudan. Chelonian Conservation Biology 18:62–67
Demaya GS, Benansio JS, Lado TF, Jubarah SK, Ladu JLC, Luiselli L (2019b) Local ecological knowledge in South Sudan can help conservation and management of Cyclanorbis elegans. Chelonian Conservation Biology 18:259–264
Dinerstein E, Olson D, Joshi A, Vynne C, Burgess ND, Wikramanayake E, … and Hansen M (2017) An ecoregion-based approach to protecting half the terrestrial realm. Bioscience 67(6):534–545
Ficetola GF, Padoa-Schioppa E, Monti A, Massa R, De Bernardi F, Bottoni L (2004) The importance of aquatic and terrestrial habitat for the European pond turtle (Emys orbicularis): implications for conservation planning and management. Canadian Journal of Zoology 82:1704–1712
Gramentz D (2008) African Flapshell Turtles – The Genera Cyclanorbis and Cycloderma. Edition Chimaira, Frankfurt am Main
Harper DAT (ed) (1999) Numerical palaeobiology. Wiley, New York
Hoogmoed MS (1980) Herpetologische waarnemingen in Ghana VIII. De schildpadden en krokodillen. Lacerta 38:112–116
IUCN (2020) The IUCN red list of threatened species. Available at https://www.iucnredlist.org Accessed 29 Dec 2020
Kessler EJ, Ash KT, Barratt SN, Larson ER, Davis MA (2020) Radiotelemetry reveals effects of upstream biomass and UV exposure on environmental DNA occupancy and detection for a large freshwater turtle. Environmental DNA 2(1):13–23
Legendre P, Legendre L (1998) Numerical Ecology, 2nd English ed. Elsevier, Paris, 853 pp
Lindeman PV (1999) Surveys of basking map turtles, Graptemys ssp in three river drainages and the importance of deadwood abundance. Biol Cons 88:33–42
Luiselli L (2006) A mega-transect along the Gulf of Guinea (West Africa) to assess the population status and the impact of human hunting activities on the hinge-back tortoises (genus Kinixys): A crucial step towards a large-scale conservation strategy for these forest species. Unpublished report to Turtle Conservation Fund (TCF) & Conservation International (CI) & Chelonian Research Foundation (CRF), Rome, 52 pp
Luiselli L (2008) Resource partitioning in freshwater turtle communities: a null model meta-analysis of available data. Acta Oecologica 34:80–88
Luiselli L, Angelici FM, Politano E (2000) Ecological correlates of the distribution of terrestrial and freshwater chelonians in the Niger Delta, Nigeria: A biodiversity assessment with conservation implications. Revue d’Ecologie (La Terre et la Vie) 55:3–23
Luiselli L, Akani GC, Politano E, Odegbune E, Bello O (2004) Dietary shifts of sympatric freshwater turtles in pristine and oil-polluted habitats of the Niger Delta, Southern Nigeria. Herpetological Journal 14:57–64
Luiselli L, Akani GC, Bello OA, Angelici FM, Ude L (2006a) Home range area may vary considerably in relation to habitat contamination in two African terrapins from pristine and oil polluted habitats. Amphibia-Reptilia 27:255–261
Luiselli L, Akani GC, Politano E (2006) Effects of habitat alteration caused by petrochemical activities and oil spill on the habitat use and interspecific relationships among four species of Afrotropical freshwater turtles. Biodiversity and Conservation 15:3751–3767
Luiselli L, Akani GC, Ebere N, Rugiero L, Vignoli L, Angelici FM, …, Behangana M (2011) Food habits of a pelomedusid turtle, Pelomedusa subrufa, in tropical Africa (Nigeria): the effects of sex, body size, season, and site. Chelonian Conservation Biology 10(1):138–144
Luiselli L, Angelici FM, Rugiero L, Akani GC, Eniang EA, Pacini N, Politano E (2008) Negative density dependence of sympatric Hinge-back Tortoises (Kinixys erosa and K. homeana) in West Africa. Acta Herpetologica 3(1):19–33
Luiselli L, Akani GC, Ajong SN, George A, Di Vittorio M, Eniang EA, Dendi D, Hema EM, Petrozzi F, Fa JE (2020) Predicting the structure of turtle assemblages along a megatransect in West Africa. Biological Journal of the Linnean Society 130:296–309
Luiselli L, Diagne T, Mcgovern P (2021) Prioritizing the Next Decade of Freshwater Turtle and Tortoise Conservation in West Africa. Journal for Nature Conservation 60:125977
Magurran AE (1988) Ecological diversity and its measurement. Princeton University Press, Princeton
Marchand MN, Litvaitis JA (2004) Effects of habitat features and landscape composition on the population structure of a common aquatic turtle in a region undergoing rapid development. Conservation Biology 18(3):758–767
Petrozzi F, Ajong SN, Pacini N, Dendi D, Gonedele Bi S, Fa JE, Luiselli L (2021) Spatial niche expansion at multiple habitat scales of a tropical freshwater turtle in the absence of a potential competitor. Diversity, submitted
Pritchard PC (2001) Observations on body size, sympatry, and niche divergence in softshell turtles (Trionychidae). Chelonian Conservation and Biology 4:5–27
R Core Team (2015) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/
Riedle JD, Kazmaier RT, Killian J, Littrell WB (2016) Habitat associations of fish and aquatic turtles in an East Texas Stream. Knowledge Management of Aquatic Ecosystems 417(8):1–10
Segniagbeto GH, Bour R, Ohler A, Dubois A, Rödel MO, Trape JF, Luiselli L (2014) Turtles and tortoises of Togo: historical data, distribution, ecology, and conservation. Chelonian Conservation and Biology 13:152–165
Shannon CE, Weaver W (1963) The mathematical theory of communication. University of Illinois Press, Urbana
Stanford CB, Rhodin AGJ, van Dijk PP, Horne BD, Blanck T, Goode E, Hudson R, Mittermeier RA, Currylow A, Eisemberg C, Frankel M, Georges A, Gibbons PM, Juvik JO, Kuchling G, Luiselli L, Haitao S, Singh S, WaldeA (2018) Turtle in trouble—the world’s 25 + most endangered tortoise and freshwater turtle. Turtle Conservation Coalition, Chelonian Research Foundation and IUCN/SSC Tortoises and Freshwater Turtles Specialist Group
Stanford CB, Iverson JB, Rhodin AGJ, van Dijk PP, Mittermeier RA, Kuchling G, Berry KH, Bertolero A, Blanck TEG, Bjorndal KA, Buhlmann KA, Burke R, Congdon J, Diagne T, Edwards T, Eisemberg C, Ennen J, Forero-Medina G, Frankel M, Fritz U, Gallego-García N, Georges A, Gibbons JW, Gong S, Goode EV, Shi HT, Hoang H, Hofmeyr MD, Horne BD, Hudson R, Juvik J, Koval P, Kiester R, Le M, Lindeman P, Lovich JE, Luiselli L, McCormack T, Meyer G, Páez VP, Platt K, Platt SG, Pritchard PCH, Quinn H, Roosenburg W, Seminoff J, Shaffer HB, Spencer R, Van Dyke JU, Vogt RG, Walde AD (2020) Turtles and tortoises are in trouble. Current Biology 30:R721–R735. https://doi.org/10.1016/j.cub.2020.04.088
Steen DA, Gibbs JP (2004) Effects of roads on the structure of freshwater turtle populations. Conservation Biology 4:1143–1148
Tesche MR, Hodges KE (2015) Unreliable population inferences from common trapping practices for freshwater turtles. Global Ecology Conservation 3:802–813
Turtle Taxonomy Working Group (2017) Turtles of the world: annotated checklist and atlas of taxonomy, synonymy, distribution, and conservation status (8th edn). In: Rhodin AGJ, Iverson JB, van Dijk PP, Saumure RA, Buhlmann KA, Pritchard PCH, Mittermeier RA (eds) Conservation biology of freshwater turtles and tortoises: a compilation project of the IUCN/SSC Tortoise and Freshwater Turtle Specialist Group. Chelonian Research Monographs 7:1–292
Vignoli L, Bologna MA, Manzini S, Rugiero L, Luiselli L (2015) Attributes of basking sites of the European pond turtle (Emys orbicularis) in central Italy. Amphibia-Reptilia 36:125–131
Weber W (2001) African Rain Forest Ecology and Conservation: An Interdisciplinary Perspective. Yale University Press, New Haven
Acknowledgements
This study was conducted with permit from the Wildlife Division of the Ghana Forestry Commission (Permit number:WD/A.30/VOL. 9/14). Our deepest appreciation goes to the Rufford Foundation, Chelonian Research Foundation, Conservation International and Turtle Conservation Fund for supporting this work and to the management and staff of the Digya National Park for field support, and to three anonymous referees for constructive comments on the submitted draft.
Funding
Rufford Foundation (to SBG), Linnaeus Fund Research Award by Chelonian Research Foundation (two grants to LL), Conservation International (to LL), Turtle Conservation Fund (two grants to LL and one to SBG), financially supported field research on West African chelonians that also allowed to carry out several steps of the present study.
Author information
Authors and Affiliations
Contributions
LL suggested the subject and the method of manuscript; SBG, SKO, PT, FP, DD, GCA, SNA, LL make the field study; SBG, MDV, LL, DD, NP analyzed the data; SBG, NP, BDH, JEF, LL prepared the first draft; all authors reviewed and approved the final draft.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflicts of Interest/Competing Interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gbewaa, S.B., Oppong, S.K., Horne, B.D. et al. Community Characteristics of Sympatric Freshwater Turtles from Savannah Waterbodies in Ghana. Wetlands 41, 61 (2021). https://doi.org/10.1007/s13157-021-01459-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13157-021-01459-w