Abstract
The Australian continent spans coastal wetland settings ranging from extensive mangrove forest and sabkha plains occupying in the tropical north, to the southern half of the continent, where high wave energy constrains wetlands within numerous barrier-fronted estuaries, drowned river valleys and coastal embayments. Only on the island of Tasmania are mangroves absent; elsewhere mangroves, Casuarina, Melaleuca and saltmarsh interact in ways illustrative of the effects of ongoing climate, tidal and sea-level change. Observations over several decades have suggested that recent anthropogenic climate change may already be impacting Australian coastal wetlands in important ways. A period of accelerating sea-level rise has been associated with saline intrusion, mangrove encroachment and Melaleuca dieback in the tropical north, punctuated by widespread mangrove mortality in drought periods. The consistent trend of mangrove encroachment and replacement of saltmarsh in the south, is associated with an “accretion deficit” in saltmarsh during contemporary sea-level rise. We review the ecological and cultural implications of these changes, including impacts on habitat provision for migratory birds, fisheries values, carbon sequestration and Indigenous cultural values. Current legislative and policy protections may not be sufficient to meet the increasingly dynamic impacts of climate change in altering wetland boundaries, composition and function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Extent and Composition of Australian Intertidal Wetlands
At the taxonomic levels of family and genus, Australian mangroves, saltmarshes, Casuarina spp. and the “paperbark” Melaleuca spp. have strong affinities with species on other continents. Australian mangroves belong to the broader biogeographic association of Old World mangroves, finding greatest diversity immediately to the north of the continent in Indonesia. Few species are endemic to Australia and there is a notable decrease in mangrove floristic diversity away from the tropics on both eastern and western Australian coastlines. Aridity is an important constraint on mangrove diversity and extent on the west coast (Duke et al. 1998). By contrast, saltmarsh floristic affinities are primarily Gondwanan, with strong similarities at family level with the saltmarsh flora of New Zealand, South Africa and temperate South America (Adam 1990; Boon et al. 2011). Saltmarsh diversity decreases towards the tropics, and the northern and southern halves of the continent, separated by 23 degrees latitude, form distinctive biogeographic provinces (Saintilan 2009). Often a feature of the landward intertidal zone, some coastal Melaleuca and Casuarina species are found across Australia and Malesia (e. g. Melaleuca cajuputi and Casuarina equisetifolia) (ABARES 2013) as well as some in New Caledonia, Papua New Guinea and Lord Howe Island (Boland et al. 2006; Brophy et al. 2013).
Intertidal areas are far larger in the tropical north due to high tidal ranges and the presence of large rivers depositing deltas and building floodplains into shallow continental shelves. Melaleuca or Casuarina-marsh-mangrove dynamics characterise the intertidal-supratidal zone which also commonly encounters hypersaline conditions during the dry season. Grassy flats, sedges and cyanobacterial “sabkha” flats are more commonly encountered than saltmarshes (Cowie et al. 2000). In the southern biogeographic provinces, coastlines are subject to high wave energy with intertidal environments constrained within estuaries behind sand barriers. The intertidal gradient here is largely characterised by saltmarsh-mangrove complexes with a transition from saltmarsh to Melaleuca or Casuarina near the supratidal zone (Rogers et al. 2017). Mean minimum daily temperature explains nearly 80% of variation in diversity of saltmarsh between the 36 coastal bioregions, with the smaller pockets of saltmarshes in southern estuaries supporting most of the 140 species recorded for the continent (Saintilan 2009; Boon et al. 2011).
This unusual sympatric distribution of mangrove, saltmarsh and Melaleuca or Casuarina across the continent, and their contrasting climatic and hydrological requirements, makes them an interesting example of climate change impacts. The contrasting latitudinal clines in diversity strongly related to temperature thresholds suggests that warming may affect species distribution and competitive advantage. For much of the continent, mangroves, saltmarsh/sabkha and Melaleuca/Casuarina form shore-normal zonation, with Melaleuca/Casuarina confined to areas above the mean high water and mangrove largely confined to areas below mean high water to approximate mean sea-level, diurnally inundated, in all but exceptionally wet climates (Bucher and Saenger 1994), though a landward mangrove fringe is common in tropical settings. The influence of sea-level rise on coastal wetlands, and the interaction of mangrove, saltmarsh and Melaleuca in particular, are best observed in wide coastal plains of gentle gradient, where vegetation communities are tightly controlled by tidal hydroperiod.
In this review, after a consideration of ecosystem services provided by Australian coastal wetlands, we present evidence that coastal wetlands in Australia are already showing the impacts of human-induced climate change. The distribution of mangrove, saltmarsh and Melaleuca/Casuarina along the Australian coastline is changing rapidly, often in ways contrary to longer-term trends through the Holocene and consistent with changes in atmospheric CO2, temperature and sea-level. These changes have implications for ecosystem services. For components of the natural environment, changes to the structure of coastal wetland communities have implications for available habitat that may already compromised by historic reclamation. The cultural dimensions of these changes are also profound, and we review recent work exploring Indigenous perspectives on the impacts of recent landscape change on biocultural values.
Ecosystem Services Associated with Australian Coastal Wetlands
Coastal wetlands perform a number of important ecosystem services in Australia, many in common with wetlands across the globe. These include shoreline protection from waves, and storms, nutrient and carbon cycling and storage, habitat provision for invertebrates, nekton, shorebirds and mammals, and cultural and recreational uses (Kelleway et al. 2017a). Here, we focus on the ecosystem services which are most important in Australia.
Migratory shorebirds utilise southern hemisphere summer feeding habitat in Australia prior to northern migration in the East-Asian Australasian flyway (Zharikov and Milton 2009; Spencer et al. 2009). Coastal wetlands form important roosting habitat for several species, and coastal saltmarsh in particular provides high tide and night-time roosting and secondary feeding habitat. Species preferentially selecting coastal saltmarsh for this purpose include the Sharp-Tailed Sandpiper (Calidris acuminata), the Eastern Curlew (Numenius madagascariensis), the Pacific Golden Plover (Pluvialis fulva) and the Red-necked Stint (Calidris ruficollis) (Spencer et al. 2009). Saltmarsh is an important feeding and breeding habitat for rare endemic species including the White Fronted Chat (Epthianura albifrons) and the critically endangered orange-bellied parrot (Neophema chrysogaster), which is confined to a small number of saltmarshes in Victoria (Mondon et al. 2009). Insectivorous microbats have also been observed feeding preferentially in saltmarshes (Gonsalves et al. 2013).
Fish, including several species of commercial importance, utilise mangrove and saltmarsh environments during high tides. During spring tide periods when the saltmarsh is inundated, fish actively feed on crab larvae released in high densities into the shallow inundating tidal water. These larvae form a particularly important component of the diet of the Ambassids (Ambassis jacksoniensis), the most numerous fish in the estuary. Stable isotope analysis has demonstrated a bottom-up trophic cascade from the saltmarsh crabs, through zooplanktivorous fishes, to commercially important species (Mazumder et al. 2011; Saintilan and Mazumder 2017). The importance of coastal mangrove and saltmarsh has also been highlighted in analysis of fisheries landing data, with the relative proportions of mangrove, saltmarsh and seagrass predicting the composition of fishing yields in multi-estuary comparisons (Saintilan 2004; Meynecke et al. 2008). For much of the tropical and subtropical north prawn harvest is associated with mangrove area (Loneragan et al. 1997; Vance et al. 2002).
Australia has the third largest extent of mangroves in the world, covering 11,500 km2, an estimated 13,825 km2 of saltmarsh/saltpan and 63,020 km2 of Melaleuca (Saintilan 2009; ABARES 2013). This represents an important store of “blue carbon” in contemporary and relict wetland soils. Estimates of coastal wetland carbon stores are heavily weighted towards more heavily populated areas, such as temperate southeast Australia where mangroves typically store 200–300 t of carbon belowground per hectare (Saintilan et al. 2013; Kelleway et al. 2016b); high by terrestrial forest standards but lower than equatorial mangrove systems (Donato et al. 2011). Further research is required to characterise and explain global variation in below-ground carbon stores in mangrove. Belowground carbon stocks in southern Australian saltmarshes are typically lower than adjacent mangroves, though stocks vary among geomorphic settings (Kelleway et al. 2016a; Macreadie et al. 2017). Carbon stores may be high beneath saltpan (sabkha) coastal plains in the tropical north, given the widespread occurrence of mangrove facies representing a prior “big swamp” phase in the early to mid-Holocene (Woodroffe et al. 1985). There is currently a paucity of information on coastal Melaleuca and Casuarina carbon stores. One published study on the range of carbon stored by all Australian Melaleucas lumped together (coastal and inland) suggested ecosystems stocks between 158 Mg C ha−1 and 286 Mg C ha−1 (Tran et al. 2013), though this was limited to the upper 30 cm of the soil profile and excludes belowground biomass. Subsequent work in Queensland Melaleuca woodland, open forest and closed forest has suggested that permanently inundated Melaleuca ecosystems from this region store 381 Mg C ha−1 on average, compared with non-inundated Melaleuca areas which store 278 Mg C ha−1 on average (Tran and Dargusch 2016).
Trends in Extent
Palaeo-stratigraphic work in both tropical and temperate SE Australia has suggested more extensive mangroves stands across the intertidal zone earlier in the Holocene than is evident today. During the latter phases of the postglacial marine transgression, mangroves colonised the flooded valleys of the Fitzroy, Ord, Daly and Alligator Rivers in tropical northern Australia (Thom et al. 1975; Woodroffe et al. 1985; Clark and Guppy 1988). Progressive infilling following high-stand led to the retreat of mangroves to channel fringes, and the development of expansive hypersaline flats and freshwater wetlands (Clark and Guppy 1988). At a smaller scale, mangrove peats are commonly encountered beneath saltmarsh in the estuaries of SE Australia, dating on the Hawkesbury River to 1200–1700 y BP, in Jervis Bay to 1300–1900 y BP (Saintilan and Hashimoto 1999; Saintilan and Wilton 2001) and Port Stephens to 750–1800 y BP (Kelleway et al. 2017b).
The upslope and head-ward expansion of mangroves and decline in Melaleuca fringes observed in recent decades in these same environments is therefore an interesting reversal of longer-term trends. Comparisons of earliest aerial photographic records with contemporary distributions document a consistent trend of saltmarsh being progressively replaced by mangrove from subtropical Queensland to the southernmost estuaries of the continent in Victoria and South Australia (Saintilan and Williams 1999; Rogers et al. 2005; Jupiter et al. 2007). Mangrove encroachment associated with an extension of tidal channel networks over successive decades has been observed in the coastal plains of Kakadu, the Gulf of Carpentaria, often co-incident with extensive dieback of floodplain fringe Melaleuca forest (Winn et al. 2006; Knighton et al. 1991; Mulrennan and Woodroffe 1998). Through both historical aerial photography analyses (Williams 1984; Bowman et al. 2011) and recent field based studies (Ens et al. 2017; Sloane 2017; Sloane et al., in review) the retreat of Melaleuca forests has largely been attributed to combinations of saltwater intrusion and the effects of feral buffalo. Williams (1984) found a 38% decline in Melaleuca forest from 1950 to 1975 in the Magela catchment while Bowman et al. (2011) found an overall 5% decline in Kakadu National Park from 1964 to 2004 noting spatial variability and greater declines at Melaleuca edges, on low-lying sites and where past buffalo activity was highest.
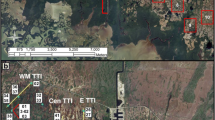
Conversely, mangroves expanded their range in the Kakadu region by 17% (Williamson et al. 2011). Similarly, across the Gulf of Carpentaria concurrent seaward and landward mangrove encroachment was observed for the period 1987–2014 (Asbridge et al. 2016) during a period of increased tidal inundation, higher freshwater flows and seasonal inundation of rivers during the monsoonal period. It is these same landward mangroves, primarily Avicennia marina, that were subject to widespread mortality during the 2015–16 summer, when lower than average rainfall coincided with regional declines in sea-level (Fig. 1). Indigenous Traditional Owners in north east Arnhem Land also suggested that feral ungulates (buffalo and pig), rising sea levels, king tides and cyclones are likely to becontributing factors of observed intertidal Melaleuca dieback (Fig. 1) (Sloane 2017; Sloane et al., in review).
Climate Change Impacts
Sea-Level and Rainfall
Climate and sea-level in Australia are subject to inter-annual and inter-decadal oscillations in oceanic and atmospheric circulation operating at hemispheric scales. Phases of the El Niño Southern Oscillation (Allen 1988; Verdon et al. 2004) and the Indian Ocean Dipole (Ummenhofer et al. 2009) exert strong influences on the timing and duration of periods of drought and flood across the continent. This modularity in prevailing conditions of atmospheric pressure and rainfall exerts a strong influence on inter-annual trends in sea-level. While the trajectory of sea-level rise across the continent has been rising at a rate comparable to global eustatic trends (White et al. 2014), there is a notable regional variability in the amplitude of sea-level trends and oscillations at annual and decadal time-scales. For example, the El Niño droughts of 2000–2008 dampened the rate of sea-level rise across the southern parts of the continent, but not in the tropical north where higher rainfall conditions prevailed.
Our understanding of the response of coastal wetlands to sea-level rise has been improved through use of the Surface Elevation Table-Marker Horizon technique (SET-MH). The elevation of coastal wetland surfaces responds dynamically to sea-level change, being influenced by the rate of sediment delivery and settlement, autotrophic plant productivity, and soil water volume. The SET-MH combines an elevation benchmark rod from which repeated measures of marsh surface elevation are taken, with a feldspar marker horizon recording surface sediment accretion. Together, the technique allows an assessment of the vertical response of a wetland to sea-level rise and the extent to which this response is a result of surface sediment accretion (Cahoon et al. 2002; Webb et al. 2013). The rapidly expanding network of sites globally has facilitated regional and international comparisons of wetland responses to sea-level rise and clarified processes controlling wetland elevation trajectory (e.g. Lovelock et al. 2015).
Within Australia several institutions have contributed to a network of SET-MH stations spanning temperate SE Australia, subtropical Queensland, and the tropical north and northwest of the continent (Fig. 2). In SE Australia, the SET-MH technique was deployed in 2000–2001 to help interpret the regional trend of mangrove encroachment into saltmarsh, and is amongst the longest-running SET networks in the world. Along some coastlines, phases of mangrove encroachment were associated with periods of higher rainfall and the freshening of the upper intertidal has been invoked as a driver of mangrove encroachment in SE Queensland (Eslami-Andargoli et al. 2009). However, as previously mentioned, rainfall and sea-level trends are tightly associated at timescales relevant to broad vegetation transitions, and are likely to have similar effects on mangrove propagule dispersal, survival and growth. The SET-MH installations in paired mangrove and saltmarsh environments have demonstrated a consistently lower rate of vertical elevation gain in saltmarsh compared to mangrove, and consistent elevation deficits in saltmarsh relative to local sea-level trends (Rogers et al. 2005, 2013, 2014a, b). Sea-level rise across the region is translating into a net competitive advantage of mangrove in saltmarsh environments regardless of rainfall trends. Patterns of encroachment into saltmarsh largely follow tidal drainage networks suggesting alterations to tidal hydro-period as an active mechanism promoting mangrove encroachment into saltmarsh.
The widespread use of the SET-MH method has also facilitated assessments of carbon sequestration associated with wetland responses to sea-level rise. The technique is conservative when used in this context, as new root growth below the feldspar horizon is not captured. Even so, estimates of below-ground carbon accumulation above feldspar horizons in mangroves in SE Australia in the range of 2–3 t per hectare per annum (Saintilan et al. 2013). Saltmarsh carbon accumulation is generally lower, especially in Sarcocornia-Sporobolus marshes (Kelleway et al. 2017b) though is consistently in the range of 2 t ha yr.−1 beneath Juncus kraussii-dominated marshes on fluvial deltas (Saintilan et al. 2013). Sedimentary methods have also emphasised the importance of geomorphic setting, and sediment grain size in particular, in predicting carbon store and accumulation in these wetlands (Kelleway et al. 2016a; Macreadie et al. 2017).
Temperature
Mangrove species distribution and diversity finds greatest expression in the tropics, with diversity decreasing steadily towards the poles on all coastlines. At the broadest scale, minimum sea-surface temperature of 20°C corresponds to poleward limits on most continents (Duke et al. 1998), though air temperatures and the occurrence of frost may regulate distributions on some coastlines. The effect of climate change on poleward range expansion of mangroves is a topic receiving considerable attention globally (Saintilan et al. 2014; Cavanaugh et al. 2015; Gabler et al. 2017).
There is little evidence in Australia for thermal tolerances playing an important role in defining the poleward limit of mangrove species. Mangroves, represented by Avicennia marina, are found south to Corner Inlet, Victoria (38.9°S), less than 30 km from Wilsons Promontory, the southernmost tip of the Australian continent. While mangroves are absent from Tasmania, this is thought to be due to dispersal constraints imposed by currents within Bass Strait, as A. marina seedlings have been observed to survive and grow in at least short-term plantings (~2 years) in locations along Tasmania’s east coast (Woodroffe and Grindrod 1991). The relatively benign thermal conditions at the southern limits of mangrove growth in Australia forms a stark contrast with the poleward limits of mangrove growth in the United States. Here, northward expanding populations are periodically checked by deep frosts causing widespread mortality (Stevens et al. 2006). Populations are recovering from freezing events, the most notable being 1989 when extensive mortality of mangroves occurred in Texas, Louisiana and Florida (Armitage et al. 2015; Giri and Long 2016).
Barriers to dispersal may also be important in defining the southern limit of individual species. Of the Rhizophoraceae, Rhizophora stylosa has the most poleward range, forming small but expanding clusters of individuals in many east coast estuaries as far south as South-West Rocks Creek in NSW (−30.88°S). Within these estuaries, the timing of leaf emergence and senescence, and the timing of propagule development and maturation are broadly comparable to tropical populations of the species (Wilson 2009; Wilson and Saintilan 2012). This contrasts to the growth characteristics of R. mangle on the US Atlantic coast, where rapidly expanding populations at the leading edge of the species range exhibit “precocious reproduction”, producing propagules on younger saplings than is observed within more established communities (Dangremond and Feller 2016).
These differences between controls on poleward range in North America and Australia can be understood in the context of continental-scale controls on extreme temperature fluctuation. Extreme cold conditions on the US Gulf Coast occur as a result of polar vortexes generated over the northern arctic tracking south across the North American land mass. In the southern hemisphere, the Southern Ocean provides a thermal buffer between extreme cold in Antarctica and the southern tips of the South American, New Zealand and Australian land masses. The southern limits of mangrove growth in Australia occur at higher latitudes than the northernmost mangroves in the US, in climates characterised by more frequent frost than the US Gulf coast but far less intense. Within Australia, Avicennia marina has evolved a higher degree of frost tolerance than has been possible for A. germinans or R. mangle in the US, where frost is less frequent but more intense (Stuart et al. 2007). A. marina and Aegiceras corniculatum, the two most poleward species in Australia, have smaller vessel diameters than A. germinans and R. mangle in the USA, with a consequent higher tolerance of mild freezing commonly encountered in temperate Australia (Stuart et al. 2007).
Mangrove populations in the Gulf and Florida coasts of the US therefore possess a latent capacity for rapid expansion in response to temperature change. Climate models project a decrease in the frequency and intensity of frost in the coming decades, removing the barrier to mangrove expansion into contiguous saltmarsh along sheltered, subtropical coastlines. The situation is different in Australia in several important respects. Mangrove species largely occupy discrete estuaries, and while long-shore currents flow predominantly southward on the east coast, threading the needle of estuarine entrances has proven to be an impediment to rapid response to climatic warming. Instead, populations appear to be expanding within their range, infilling saltmarsh within estuaries and, in the case of Rhizophora, backfilling into estuaries that may have previously been uninhabited (Wilson and Saintilan 2012). Observations of mangrove range expansion from Australia generally support the findings of range conservatism with respect to thermal limits noted elsewhere (Quisthoudt et al. 2012).
Unlike mangrove, saltmarsh diversity in Australia increases poleward, strongly correlating with monthly minimum temperatures (Saintilan 2009). The effect of cold temperatures on saltmarsh germination deserves further study, given the possibility that warming may inhibit germination and survival of some species (Greenwood and MacFarlane 2006). On the Australian east coast, a 2-degree warming would, on the basis of current mangrove and saltmarsh distribution, lead to the loss of several species finding their northern limit in Sydney region (e.g. Rhagodia baccata; Distichlis distichophylla, Austrastipa stipoides, Wilsonia backhausii: Fig. 3) and the expansion of Rhizophoraceae south to occupy the shorelines of estuaries. This modelling assumes unrestricted dispersal which we have indicated is unlikely to be the case, though human agents have facilitated poleward range expansion in other settings (e.g. south to the Nahoon estuary in South Africa: Hoppe-Speer et al. 2015).
Changes in the diversity of saltmarsh species with mean minimum temperate for bioregions on the Australian east coast. Blue lines represent the shift in mean minimum temperature from current to 2040 (an increase of 2 °C, averaged for winter months: Olson et al. 2016)
Distributional shifts in coastal Melaleuca and Casuarina have not been well studied and require further investigation. However, the biogeography of Melaleuca at the species level, at least in northern Australia, reflects a distinct pattern of local flooding and salinity tolerance (Cowie et al. 2000). For example, Melaleuca acacioides, occurs at the limit of Melaleuca spp. salinity tolerance in this region on the landward side of mangroves (Levitt 1981). Such is the link between Melaleuca and salinity, that the limits to salinity tolerance of Melaleuca spp. have been exploited in the Alligator Rivers Region of northern Australia, in which Melaleuca spp. dieback was utilised as a bioindicator of saltwater intrusion into previously predominantly freshwater wetlands (Bell et al. 2001). More recently, field-based assessment in north east Arnhem Land has correlated ~70% of Melaleuca dieback (across three species: M. cajuputi, M. viridiflora and M. acacioides) with soil salinity, feral pig (Sus scrofa) and feral buffalo (Bubalus bubalis) impacts; which was in strong alignment with explanations by local Aboriginal people (Sloane 2017; Sloane et al., in review).
Biophysical and Cultural Implications
The encroachment of mangrove into saltmarsh and Melaleuca forest has several important implications for the provision of habitat (Kelleway et al. 2017a). Species of migratory shorebirds occupying coastal saltmarsh prefer open roosting habitat, in particular shallow open pools useful for secondary foraging opportunities and thermal regulation (Spencer et al. 2009). Trees provide ambush hides for predatory birds (Dekker and Ydenberg 2004), which may be one reason Lawler (1998) noted a preference for saltmarsh habitat more than 30 m from tree-form vegetation. Mangrove encroachment therefore compromises the habitat value of saltmarsh for shorebirds (Guo et al. 2017).
Several species are known to inhabit coastal Melaleuca forest such as colonies of flying foxes (Pteropus scapulatus and Pteropus alecto) and the Northern Blossom Bat (Macroglossus minimus) (Tidemann et al. 1999; Finlayson et al. 2006); however again, more research is needed to document that habitat values of Melaleuca as well Casuarina, even in high profile locations such as the World Heritage Area Kakadu National Park (Finlayson et al. 2006). In northern Australia, the salinisation of Melaleuca forests is likely to have profound impacts on flora and fauna that inhabit these areas including Magpie Goose (Anseranus semipalmata) and the water chestnut Eleocharis spp. which also have great Indigenous cultural significance.
Relationships to plants and animals are important for emotional, social and physical wellbeing of Australian Indigenous people (Kingsley et al. 2010). Melaleuca species exemplify this relationship as they are valued by Indigenous people for a range of ethnobotanical reasons. One such important use is as bush medicine. Crushed leaves of many species can be placed in hot water and the resulting steam is inhaled to treat coughs, colds and nasal congestion. Alternatively, a wash made from the leaves and water can be applied to relieve skin irritation. Water from swellings in the trunk of some species can be utilised as a freshwater source. Bark can be used as blankets, for shelter or for carrying things. Wood of some of the larger species can be used to make canoes. Leaves of M. acacioides can flavour food or function as mosquito repellent when burned. Flowering of M. viridiflora can indicate good turtle hunting (Levitt 1981; Wrigley and Fagg 1993; Yunupiŋu et al. 1995; Winn 2001; Ens et al. 2017).
Recent research by (Sloane 2017; Sloane et al. in review) showed that Yolngu Traditional Owners of north east Arnhem land were very concerned about the loss of coastal swamps and patches of culturally significant coastal Melaleuca. For recent observations of decline, a Senior Traditional Owner and Senior Cultural Advisor for The Yirralka Rangers, Jimmy Wunungmurra said that people are hurting because the land (notably Melaleuca spp. and Eleocharis spp.) are being damaged by feral pigs, feral buffalo and saltwater intrusion. He had observed these feral animals creating erosion channels and trampling which facilitated intrusion of saltwater and movement of mangrove propagules further toward the landward side of the floodplain than he had observed in the past. He was wondering how to bring the land “back to life”. Therefore, the potential cultural impacts of the conversion of Melaleuca to mangrove forest warrants further study.
The cultural and commercial implications of mangrove encroachment in saltmarshes is also not clear. The production of crab zoea also occurs in the mangrove, and so may not be compromised by mangrove encroachment (Saintilan and Mazumder 2017). Indeed, Moussalli and Connolly (1998) found that fish diversity was higher in mangrove than in saltmarsh at comparable hydroperiod in SE Queensland. There is some evidence that saltmarsh area may be important in providing habitat for the commercially important mud crab Scylla serrata, though predictive models suggest both mangrove and saltmarsh are important (Saintilan 2004). This latter point highlights the potential significance of habitat connectivity and diversity in meeting species habitat requirements, rather than saltmarsh or mangrove alone. Fish within the estuary utilise multiple habitats at different stages of the tidal cycle (Saintilan et al. 2007), and microbat utilisation of saltmarsh habitat may depend on the mangrove-saltmarsh boundary, against which insects are concentrated (Gonsalves et al. 2012). Bats appear to use echo-location in the open habitat to identify insects in front of the mangroves.
Given the general trend of higher carbon storage and accumulation rates in Australian mangrove relative to saltmarsh, we expect encroachment by mangrove may have a beneficial carbon sequestration outcome. To date, only one study has directly quantified this in SE Australia, showing significant increases in both aboveground biomass and belowground (roots, rhizomes and soil) carbon pools with mangrove encroachment (Kelleway et al. 2016b). Importantly, these changes were not apparent until after >30 years of encroachment, and were larger belowground, than aboveground. That is, annual increases in belowground carbon storage of up to 2.30 Mg C ha−1 y−1 were reported over the entire 70-year study sequence, compared to increases equating to 0.58 Mg C ha−1 y−1 in aboveground carbon pools (Kelleway et al. 2016b). The recent dieback of Melaleuca and encroachment of mangroves, especially in northern Australia, warrants further investigation.
Policy Directions and Conclusions
By global standards Australian coastal wetlands enjoy strong legislative protection in most states and territories (Rogers et al. 2016). At a National level, the Environment Protection and Biodiversity Conservation Act 1999 affords protection of Ramsar sites (19 of which contain mangrove and/or saltmarsh in Australia), and the coastal salt marsh ecological community south of 23 degrees latitude. Most Australian states protect coastal wetland vegetation under legislation targeting fish habitat (e.g. Fisheries Management Act NSW 1994; Fisheries Act Qld 1994; Fisheries Management Act South Australia 2007; Fisheries Resource Management Act Western Australia 1994), or special area protection (State Environmental Planning Policy (SEPP) 14 NSW, Marine Estate Management Act NSW 1974; Marine Parks Act Qld 2004; Flora and Fauna Guarantee Act Vic 1988; Nature Conservation Act (Tas.) 2002 etc.). The cultural significance of the intertidal zone is also starting to be recognised through Native Title legislation and Australia’s Indigenous Protected Area system, although much more work needs to be done to fully acknowledge Indigenous rights and values in these ecosystems. These legislative and policy initiatives were implemented to arrest the widespread clearance and reclamation of coastal wetlands primarily in eastern states in the twentieth Century. Flood control works on large east coast rivers in NSW and Queensland led to substantial losses of coastal wetlands in the 1950’s and 1960’s. Though the extent of historic losses is difficult to quantify, as many as 4200 structures have been identified impeding tidal flow in NSW alone (Williams and Watford 1997).
While this legislative and policy framework has been effective in preventing further net decline in coastal wetlands in eastern Australia, they may not be sufficient to address the emerging threat of climate change across the nation. A suite of climate change drivers, potentially including elevated CO2, temperature and sea-level, are altering the composition of coastal wetlands in important ways, and while coastal wetland area has stabilised in eastern Australia, the decline of coastal saltmarsh has continued unabated. Modelling of coastal wetland responses to projected sea-level rise has demonstrated the importance of migration pathways to facilitate mangrove and saltmarsh colonisation of coastal lowlands, even factoring in vertical elevation gains documented by the SET network (Rogers et al. 2014a; b). Many of the policy instruments identify wetlands as discrete mapped locations, an approach that while providing unequivocal identification of the protected asset, has the disadvantage of growing obsolescence in dynamically evolving landscapes. In particular, “coastal squeeze”, where by landward encroaching wetlands meet hard human infrastructure barriers, is not currently addressed within the delineation approach of Ramsar or local planning controls such as SEPP14 in NSW (Rogers et al. 2014b; Rogers et al. 2016).
While coastal wetland protection in Australia has focussed on the fisheries and biodiversity values of wetlands, consideration is currently being given to carbon sequestration (blue carbon) and cultural values as a tool for promoting wetland preservation and climate change mitigation in Australia. Wetland conservation and restoration activities are relevant to National Greenhouse Gas inventory reporting (now possible under the IPCC wetlands supplement guidelines; Hiraishi et al. 2014), as well as investment through carbon mitigation activities. Australia has in recent years moved away from emissions trading towards direct investment by government through the Emissions Reduction Fund and Direct Action programs. While these have largely focussed on terrestrial carbon sequestration and emission reductions in agricultural and industrial sectors, blue carbon opportunities are being actively scoped. Until blue carbon methodologies are included under national carbon mitigation policies, voluntary carbon market opportunities will offer the best prospect of economic incentive for wetland preservation, restoration and creation.
One of the attractive features of blue carbon is the potential economic incentive provided to tidal wetland reinstatement and wetland encroachment in private and Indigenous landholdings. Further research is required to quantify these benefits and possible hydrological challenges (see Rodríguez et al. 2017) and further policy work required to facilitate market-based approaches to carbon trading in Australia. However, it is likely that the true value of coastal wetlands, encompassing carbon sequestration, fisheries value, biodiversity conservation, cultural values and shoreline protection will need to be recognised to allow their preservation in coastal lowlands facing the challenge of retreat. Ongoing preservation will therefore require public-private partnerships, incorporating the perspectives and values of Indigenous communities, agriculturalists, local communities and fisheries.
References
ABARES (2013) Australia’s state of the forests report 2013. Australian Bureau of Agricultural and Resource Economics and Sciences, Canberra
Adam P (1990) Saltmarsh ecology. Cambridge University Press, Cambridge
Allen RJ (1988) El Nino southern oscillation influences in the Australasian region. Prog Phys Geogr 12:313–348
Armitage AR, Highfield WE, Brody SD, Louchouarn P (2015) The contribution of mangrove expansion to salt marsh loss on the Texas Gulf Coast. PLoS One 10(5):e0125404
Asbridge E, Lucas R, Ticehurst C, Bunting P (2016) Mangrove response to environmental change in Australia's gulf of Carpentaria. Ecology and Evolution 6(11):3523–3539
Bell D, Menges CH, Bartolo RE (2001) Assessing the extent of saltwater intrusion in a tropical coastal environment using radar and optical remote sensing. Geocarto International 16:45–52
Boland DJ, Brooker MIH, Chippendale GM, Hall N, Hyland BPM, Johnston RD, Kleinig DA, McDonald MW, Turner JD (2006) Forest trees of Australia. CSIRO publishing, Melbourne
Boon PI, Allen T, Brook J, Carr G, Frood D, Harty C, Hoye J, McMahon A, Mathews S, Rosengren N, Sinclair S, White M, Yugovic J (2011) Mangroves and coastal saltmarsh of Victoria: distribution, condition, threats and management. Institute for Sustainability and Innovation, Victoria University, Melbourne
Bowman DMJS, Prior LD, De Little SC (2011) Retreating melaleuca swamp forests in Kakadu National Park: evidence of synergistic effects of climate change and past feral buffalo impacts. Austral Ecology 35:898–905
Brophy JJ, Craven, LA Doran JC (2013) Melaleucas: their botany, essential oils and uses. ACIAR, Canberra
Bucher D, Saenger P (1994) A classification of tropical and subtropical Australian estuaries. Aquat Conserv Mar Freshwat Ecosyst 4(1):1–19
Cahoon DR, Lynch JC, Perez BC, Segura B, Holland RD, Stelly C et al (2002) High-precision measurements of wetland sediment elevation: II. The rod surface elevation table. J Sediment Res 72(5):734–739
Cavanaugh KC, Parker JD, Cook-Patton SC, Feller IC, Williams AP, Kellner JR (2015) Integrating physiological threshold experiments with climate modeling to project mangrove species’ range expansion. Glob Chang Biol 21(5):1928–1938
Clark RL, Guppy JC (1988) A transition from mangrove forest to freshwater wetland in the monsoon tropics of Australia. J Biogeogr 15:665–684
Cowie ID, Short PS, Ostercamp Madsen M (2000) Floodplain Flora: a flora of the coastal floodplains of the northern territory. Australia, ABRS, Canberra/PWCNT, Darwin
Dangremond EM, Feller IC (2016) Precocious reproduction increases at the leading edge of a mangrove range expansion. Ecology and Evolution 6(14):5087–5092
Dekker D, Ydenberg R (2004) Raptor predation on wintering dunlins in relation to the tidal cycle. Condor 106:415–419
Donato DC, Kauffman JB, Murdiyarso D, Kurnianto S, Stidham M, Kanninen M (2011) Mangroves among the most carbon-rich forests in the tropics. Nat Geosci 4(5):293–297
Duke NC, Ball MC, Ellison JC (1998) Factors influencing biodiversity and distributional gradients in mangroves. Glob Ecol Biogeogr Lett 7:27–47
Ens EJ, Bentley-Toon S, Campion F, Campion S, Kelly J, Towler G (2017) Rapid appraisal links feral buffalo with kunkod (melaleuca spp.) decline in freshwater billabongs of tropical northern Australia. Mar Freshw Res 68:1642–1652
Eslami-Andargoli L, Dale PER, Sipe N, Chaseling J (2009) Mangrove expansion and rainfall patterns in Moreton Bay, southeast Queensland, Australia. Estuar Coast Shelf Sci 85(2):292–298
Finlayson C, Lowry J, Bellio M, Nou S, Pidgeon R, Walden D, Humphrey C, Fox G (2006) Biodiversity of the wetlands of the Kakadu region, northern Australia. Aquatic Sciences - Research Across Boundaries 68:374–399
Gabler CA, Osland MJ, Grace JB, Stagg CL, Day RH, Hartley SB, Enwright NM, From AS, McCoy ML, McLeod JL (2017) Macroclimatic change expected to transform coastal wetland ecosystems this century. Nat Clim Chang 7:142–147
Giri C, Long J (2016) Is the geographic range of mangrove forests in the conterminous United States really expanding? Sensors 16(12):2010
Gonsalves L, Law B, Webb C, Monamy V (2012) Are vegetation interfaces important to foraging insectivorous bats in endangered coastal saltmarsh on the central coast of new South Wales? Pac Conserv Biol 18(4):282–292
Gonsalves L, Law B, Webb C, Monamy V (2013) Foraging ranges of insectivorous bats shift relative to changes in mosquito abundance. PLoS One 8(5):e64081
Greenwood ME, MacFarlane GR (2006) Effects of salinity and temperature on the germination of Phragmites Australis, Juncus Kraussii, and Juncus Acutus: implications for estuarine restoration initiatives. Wetlands 26:854–861
Guo H, Weaver C, Charles S, Whitt A, Dastidar S, D'Odorico P, Fuentes JD, Kominoski JE, Armitage AR, Pennings SC (2017) Coastal regime shifts: rapid responses of coastal wetlands to changes in mangrove cover. Ecology 98:762–772
Hiraishi T, Krug T, Tanabe K, Srivastava N, Baasansuren J, Fukuda M, Troxler TG (2014) 2013 supplement to the 2006 IPCC guidelines for national greenhouse gas inventories: wetlands. IPCC, Switzerland
Hoppe-Speer SC, Adams JB, Rajkaran A (2015) Mangrove expansion and population structure at a planted site, East London, South Africa. Southern Forests: a Journal of Forest Science 77(2):131–139
Jupiter SD, Potts DC, Phinn SR, Duke NC (2007) Natural and anthropogenic changes to mangrove distributions in the Pioneer River estuary (QLD, Australia). Wetl Ecol Manag 15(1):51–62
Kelleway JJ, Saintilan N, Macreadie PI, Ralph PJ (2016a) Sedimentary factors are key predictors of carbon storage in SE Australian saltmarshes. Ecosystems 19:865–880
Kelleway JJ, Saintilan N, Macreadie PI, Skilbeck CG, Zawadzki A, Ralph PJ (2016b) Seventy years of continuous encroachment substantially increases ‘blue carbon’ capacity as mangroves replace intertidal salt marshes. Glob Chang Biol 22:1097–1109
Kelleway JJ, Cavanaugh K, Rogers K, Feller IC, Ens E, Doughty C, Saintilan N (2017a) Review of the ecosystem service implications of mangrove encroachment into salt marshes. Glob Chang Biol 23:3967–3983
Kelleway JJ, Saintilan N, Macreadie P, Baldock J, Heijnis H, Zawadzki A, Gadd P, Jacobsen G, Ralph PJ (2017b) Importance of environmental history to blue carbon stocks revealed by geochemical analyses. J Geophys Res Biogeosci 122:1789–1805
Kingsley JY, Phillips R, Townsend M, Henderson-Wilson C (2010) Using a qualitative approach to research to build trust between a non-aboriginal researcher and aboriginal participants (Australia). Qual Res J 10:2–12
Knighton AD, Mills K, Woodroffe CD (1991) Tidal-creek extension and saltwater intrusion in northern Australia. Geology 19:831–834
Lawler W (1998) Guidelines for management of migratory shorebird habitat in southern east coast estuaries, Australia. University of New England
Levitt DL (1981) Plants and people: aboriginal uses of plants on Groote Eyelandt. Canberra, Australian Institute of Aboriginal Studies
Loneragan NR, Bunn SE, Kellaway DM (1997) Are mangroves and seagrasses sources of organic carbon for penaeid prawns in a tropical Australian estuary? A multiple stable-isotope study. Mar Biol 130:289–300
Lovelock CE, Cahoon DR, Friess DA, Guntenspergen GR, Krauss KW, Reef R et al (2015) The vulnerability of indo-Pacific mangrove forests to sea-level rise. Nature 526:559–563
Macreadie PI, Ollivier QR, Kelleway JJ, Serrano O, Carnell PE, Ewers Lewis CJ, Atwood TB, Sanderman J, Baldock J, Connolly RM, Duarte CM, Lavery PS, Steven A, Lovelock CE (2017) Carbon sequestration by Australian tidal marshes. Sci Rep 7:44071
Mazumder D, Saintilan N, Williams RJ, Szymczak R (2011) Trophic importance of a temperate intertidal wetland to resident and itinerant taxa: evidence from multiple stable isotope analyses. Mar Freshw Res 62:11–19
Meynecke JO, Lee SY, Duke NC (2008) Linking spatial metrics and fish catch reveals the importance of coastal wetland connectivity to inshore fisheries in Queensland, Australia. Biol Conserv 141(4):981–996. https://doi.org/10.1016/j.biocon.2008.01.018
Mondon J, Morrison K, Wallis R (2009) Impact of saltmarsh disturbance on seed quality of sarcocornia (Sarcocornia Quinqueflora), a food plant of an endangered Australian parrot. Ecological management & restoration 10(1):58–60
Moussalli A, Connolly RM (1998) Fish use of the inundated waters of a subtropical saltmarsh-mangrove complex in Southeast Queensland, Australia. Short communication In: Tibbetts, IR., Hall, NJ. & Dennison, WD (eds) Moreton Bay and Catchment. School of Marine Science, The University of Queensland, Brisbane, 471-472
Mulrennan ME, Woodroffe CD (1998) Saltwater intrusion into the coastal plains of the lower Mary River, northern territory, Australia. J Environ Manag 54:169–188
Olson R, Evans JP, Di Luca A, Argüeso D (2016) The NARCliM project: model agreement and significance of climate projections. Clim Res 69(3):209–227
Quisthoudt K, Schmitz N, Randin CF, Dahdouh-Guebas F, Robert EM, Koedam N (2012) Temperature variation among mangrove latitudinal range limits worldwide. Trees 26(6):1919–1931
Rodríguez JF, Saco PM, Sandi S, Saintilan N, Riccardi G (2017) Potential increase in coastal wetland vulnerability to sea-level rise suggested by considering hydrodynamic attenuation effects. Nat Commun 8:16094
Rogers K, Saintilan N, Heijnis H (2005) Monitoring of mangrove and saltmarsh resources in Westernport Bay, Australia. Estuaries 28:551–559
Rogers K, Saintilan N, Howe AJ, Rodríguez JF (2013) Sedimentation, elevation and marsh evolution in a southeastern Australian estuary during changing climatic conditions. Estuar Coast Shelf Sci 133:172–181
Rogers K, Saintilan N, Woodroffe CD (2014a) Surface elevation change and vegetation distribution dynamics in a subtropical coastal wetland: implications for coastal wetland response to climate change. Estuar Coast Shelf Sci 149:46–56
Rogers K, Saintilan N, Copeland C (2014b) Managed retreat of saline coastal wetlands: challenges and opportunities identified from the Hunter River estuary, Australia. Estuar Coasts 37(1):67–78
Rogers K, Boon PI, Branigan S, Duke NC, Field CD, Fitzsimons JA, Kirkman H, Mackenzie JR, Saintilan N (2016) The state of legislation and policy protecting Australia's mangrove and salt marsh and their ecosystem services. Mar Policy 72:139–155
Rogers K, Boon P, Lovelock C, Saintilan N (2017) Coastal halophytic vegetation. In: Vegetation A (ed) D. A. Keith. Cambridge University Press, New York
Saintilan N (2004) Relationships between estuarine geomorphology, wetland extent and fish landings in new South Wales estuaries. Estuar Coast Shelf Sci 61(4):591–601. https://doi.org/10.1016/j.ecss.2004.07.002
Saintilan N (2009) Biogeography of Australian saltmarsh plants. Austral Ecology 34:929–937
Saintilan N, Hashimoto R (1999) Mangrove-saltmarsh dynamics on a prograding bayhead delta in the Hawkesbury River estuary, new South Wales, Australia. Hydrobiologia 413:95–102
Saintilan N, Mazumder D (2017) Mass spawning of crabs: ecological implications in subtropical Australia. Hydrobiologia 803(1):239–250
Saintilan N, Williams RJ (1999) Mangrove transgression into saltmarsh environments in south-east Australia. Glob Ecol Biogeogr Lett 8:117–124
Saintilan N, Wilton K (2001) Changes in the distribution of mangroves and saltmarshes in Jervis Bay, Australia. Wetl Ecol Manag 9:409–420
Saintilan N, Hossain K, Mazumder D (2007) Linkages between seagrass, mangrove and saltmarsh as fish habitat in the Botany Bay estuary, new South Wales. Wetl Ecol Manag 15:277–286
Saintilan N, Rogers K, Mazumder D, Woodroffe C (2013) Allochthonous and autochthonous contributions to carbon accumulation and carbon store in southeastern Australian coastal wetlands. Estuar Coast Shelf Sci 128:84–92
Saintilan N, Wilson NC, Rogers K, Rajkaran A, Krauss KW (2014) Mangrove expansion and salt marsh decline at mangrove poleward limits. Glob Chang Biol 20:147–157
Sloane DR (2017) An eco-cultural investigation of melaleuca spp. dieback in north-east Arnhem land, Australia. Master of Research, Macquarie University
Spencer J, Monamy V, Breitfuss M (2009) Saltmarsh as habitat for birds and other vertebrates. In: Saintilan N (ed) Australian saltmarsh ecology. CSIRO Publishing, Collingwood, pp 149–165
Stevens PW, Fox SL, Montague CL (2006) The interplay between mangroves and saltmarshes at the transition between temperate and subtropical climate in Florida. Wetl Ecol Manag 14:435–444
Stuart SA, Choat B, Martin KC, Holbrook NM, Ball MC (2007) The role of freezing in setting the latitudinal limits of mangrove forests. New Phytol 173:576–583
Thom BG, Wright LD, Coleman JM (1975) Mangrove ecology and deltaic-estuarine geomorphology, Cambridge gulf-Ord River, Western Australia. J Ecol 63:203–222
Tidemann CR, Vardon MJ, Loughland RA, Brocklehurst PJ (1999) Dry season camps of flying-foxes (Pteropus spp.) in Kakadu world heritage area, north Australia. J Zool 247:155–163
Tran DB, Dargusch P (2016) Melaleuca forests in Australia have globally significant carbon stocks. For Ecol Manag 375:230–237
Tran DB, Dargusch P, Herbohn J, Moss P (2013) Interventions to better manage the carbon stocks in Australian melaleuca forests. Land Use Policy 35:417–420
Ummenhofer CC, England MH, McIntosh PC, Meyers GA, Pook MJ, Risbey JS, Gupta AS, Taschetto AS (2009) What causes southeast Australia's worst droughts? Geophys Res Lett 36:L04706
Vance DJ, Haywood MDE, Heales DS, Kenyon RA, Loneragan NR, Pendrey RC (2002) Distribution of juvenile penaeid prawns in mangrove forests in a tropical Australian estuary, with particular reference to Penaeus Merguiensis. Mar Ecol Prog Ser 228:165–177
Verdon DC, Wyatt AM, Kiem AS, Franks SW (2004) Multidecadal variability of rainfall and streamflow: eastern Australia. Water Resources Research 40:1–8
Webb EL, Friess DA, Krauss KW, Cahoon DR, Guntenspergen GR, Phelps J (2013) A global standard for monitoring coastal wetland vulnerability to accelerated sea-level rise. Nat Clim Chang 3(5):458–465
White NJ, Haigh ID, Church JA, Koen T, Watson CS, Pritchard TR et al (2014) Australian sea levels—trends, regional variability and influencing factors. Earth Sci Rev 136:155–174
Williams AR (1984) Changes in melaleuca forest density on the Magela floodplain, northern territory, between 1950 and 1975. Aust J Ecol 9:199–202
Williams RJ, Watford FA (1997) Identification of structures restricting tidal flow in new South Wales, Australia. Wetl Ecol Manag 5(1):87–97
Williamson GJ, Boggs GS, Bowman DM (2011) Late 20th century mangrove encroachment in the coastal Australian monsoon tropics parallels the regional increase in woody biomass. Reg Environ Chang 11(1):19–27
Wilson NC (2009) The distribution, growth and population genetics of a mangrove species Rhizophora stylosa in NSW, Australia. PhD Thesis, School of Arts and Sciences, Australian Catholic University
Wilson NC, Saintilan N (2012) Growth of the mangrove species Rhizophora Stylosa Griff. At its southern latitudinal limit in eastern Australia. Aquat Bot 101:8–17
Winn KO (2001) Saltwater intrusion and morphological change at Point Farewell, Alligator Rivers region. BSc (Hons) Honours Thesis, The University of Western Australia
Winn KO, Saynor MJ, Eliot MJ, Eliot I (2006) Saltwater intrusion and morphologicvla change at the mouth of the east Alligator River, northern territory. J Coast Res 22:137–149
Woodroffe CD, Grindrod J (1991) Mangrove biogeography: the role of quaternary environmental and sea-level change. J Biogeogr 18:479–492
Woodroffe CD, Thom BG, Chappell J (1985) Development of widespread mangrove swamps in mid-Holocene times in northern Australia. Nature 317(6039):711–713
Wrigley JW, Fagg M (1993) Bottlebrushes, paperbarks & tea trees: and other plants in the leptospermum alliance. Angus & Robertson, Sydney
Yunupiŋu B, Yunupiŋu-Marika L, Marika D, Marika B, Marika B, Marika R, Wightman G (1995) Rirratjiiŋu ethnobotany: aboriginal plant use from Yirrkala, Arnhem land, Australia. Parks and Wildlife Commission of the Northern Territory, Palmerston
Zharikov Y, Milton DA (2009) Valuing coastal habitats: predicting high-tide roosts of non- breeding migratory shorebirds from landscape composition. Emu 109:107–120
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saintilan, N., Rogers, K., Kelleway, J.J. et al. Climate Change Impacts on the Coastal Wetlands of Australia. Wetlands 39, 1145–1154 (2019). https://doi.org/10.1007/s13157-018-1016-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-018-1016-7