Abstract
We investigated the variation in macroinvertebrate and bird fauna of 12 different freshwater wetlands located in three different climatic zones of southwestern Ethiopia. Data on macroinvertebrates, birds, physico-chemical water quality variables, human disturbance and vegetation cover were collected from 62 sampling sites during the dry and wet season of 2015. Generalized linear mixed models (GLMMs) were used to identify the most important variables explaining the variation in macroinvertebrates and birds. Twenty four percent of the variation in macroinvertebrate richness was explained by a combination of vegetation cover and dissolved oxygen, whereas 34% of the variation in macroinvertebrate abundance was explained by a combination of dissolved oxygen saturation, electric conductivity, total phosphorus and vegetation cover. A combination of water depth, dissolved oxygen, human disturbance and macroinvertebrate abundance explained about 34% and 31% of variation in bird species richness and abundance, respectively. Richness and abundance of macroinvertebrate and wetland dependent birds were significantly (P < 0.05) different between wetlands. When investigating the ecological status of wetlands, local environmental conditions of wetland should be taken into account for the development of wetland conservation strategies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wetlands are among the most productive ecosystems on earth and provide many ecological services and socio-economic benefits (Keddy et al. 2009; Lavoie et al. 2016). Wetlands are responsible for water quality regulation (Daneshvar et al. 2017), climate change mitigation (Roulet 2000) and ground water recharge (Hayashi et al. 2003). An important contribution of wetlands to the livelihood of people and many functions in agriculture such as livestock grazing, water supply and wood production have been reported (Schuyt 2005; Getachew et al. 2012). Furthermore, wetlands are considered as biodiversity hotspots and provide habitat for numerous species of waterfowl (Beatty et al. 2014), macroinvertebrates (Balcombe et al. 2005) and fish (Clavero et al. 2015).
Despite their high ecosystem and economic value, wetlands and their associated species have been rapidly declining during the past decades due to anthropogenic disturbances (Millennium Ecosystem Assessment 2005). Around 64–71% of the global wetland area has disappeared during the 20th and early twenty-first century (Davidson 2014). Agriculture is considered one of the main causes for gradual loss of wetlands since the 1950s (Teferi et al. 2010; Davidson 2014). One of the countries in sub-Saharan Africa that suffers from continuous degradation and loss of wetlands due to anthropogenic activities is Ethiopia (Gebresllassie et al. 2014). As a result, the species richness, abundance and distribution of wetland biological communities is highly affected in different parts of the country (Abebe and Geheb 2003; Mooney et al. 2009; Getachew et al. 2012).
Ethiopia is a country harbouring many different habitats including enormous wetland ecosystems and is divided in 11 climatic zones based on Koeppen’s classification method (Gonfa 1996; Abebe and Geheb 2003). Except coastal and marine related wetlands and extensive swamp-forest complexes, all types of wetlands are found in Ethiopia including alpine formations, riverine, lacustrine and palustrine wetlands (Abebe and Geheb 2003). A large number of freshwater wetlands are found in southwestern Ethiopia, which support a high number of bird species and macroinvertebrates (Mereta et al. 2013). However, these wetlands are prone to degradation due to over grazing, clay mining, disposal of domestic sewage and farming (Mereta et al. 2013).
Wetland biological communities can be used to assess wetland conditions and to develop appropriate wetland conservation methods (Seilheimer et al. 2009). Various studies have been conducted using different biological communities such as macroinvertebrates, fish or birds (Green and Figuerola 2005; Larsen et al. 2012; Guareschi et al. 2015). Macroinvertebrates and birds are considered major bio-indicators of ecosystem health (Reid et al. 2014; Gebrehiwot et al. 2017; Gezie et al. 2017). Macroinvertebrates are used in wetland bio-assessment because of their short life cycle, their influence on nutrient and energy flows and their response to oxygen availability and habitat structure (Covich et al. 1999; Steinman et al. 2003; Saloom and Duncan 2016). Wetland birds are also considered as important ecological indicators as they are sensitive to environmental change, can be sampled easily and their taxonomy is well-known (Timothy et al. 2000; Gregory et al. 2003; Herrando et al. 2014). Moreover, macroinvertebrates and wetland birds are interacting with each other for example via the trophic web structure (Tomankova et al. 2014) or via dispersal interactions (Green and Figuerola 2005).
It is reported that local environmental conditions and human disturbance structure biodiversity of freshwater ecosystems (Al-shami et al. 2013; Heino 2013; Hawkins et al. 2015). In addition to local environmental conditions, temporal variability such as season and regional (spatial) determinants such as dispersal, geology and climate play a role in the biodiversity patterns observed in freshwater biological communities (Dolédeca et al. 2017; Baselga et al. 2012). An in-depth investigation of wetland biodiversity across climatic zones plays a crucial role for effective conservation of wetlands and its biodiversity (Erwin 2009). It has been reported that climatic conditions of wetlands affect the ecosystem structure and functioning through a change in precipitation and temperature (Erwin 2009; Ruhí et al. 2013). However, previous studies conducted on wetlands found in south-western Ethiopia, have been focusing on the development of biological indices (Yimer and Mengistou 2009; Mereta et al. 2013), on environmental characterization and composition of macroinvertebrate communities (Mereta et al. 2012) and on the perception of humans to wetland management (Moges et al. 2016). To our knowledge, no studies are present on how the composition of macroinvertebrates and birds vary between different climatic zones and how human disturbance interferes with the natural variation of wetlands in southwestern Ethiopia. Albeit interesting and important, using multiple biological communities to assess the status of wetland ecosystems has only been performed for the north-eastern part of the country (Getachew et al. 2012). The aim of this study was (1) to investigate the spatial differences in macroinvertebrate and bird fauna of wetlands located in three different climatic zones of southwestern Ethiopia and (2) to identify important environmental variables affecting these biological communities. To investigate this, we selected wetlands situated in a tropical rainforest climate, a warm temperate rainy climate and a tropical rainy climate based on the climatic classification of Ethiopia as reported by Gonfa (1996). We expected differences in diversity and abundance of macroinvertebrates and wetland birds due to climatic differences and varying local environmental conditions. We also expected differences in species richness and abundance of macroinvertebrates and birds between different wetlands. The results of this study can help to assess the status of wetlands in Ethiopia and therefore can be used to support the conservation and management of wetlands in an understudied area.
Materials and Methods
Study Area
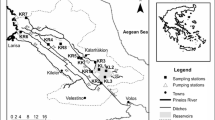
This study was carried out in wetlands located in the southwestern part of Ethiopia in three different climatic zones. Several wetlands situated in a warm temperate rainy climate, a tropical rainforest climate and a tropical rainy climate were selected (Fig. 1). In the warm temperate rainy climate, six different wetlands were investigated: Awetu; Boye; Kofe; Bulbul; Kito and Haro, which are located in the Gilgel Gibe watershed situated between latitudes 7°37’ N and 7°45’ N and longitudes 36°47′ E and 37°15′ E. All of these wetlands are freshwater wetlands according to the wetland classification of Cowardin et al. (1979). Four of them, however, are totally covered by vegetation (Awetu; Boye; Kofe and Kito), whereas Bulbul and Haro are wetlands characterized by open water (Mereta et al. 2013). The average annual temperature of the area ranges between 22 and 32 °C (National Meteorological Agency 2015). The mean annual precipitation ranges between 1000 mm and 2800 mm (Gonfa 1996; National Meteorological Agency 2015). The dominant soil types in this region are Nitisols, Acrisols, Ferralsols, Vertisols and Planosols (Van Ranst et al. 2011). The altitude in the catchment varies between 1096 and 3259 m a.s.l.. The land use includes cultivation, clay mining, plantation, disposal of domestic sewage and grazing (Table 1). In the tropical rainforest climate, five wetlands were sampled: Shimbira; Alemgono; Afala; Ottra and Nech Wuha which are situated between 7°19’ N and 07°24’ N and 36°12′ E and 36°14′ E. All selected wetlands in this climatic zone are classified as freshwater wetlands and they are almost totally covered by vegetation. According to Gamachu (1977) this region is the most humid part of the country, with only two to four dry months per the year. The area has an average annual rainfall of 1200–2800 mm and mean temperature of 19.4 °C (Gonfa 1996; National Meteorological Agency 2015). The region is characterized by Afromontane cloud forests and rainforests, which contain wild Coffee Arabica, bamboo forests, grasslands and shrublands (NABU 2015). The dominant soil types occurring in this area are dystric nitisols (WBISPP 2004). The altitude in this region varies between 1500 and 2600 m a.s.l.. Chalalaki wetland, which is found in tropical rainy climate, is situated between latitude 08°14’ N and longitude 37° 01′ E and is characterized as a freshwater wetland with open water. The mean temperature in this region is 26 °C and the annual rainfall ranges between 680 mm and 2000 mm (Gonfa 1996; National Meteorological Agency 2015). The dominant soil type in this region is Chromic, Pellic, Vertisols, Orthic Acrisols and Dystric Nitisols (Tegegn 2017). The altitude ranges between1573–1577 m a.s.l.. The main land use practice around this wetland is grazing. A summary of the human impact, climatic condition, size and number of sampling locations monitored in the studied wetlands can be found in Table 1.
Macroinvertebrate Sampling and Identification
Macroinvertebrate samples were collected twice from a total of 62 sampling locations, once during January to April 2015 (dry season) and once during September to November 2015 (wet season). The sampling locations were selected based on previous research, the distance between sites, the level of human disturbance and based on the confluence of rivers. Equal sampling effort was ensured in all sites by allotting proportional time to cover different meso-habitats such as open water or emergent vegetation (Mereta et al. 2013). A rectangular frame net (20 × 30 cm) with a mesh size of 300 μm was used to sample for 10 min along a 10 m stretch. The net was swept while the bottom sediment was disturbed by foot in order to collect the benthic macroinvertebrates. Then the sample was emptied into a rectangular tray in order to sort macroinvertebrates. All collected macroinvertebrates were sorted according to broad taxonomic groups in the field and stored in 80% ethanol. Afterwards, all macroinvertebrates were transported to the laboratory and examined using a stereomicroscope (10× magnification). Identification was conducted to family level using the identification key of Bouchard (2004). Identification to a lower taxonomic level was impossible due to the lack of proper identification literature and reference collections for Ethiopia.
Bird Survey
A bird survey was conducted twice a year during the wet and dry season similar to the macroinvertebrate sampling period. Surveys were carried out between 6 and 10 am and between 4 and 6 pm local standard time. The point count method was used to collect data within a 100 m radius of the site where macroinvertebrates and environmental conditions were sampled (Lee and Marsden 2008). This method was justified as we mainly visually observed birds near open grassland and not dense forest. All species visually observed during 15 min were recorded using binoculars (Kite, 10 × 42) and identified using the key of Van Perlo (2009). The first 5 min were used to allow birds to settle and return to their natural behavior, whereas the remaining 10 min were used to record all bird species seen (Ralph et al. 1995). The recorded bird species were classified as wetland dependent and wetland associated birds based on Almaw (2012). Birds that flew over were not included. The number of species as well as the abundance of each particular species was recorded at each site.
Environmental Variables
To assess the hydro-morphological habitat characteristics of each site, the United States Environmental Protection Agency (USEPA) wetland habitat assessment protocol was used (Baldwin et al. 2005), which was modified by Mereta et al. (2013) for specific conditions present in Ethiopian wetlands. A measure of human disturbance was obtained by assessing hydrological modifications, habitat alteration and land-use practices. Hydrological modifications included ditching or draining, filling and abstracting of water in the wetland. Habitat alteration included grazing, tree plantation and vegetation removal. Land use practices in the wetlands included farming, waste dumping and clay mining. The degree of intensity of land use practices, which was indicated as human disturbance, was quantified based on the protocol described by Hruby (2004), which was modified by Mereta et al. (2013). A score of 1 was assigned to no or minimal disturbance, 2 to moderate and 3 to high disturbance. The overall human disturbance for each site was calculated by summing the individual disturbance values of land use activities (9 different activities in total). Each of the 9 scores were summed to calculate the overall human disturbance at each site, so the minimum disturbance score was 9 and the maximum disturbance score was 27. The percentage of vegetation cover was visually estimated at each sampling site within a radius of 100 m from the sampling site. Dissolved oxygen, electrical conductivity (EC), pH, turbidity and water temperature were measured in the field using a multi-probe meter (HQ30d Single-Input Multi-Parameter Digital Meter, Hach). Total nitrogen (TN), total phosphorus (TP) and chemical oxygen demand (COD) were analyzed in the laboratory using NANOCOLOR® test kits. Step by step instructions provided with the test kits were followed for all chemical analyses. Physical variables such as sediment and water depth were measured at each site.
Data Analysis
Richness and abundance were calculated at family level for macroinvertebrates and at species level for birds. The total richness per site was obtained for macroinvertebrates by counting the number of recorded families at each site, whereas for birds the number of species per site was counted. The total abundance was obtained by summing all individuals at each site. Total family richness, total family abundance, number of Ephemeroptera, Odonata and Trichoptera (EOT) taxa, abundance of EOT taxa and percentage of Chironomidae was calculated to characterize a sampling site. These metrics were selected as these are the sub-metrics used to calculate the biotic index developed by Mereta et al. (2013) for natural wetlands in Ethiopia. The relative abundance (%) was calculated by dividing the abundance of individual taxa by the total abundance and multiplying the outcome by 100.
Normality was checked for richness and abundance of macroinvertebrates and birds first, using a Shapiro Wilk Normality test. Since the data were not normally distributed we used a non-parametric Kruskal-Wallis test to determine significant differences in richness and abundance of macroinvertebrates and birds between wetlands. All analyses were performed in R (R Development Core Team 2015). Boxplots were used to visualise richness and abundandance of macroinvertebrate families and bird species.
Generalized linear mixed models (GLMM) were built to identify the response of richness and abundance of macroinvertebrates and birds to environmental variables. Season was set as random variable, whereas fixed variables included: climatic zone (warm temperate and rainy climate, tropical rainforest climate and tropical rainy climate), water depth, sediment depth, water temperature, ambient temperature, dissolved oxygen saturation, electrical conductivity (EC), pH, total nitrogen (TN), total phosphorus (TP), chemical oxygen demand (COD), human disturbance, abundance of macroinvertebrates (for wetland birds) and vegetation cover. Prior to the development of the GLMM, strongly correlated predictor variables were identified using the pairs function in R to avoid problems of correlation (Zuur et al. 2009). If variables were strongly correlated, only one of the variables in the pair was included in the model. Also, the spatial autocorrelation was checked for locations to identify if data in the same wetland was spatially auto-correlated using a correlogram (Zuur et al. 2009). If positive spatial autocorrelations were observed, the variable was set as a random variable. All possible models were constructed and the model with the lowest Akaike Information Criterion (AIC) was selected. GLMMs were run using a Poisson error distribution. The model was considered reliable if the residuals were normally distributed and rejected if not.
Results
A total of 14,480 individuals from 82 macroinvertebrate families were collected from the three climatic zones. The most commonly found macroinvertebrate families were Chironomidae and Dytiscidae followed by Coenagrionidae with a relative abundance of 10.3%, 9.7% and 8.5%, respectively. For a complete list of recorded macroinvertebrates we refer to Appendix Table 4.
Based on the correlation analysis, climatic zone showed a strong correlation with temperature (R = 0.7) and water temperature (R = 0.8), so we removed both ambient temperature and water temperature, as we considered climatic zone as a variable of interest. No spatial autocorrelation was observed between sampling locations. The GLMM model did not select climatic zones, pH and COD as important variables for both richness and abundance of macroinvertebrates and wetland birds.
Based on the GLMM we found that taxa richness of macroinvertebrates was positively correlated with vegetation and dissolved oxygen (Table 2). Taxa abundance of macroinvertebrates was negatively correlated with dissolved oxygen, EC and vegetation cover, whereas positively correlated with TP (Table 2). Twenty four percent of the variation in macroinvertebrate richness was explained by a combination of vegetation cover and dissolved oxygen, whereas 34% of the variation in macroinvertebrate abundance was explained by a combination of dissolved oxygen saturation, electric conductivity, total phosphorus and vegetation cover.
Variation in richnes and abundance of macroinvertebrates was observed between wetlands. Total taxa richness did not significantly differ (P = 0.14) between wetlands. Total taxa abundance was significantly higher (P = 0.02) in Chalalaki and Otra wetland compared to other wetlands. EOT richness and EOT abundance were significantly higher (P = 0.01 and P < 0.001, respectively) in Chalalaki and Nech Wuha wetland, whereas the percentage of Chironomidae was significantly higher (P = 0.01) in Awetu wetland followed by Afala and Otra wetland (Fig. 2).
Box plots of taxa richness and abundance of macroinvertebrates for the different wetlands studied in southwestern Ethiopia: (a) total richness, (b) total abundance, (c) EOT richness, (d) EOT abundance, (e) percentage of Chironomidae. Where: Ch = Chalalaki, Bu = Bulbul, Ha = Haro, Bo = Boye, Kt = Kito, Kf = Kofe, Aw = Awetu, NW = Nech Wuha, Af = Afala, Ot = Otra, Al = Alamgano and Sh = Shimbira
A total of 168 bird species were recorded in the three climatic zones, of which 59 species were categorized as wetland dependent birds and 109 as wetland associated birds. The most common wetland dependent bird was the Cattle Egret, followed by the Yellow-billed Duck with a relative abundance of 11% and 6.64%, respectively. The highest species richness and abundance of wetland dependent birds was observed for wetlands found in the tropical rainy climate. The most common wetland associated bird was the Hadada Ibis and Bronze Mannikin with a relative abundance of 11.5% and 9.31%, respectively. For a complete list of recorded bird species, we refer to Appendix Table 5.
Based on the GLMM we found that water depth, dissolved oxygen saturation and macroinvertebrate abundance were positively correlated with species richness and abundance of wetland dependent birds, whereas human disturbance was negatively correlated with both bird abundance and richness (Table 3). A combination of water depth, dissolved oxygen, macroinvertebrate abundance and human disturbance explained about 34% and 31% of variation in bird species richness and abundance respectively.
Wetlands with open water such as Chalalaki, Bulbul and Haro were characterized by a higher species richness and abundance of wetland dependent birds compared to wetlands totally covered by vegetation. Species richness and abundance of wetland associated birds appeared to be more or less similar in wetlands with open water and wetlands totally covered by vegetation (Fig. 3).
Box plots of species richness and abundance of wetland dependent and wetland associated birds for the different wetlands studied in southwestern Ethiopia: (a) species richness of wetland dependent birds (b) species abundance of wetland dependent birds, (c) species richness of wetland associated birds and (d) species abundance of wetland associated birds. Where: Ch = Chalalaki, Bu = Bulbul, Ha = Haro, Bo = Boye, Kt = Kito, Kf = Kofe, Aw = Awetu, NW = Nech Wuha, Af = Afala, Ot = Otra, Al = Alamgano and Sh = Shimbira
Discussion
Our model results indicated that several environmental variables were important for describing the occurrence of macroinvertebrates. Vegetation cover was positively correlated with macroinvertebrate richness and negatively correlated with abundance. This result is in contrast with the result of Michaletz et al. (2005) who reported no relation of macroinvertebrate abundance with vegetation. However, our results seem in agreement with the result of Oyague Passuni and Maldonado Fonken (2014) who reported a negative correlation of total vegetation cover with macroinvertebrate assemblages occurring in Andean peatland systems. Electric conductivity negatively correlated with abundance of macroinvertebrates. Although the maximum values recorded in our study area were rather low, our result is in agreement with the result of Kefford (1998), who reported a decrease in abundance of macroinvertebrates at higher electric conductivities. Our model indicated that total taxa richness of macroinvertebrates is positively correlated to dissolved oxygen, suggesting that conditions favouring a high dissolved oxygen concentration promote a higher macroinvertebrate richness. Abundance of macroinvertebrates on the other hand, was negatively correlated with DO saturation and positively correlated with total phosphorus. This might be explained by the higher relative abundance (10.3%) of Chironomidae taxa in our study area. The majority of Chrironomidae was collected at Awetu wetland which receives high amounts of domestic waste and is characterised by a higher nutrient concentration and lower dissolved oxygen. It has been reported that some genera of this taxon can thrive well at sites with a high organic enrichment due to anthropogenic activity (Silva et al. 2009). Moreover, this taxon is often characterized by a fast colonization allowing it to reach high abundances (Silveira et al. 2013). However, our result needs further styudy as identification was only performed at family level and there are also pollution sensitive Chironomidae species.
The average richness and abundance of macroinvertebrate taxa differed between wetlands. Higher total taxa richness, total taxa abundance, EOT richness and EOT abundance was observed in wetlands with a low human disturbance such as Chalalaki and Otra. Gezie et al. (2017) also reported a high macroinvertebrate richness in wetlands with a low human disturbance. Nech Wuha wetland, was characterized by a high EOT richness and abundance. This might be due to the high dissolved oxygen concentration present in this wetland compared to others. It has been reported that EOT taxa benefit from high dissolved oxygen concentrations (Hofmann and Mason 2005; Narangarvuu et al. 2015).
Our study revealed that dissolved oxygen concentration was, besides for macroinvertebrates, an important variable explaining the variation observed in birds. Dissolved oxygen concentration was positively correlated with richness and abundance of wetland dependent birds. This finding is consistent with the finding of Patra et al. (2010) who reported a positive influence of dissolved oxygen on water birds. The positive correlation of water depth with the richness and abundance of wetland dependent birds found in our study could be ascribed to the high number of wading and diving birds in our study sites, which prefer open water. Next to the effect of physico-chemical variables on birds, we found a negative correlation of human disturbance (physical variable) and a positive correlation of macroinvertebrate abundance (biotic variable) with species richness and abundance of wetland dependent birds. Previous studies reported a low bird species diversity in high disturbed wetlands (DeLuca et al. 2004; Ntongani and Andrew 2013) and a high bird abundance at sites with greater macroinvertebrate diversity and abundance Getachew et al. (2012). Furthermore, high species richness and abundance of wetland dependent birds was observed in wetlands with a high percentage of open water such as Chalalaki, Haro and Bulbul which are also characterized by a high macroinvertebrate richness and abundance. In our study area, wetlands totally covered by vegetation were characterized by a higher level of human disturbance, a lower availability of food such as fish (Mereta et al. unpublished data) and consequently also a lower richness and abundance of wetland dependent birds.
In contrary to our hypothesis, the GLMM model did not select climatic zones as important variable to explain variation in richness and abundance of both macroinvertebrates and birds. In contrast, local environmental conditions seem more important than climatic conditions in explaining the variation in richness and abundance of macroinvertebrate and birds in our study area. Previous studies investigating birds or macroinvertebrates support our results. Zhang et al. (2014) reported that especially local environmental conditions affect the macroinvertebrate assemblage. Whereas, Hiley et al. (2016) reported that the diversity of birds was affected by habitat modification in different climatic zones in Mexico.
Despite the importance of freshwater wetlands as biodiversity hot spots, they are continuously under pressure due to expanding human activities. The record of 169 bird species including IUCN red list and endemic species and 82 macroinvertebrate taxa indicates that the wetlands in the study area are an important biodiversity hotspot. Previous studies reported that local environmental conditions such as change in habitat or water quality affect the occurrence of macroinvertebrates (Mereta et al. 2012; Gezie et al. 2017) and that regional and global factors such as climate affect bird diversity and abundance (Valiela and Bowen 2003). The present study indicates that local environmental conditions are important when assessing the richness and abundance of birds and macroinvertebrates, rather than the spatial differences between different climatic zones. Therefore, the selected environmental variables can be considered important when monitoring wetland ecosystems and assessing the wetland status. These results could help to inform decision-makers and stakeholders, when developing wetland conservation strategies, since local environmental conditions should be taken into account. Moreover, further study is needed on the composition and structure of biological communities in these wetlands, as species richness and abundance alone is not enough to measure ecosystem health and function.
References
Abebe YD, Geheb K (2003) Wetlands of Ethiopia. Proceedings of a seminar on the resources and status of Ethiopia’s wetlands. pp 28–30
Almaw R (2012) A checklist of the birds of the Abijata-Shalla lakes national park (Central Rift-Valley, Ethiopia). Ethiopian Wildlife Conservation Authority, Addis Abeba
Al-Shami SA, Heino J, Che Salmah MR, Hassan AA, Suhaila AA, Madras MR (2013) Drivers of beta diversity of macroinvertebrate communities in tropical forest streams. Freshwater Biology 58:1126–1137
Balcombe CK, Anderson JT, Fortney RH, Kordek WS (2005) Aquatic macroinvertebrate assemblages in mitigated and natural wetlands. Hydrobiologia 541:175–188
Baldwin DS, Nielsen DL, Bowen PM, Williams J (2005) Recommended methods for monitoring floodplains and wetlands. Berkeley Planning Journal 1921038 20 9. MDBC publication no. 72/ 04
Baselga A, Lobo JM, Svenning J, et al (2012) Dispersal ability modulates the strength of the latitudinal richness gradient in European Beetles. Global Ecology and Biogeography 21:1106–1113
Beatty WS, Kesler DC, Webb EB Raedeke AH, Naylor LW, Humburg DD (2014) The role of protected area wetlands in waterfowl habitat conservation: implications for protected area network design. Biological Conservation 176:144–152
Bouchard RW (2004) Guide to aquatic maro-invertebrates of the upper Midwest. University of Minnesota, St. Paul, Water Resources Centre
Clavero M, López V, Franch N et al (2015) Use of seasonally flooded rice fields by fish and crayfish in a Mediterranean wetland. Agriculture, Ecosystems and Environment 213:39–46
Covich AP, Palmer MA, Crowl TA (1999) The role in of species invertebrate freshwater ecosystems benthic zoobenthic species influence energy flows and nutrient cycling. BioScience 49:119–127
Cowardin LM, Carter V, Golet FC, LaRoe ET (1979). Classification of wetlands and deepwater habitats of the United States. U.S. Department of the Interior, Washington, DC, USA. FWS/OBS-79/31
Daneshvar F, Nejadhashemi AP, Adhikari U et al (2017) Evaluating the significance of wetland restoration scenarios on phosphorus removal. Journal of Environmental Management 192:184–196
Davidson NC (2014) How much wetland has the world lost ? Long-term and recent trends in global wetland area. Marine and Freshwater Research 65:936–941
DeLuca WV, Studds CE, Rockwood LL, Marra PP (2004) Influence of land use on the integrity of marsh bird communities of Chesapeake Bay, USA. Wetlands 24:837–847
Dolédeca S, Tilbiana J, Bonadab N (2017) Temporal variability in taxonomic and trait compositions of invertebrate assemblages in two climatic regions with contrasting flow regimes. Science of The Total Environment 599–600:1912–1921
Erwin KL (2009) Wetlands and global climate change : the role of wetland restoration in a changing world. Wetlands Ecology and Management 17:71–84
Gamachu D (1977) Aspects of climate and water budget in Ethiopia. Addis Ababa University Press, Addis Ababa, p 71
Gebrehiwot M, Awoke A, Beyene A et al (2017) Limnologica macroinvertebrate community structure and feeding interactions along a pollution gradient in Gilgel Gibe watershed, Ethiopia : implications for biomonitoring. Limnologica 62:68–76
Gebresllassie H, Gashaw T, Mehari A (2014) Wetland degradation in Ethiopia : causes, consequences and remedies. Journal of Environment and Earth Science 4:40–49
Getachew M, Ambelu A, Tiku S et al (2012) Ecological assessment of Cheffa wetland in the Borkena Valley, northeast Ethiopia: macroinvertebrate and bird communities. Ecological Indicators 15:63–71
Gezie A, Anteneh W, Dejen E, Mereta ST (2017) Effects of human-induced environmental changes on benthic macroinvertebrate assemblages of wetlands in Lake tana. Environmental Monitoring and Assessment 189:152
Gonfa L (1996) Climate classification of Ethiopia. National Meteorological Agency, Addis Ababa
Green AJ, Figuerola J (2005) Recent advances in the study of long- distance dispersal of aquatic invertebrates via birds. Diversity and Distributions 11:149–156
Gregory RD, Noble D, Field R et al (2003) Using birds as indicators of biodiversity. Ornis Hungarica 12–13:11–24
Guareschi S, Abellán P, Laini A et al (2015) Cross-taxon congruence in wetlands : assessing the value of waterbirds as surrogates of macroinvertebrate biodiversity in Mediterranean Ramsar sites. Ecological Indicators 49:204–215
Hawkins CP, Mykrä H, Oksanen J, Vander LJJ (2015) Environmental disturbance can increase beta diversity of stream macroinvertebrate assemblages. Global Ecology and Biogeography 24:483–494
Hayashi M, Kamp GVD, Schmidt R (2003) Focused infiltration of snowmelt water in partially frozen soil under small depressions. Journal of Hydrology 270:214–229
Heino J (2013) The importance of metacommunity ecology for environmental assessment research in the freshwaterrealm. Biological Reviews 88:166–178
Herrando S, Anton M, Sardà-Palomera F et al (2014) Indicators of the impact of land use changes using large-scale bird surveys: land abandonment in a Mediterranean region. Ecological Indicators 45:235–244
Hiley JR, Bradbury RB, Thomas CD et al (2016) Impacts of habitat change and protectedareas on alpha and beta diversity of Mexican birds. Diversity and Distributions 22:1245–1254
Hofmann TA, Mason CF (2005) Habitat characteristics and the distribution of Odonata in a lowland river catchment in eastern England. Hydrobiologia 539:137–147
Hruby T (2004) Washington state wetland rating system for eastern Washington - revised. Washington State Department of ecology publication no. 04-06-15
Keddy PA, Fraser LH, Solomeshch AI et al (2009) Wet and wonderful: the world’s largest wetlands are conservation priorities. BioScience 59:39–51
Kefford BJ (1998) The relationship between electrical conductivity and selected macroinvertebrate communities in four river systems of south-west Victoria, Australia. International Journal of Salt Lake Research 7:153–170
Larsen S, Mancini L, Pace G et al (2012) Weak concordance between fish and macroinvertebrates in Mediterranean streams. PLoS One 7(12):e51115
Lavoie R, Deslandes J, Proulx F (2016) Assessing the ecological value of wetlands using the MACBETH approach in Quebec City. Journal for Nature Conservation 30:67–75
Lee DC, Marsden SJ (2008) Adjusting count period strategies to improve the accuracy of forest bird abundance estimates from point transect distance sampling surveys. Ibis 150:315–325
Mereta ST, Boets P, Bayih AA et al (2012) Analysis of environmental factors determining the abundance and diversity of macroinvertebrate taxa in natural wetlands of Southwest Ethiopia. Ecological Informatics 7:52–61
Mereta ST, Boets P, De Meester L, Goethals PLM (2013) Development of a multimetric index based on benthic macroinvertebrates for the assessment of natural wetlands in Southwest Ethiopia. Ecological Indicators 29:510–521
Michaletz PH, Doisy KE, Rabeni CF (2005) Influences of productivity, vegetation, and fish on macroinvertebrate abundance and size in Midwestern USA impoundments. Hydrobiologia 543:147–157
Millennium Ecosystem Assessment (2005) Ecosystem and human well-being. Wetlands and water synthesis, Washington, DC
Moges A, Beyene A, Triest L et al (2016) Imbalance of ecosystem services of wetlands and the perception of the local community towards their restoration and management in Jimma highlands, Southwestern Ethiopia. Wetlands. https://doi.org/10.1007/s13157-016-0743-x
Mooney H, Larigauderie A, Cesario M et al (2009) (2009) biodiversity, climate change, and ecosystem services. Current Opinion in Environmental Sustainability 1:46–54
NABU (2015) Conservation and sustainable use of the last wild coffee forests of Ethiopia. https://en.nabu.de/projects/ethiopia/kafa/biodiversity/index.html. Accessed 15 Sept 2017
Narangarvuu D, Oyunbileg J, Yang P, Boldgiv B (2015) Distribution of Ephemeroptera, Plecoptera, and Trichoptera assemblages in relation to environmental variables in headwater streams of Mongolia. Environmental Earth Sciences 73:835–847
National Meteorological Agency (2015) Unpublished report. Addis Abeba, Ethiopia
Ntongani WA, Andrew SM (2013) Bird species composition and diversity in habitats with different disturbance histories at Kilombero wetland, Tanzania. Open Journal of Ecology 3:482–488
Oyague Passuni E, Maldonado Fonken MS (2014) Relationships between aquatic invertebrates, water quality and vegetation in an Andean peatland system. Mires and Peat 15:1–21
Patra A, Santra KB, Manna CK (2010) Relationship among the abundance of waterbird species diversity, macrophytes, macroinvertebrates and physico-chemical characteristics in Santragachi Jheel, Howrah, W.B. India. Acta Zoologica Bulgarica 62:277–300
R Development Core Team (2015) R. A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/
Ralph CJ, Sauer JR, Droege S (technical eds) (1995) Monitoring bird populations by point counts. Gen. Tech. Rep. PSW-GTR-149. Albany, CA: Pacific southwest Research Station, Forest Service, US. Department of Agriculture, p 187
Reid JL, Mendenhall CD, Rosales JA et al (2014) Landscape context mediates avian habitat choice in tropical forest restoration. PLoS One. https://doi.org/10.1371/journal.pone.0090573
Roulet NT (2000) Peatlands, carbon storage, greenhouse gases, and the kyoto protocol : prospects and significance for Canada. Wetlands 20:605–615
Ruhí A, Boix D, Gascón S et al (2013) Functional and phylogenetic relatedness in temporary wetland invertebrates : current macroecological patterns and implications for future climatic change scenarios. PLoS One 8:e81739. https://doi.org/10.1371/journal.pone.0081739.
Saloom ME, Duncan RS (2016) Low dissolved oxygen levels reduce anti-predation behaviours of the freshwater clam Corbicula Fluminea. Freshwater Biology 50:1233–1238
Schuyt KD (2005) Economic consequences of wetland degradation for local populations in Africa. Ecological Economics 53:177–190
Seilheimer TS, Mahoney TP, Chow-fraser P (2009) Comparative study of ecological indices for assessing human-induced disturbance in coastal wetlands of the Laurentian Great Lakes. Ecological Indicators 9:81–91
Silva FL, Silveira AD, Talamoni JLB, Ruiz SS (2009) Temporal variation of Chironomidae larvae ( Insecta, Diptera ) in the Batalha River, Midwestern São Paulo state, Brazil. Ciência et Praxis 2:3
Silveira LS, Martins RT, Silveira GA et al (2013) Colonization by Chironomidae larvae in decomposition leaves of Eichhornia Azurea in a lentic system in southeastern Brazil. Journal of Insect Science 13:1–13
Steinman AD, Conklin J, Bohlen PJ, Uzarski DJ (2003) Influence of cattle grazing and pasture land use on macroinvertebrate communities in freshwater wetlands. Wetlands 23:877–889
Teferi E, Uhlenbrook S, Bewket W et al (2010) The use of remote sensing to quantify wetland loss in the Choke Mountain range, upper Blue Nile basin, Ethiopia. Hydrology and Earth System Sciences 14:2415–2428
Tegegn DA (2017) Historical survey of Limmu Genet town from its foundation up to present. International Journal of Scientific and Technology Research 6:2277–8616
Timothy JO, Laura EJ, Robert PB (2000) Bird guilds as indicators of ecological condition in the central Appalachians. Ecological Applications 10:1706–1721
Tomankova I, Harrod C, Fox AD, Reid N (2014) Chlorophyll- a concentrations and macroinvertebrate declines coincide with the collapse of overwintering diving duck populations in a large eutrophic lake. Freshwater Biology 59:249–256
Valiela I, Bowen JL (2003) Shifts in winter distribution in birds: effects of global warming and local habitat change. Ambio 32:476–480
Van Perlo B (2009) Birds of Eastern Africa. Princeton University press, New Jersey 08549, United States
Van Ranst E, Dumon M, Tolossa AR, Cornelis JT, Stoops G, Vandenberghe RE, Deckers J (2011) Revisiting ferrolysis processes in the formation of Planosols for rationalizing the soils with stagnic properties in WRB. Geoderma 163:265–274
WBISPP (2004) Woody biomass inventory and strategic planning project report, Addis Abeba
Yimer HD, Mengistou S (2009) Water quality parameters and macroinvertebrates index of biotic integrity of the Jimma wetlands, southwestern Ethiopia. Journal of Wetlands Ecology 3:77–93
Zhang Y, Zhang J, Wang L et al (2014) Influences of dispersal and local environmental factors on stream macroinvertebrate communities in Qinjiang River, Guangxi, China. Aquatic Biology 20:185–194
Zuur AF, Ieno EN, Walker NJ, et al (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgements
This work was supported by Schlumberger Foundation The Hague, The Netherlands, grant number: 41167008. The first author is a recipient of a PhD scholarship grant from the Schlumberger Foundation. The authors wish to thank all people who helped with fieldwork.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Chawaka, S.N., Boets, P., Mereta, S.T. et al. Using Macroinvertebrates and Birds to Assess the Environmental Status of Wetlands across Different Climatic Zones in Southwestern Ethiopia. Wetlands 38, 653–665 (2018). https://doi.org/10.1007/s13157-018-1008-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-018-1008-7