Abstract
Microbial communities, enzyme activities and soil physiochemical characteristics were investigated in alpine wetlands with altitude changes in the Nyainqentanglha Mountains on the Tibetan plateau. The results showed that with an increasing altitude gradient, soil physicochemical properties such as total organic carbon (TOC), total nitrogen (TN), water-soluble organic carbon (WSOC), water-soluble organic nitrogen (WSON) and pH decreased coincident with a decrease in mean annual temperature (MAT). Bacteria biomass, fungal biomass and actinobacterial biomass all decreased with an increase in altitude gradient. The ratio of Gram-positive bacteria to Gram-negative bacteria and the ratio of cyclopropyl to precursor fatty acids all indicate that the level of environmental harshness intensified with an increase in altitude. We also found that soil enzyme activities such as phenol oxidase, peroxidase, L-asparaginase, protease, urease and alkaline phosphatase all consistently decreased with an increase in altitude gradient. Additionally, the activities of peroxidase, protease and alkaline phosphatase declined, mainly due to a reduction in enzyme activities with temperature rather than the reduction of associated microbial biomass. Statistical analysis showed that both microbial biomass and enzyme activities were significantly influenced by mean annual temperature, suggesting that temperature is a key factor that affects microbial communities and soil enzyme activities in alpine wetlands on the Tibetan Plateau and indicating that climate warming may significantly impact these areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
To better understand the impact of global climate change on a variety of ecosystems, knowledge of altitudinal diversity patterns is needed (Lomolino 2001; Rahbek 2005). Soil microorganisms play an important role in soil organic turnover and biogeochemical cycling. Additionally, soil microbial community composition and activity are influenced by biotic and abiotic factors. Many recent studies have begun to focus on latitude distribution of microbial communities and activities (Fierer and Jackson 2006; Griffiths et al. 2011; Rousk et al. 2010; Margesin et al. 2009), whereas little information on microbial composition or diversity patterns is available for altitudinal gradients. Previous studies showed that fungal biomass and diversity decreased with an increase in altitude in the Austrian Central Alps (Margesin et al. 2009; Schinner and Gstraunthaler 1981). Similarly, a decrease in the bacteria population with an increase in altitude was observed in the cold temperate Kalasi Lake and a tropical dry deciduous forest (Giri et al. 2007; Ma et al. 2004). In contrast, Mannisto et al. (2007) observed that microbial communities were affected by soil pH rather than altitude in the Arctic fjelds of Finnish Lapland. Shen et al. (2013) also observed that soil pH was the key factor that drove the spatial distribution of microbial communities along an altitudinal gradient at Changbai Mountain. Moreover, Djukic et al. (2010) suggested that microbial community structure was controlled by decomposition conditions and vegetation composition along an altitudinal gradient in the Austrian Limestone Alps.
Studies on soil microbial communities in wetlands along altitude gradients are still very limited. Wetlands are special ecosystems at the interface between terrestrial and aquatic ecosystems, providing a unique habitat for a diverse range of microbial species. However, wetlands are very sensitive to global climate change (Erwin 2009) and respond to it through alteration of energy, hydrological or carbon patterns. Wetlands also play an important role in the regulation of global climate change by sequestering and releasing a major proportion of atmospheric carbon in the biosphere (Limpens et al. 2008; Ma et al. 2016). Therefore, a better understanding of the changes in wetland microbial communities and their associated activities in response to altitudinal gradients and underlying mechanisms is of scientific and economic importance, especially in the context of potential climate change.
The Tibetan Plateau, known as “the third pole” of the world, is located in the middle of the Asian continent with a total area of over 2.5 million square kilometres. Because of its extreme cold and semiarid-arid environmental features, the Tibetan Plateau is very sensitive to climate change (Yao et al. 2000). The total wetland area of the Tibetan Plateau is very large and has important ecological functions such as climatic regulation and water storage. The Nyainqentanglha Mountains, marked by a wide distribution of extremely tall mountains, are located in the middle and south of the Tibetan Plateau. There are multiple wetlands in the Nyainqentanglha Mountains ranging from 4200 m to 5100 m, accompanied by changes in geographic factors. Therefore, the Tibetan Plateau provides an ideal place for the study of the structure of microbial communities along an elevation gradient.
Phospholipid fatty acid (PLFA) technology uses the cellular membrane lipids of microorganisms as biomarkers for specific organisms, and thus is commonly used to create the fingerprint of studied microbial communities or indicators of environmental stress (Guckert et al. 1986). The total concentration of PLFA is used to measure viable microbial biomass, since phospholipids are readily degraded after cells die (Zelles 1997). Soil enzyme activities play a very important role in many soil functions such as nutrient cycling, decomposition and organic matter decomposition. Moreover, soil enzyme activities are related to soil physiochemical characters (Amador et al. 1997), the structure of microbial community (Kourtev et al. 2002; Waldrop et al. 2000), vegetation type (Kourtev et al. 2002; Sinsabaugh et al. 2002), disturbance (Boerner et al. 2000; Bolton et al. 1993; Garcia and Hernandez 1997), and successional stage (Tscherko et al. 2003). We integrated these two methods to study microbial activities with an increase of elevation. Also, we want to decipher whether the change of microbial activities is cased by the change of microbial biomass or the response of enzymes to a decrease of temperature.
Accordingly, the aims of our research are (1) to examine the soil microbial community structure and soil enzyme activities with altitude changes in the Nyainqentanglha mountains, and (2) to study the relationship between community structure and soil enzyme activities and their driving factors.
Materials and Methods
This study was conducted along the south-facing slope of the Nyainqentanglha Mountains in the central Tibetan Plateau (30°29′–30°43’N, 91°05′–91°08’E). To study the impact of environmental factors on microbial activities, we chose alpine wetlands located along Dangqu river in an altitudinal gradient in Damxung County (Fig. 1). With the increase of altitude, environmental factors, such as temperature, precipitation and radiation, change coincidently. To minimize potential influences of precipitation, alpine wetlands were chosen because soils in alpine wetlands are immersed with large amount of water so that precipitation may not strongly affect soil water content. There are many alpine wetlands located in Damxung County with a range of altitude between 4200 m and 5000 m a.s.l. (30°29′–30° 43’N, 91°05′–91°08’E). Seven alpine wetlands lied in different altitudes were chosen (Table 1). According to the meteorological observation between 1963 and 2008 at Damxung station (4288 m a.s.l.), the mean annual air temperature (MAT) in January and July was 1.8 °C and 10.9 °C, respectively. Also, with an increase of additional 100-m above sea level, the temperature dropped more than 0.5 °C (Table 1). The annual precipitation was 479 mm (290–700 mm, with >85% rainfall occurring in the growing season between May and September), which was much less than the annual pan evaporation of 1726 mm (Wang et al. 2013). The major vegetation types include alpine meadow and sedge, with Oxytropis ochrocephala and Kobresia as dominant species. No specific permissions were required for the field survey which was done in a small plot in a pastureland which is not privately-owned or protected in any way. The field studies did not involve endangered or protected species.
Study Sites and Sample Collections
Soil samples were taken from 0 to 10 cm depth on hummocks in wetlands in August, 2011. Details of the location, vegetation, climate and soil characteristics are provided in Table 1. We selected representative types of wetland to determine final sample sites with minimum amount of disturbance. In order to minimize spatial heterogeneity, seven soil cores were collected along a diagonal line across each plot, and were subsequently mixed to form one composite sample. Four replicates samples were taken per wetland at each altitude. Soils were sieved to remove plant roots and stones (sieve mesh 2 mm) at sampling sites, and the subsamples were then packed in ice blocks and sent back to the laboratory. Each sample was divided into two subsamples, with one part stored at −80 °C for microbial analysis and the rest preserved at 4 °C for physicochemical analysis.
Soil Physicochemical Analysis
Air-dried soil samples were used to measure soil pH, electrical conductivity, particle size, total C, N and P. Gravimetric soil moisture was calculated on 5 g fresh subsamples after drying in a 105 °C oven for 12 h. Soil pH was detected in a 1:2.5 mixture of soil and deionized water using a sartorius PB–10 digital pH meter. Soil electrical conductivity was measured in a 1:5 mixture of soil and deionized water using DDBJ–350 conductivity meter (Precision and Scientific Corp. Shanghai, China). Soil texture (Clay/silt/sand percentage) was determined with a Malvern Mastersizer 2000 with a measurement range of 0.02–2000 μm. Soil total organic C was measured using a Shimadzu TOC–TN analyzer (Shimadzu Corp., Kyoto, Japan). 0.5 g air-dried soil which was sieved by 100 meshes was digested 1.5 h by 1.5 g mixed catalyst and 4 mL concentrated sulphuric acid, and then dilute with deionized water to 50 mL to analyze total N and P. Total N was quantified using a continuous flow analyzer. Total P in soils was detected by ultraviolet spectrophotometer.
Soil water–soluble carbon (WSOC) and nitrogen (WSON) were measured by the method of Jones and Willett (2006). Briefly, 2.5 g of fresh soil was shaken with 25 mL of distilled water (1:10 w/v soil–to–solution ratio) for 15 min in 50 mL polypropylene bottles on a reciprocating shaker at a speed of 200 rpm. The soil extracts were then centrifuged at 8000×g for 10 min and the supernatant was recovered and stored in polypropylene bottles in a 4 °C freezer prior to analysis. Concentrations of WSOC and WSON were determined with a Shimadzu TOC–TN analyzer (Shimadzu Corp., Kyoto, Japan). Water extracted of Cl−, NO3−, SO4 2− from soil were measured using ion chromatograph (Binex ICS900).
Soil Enzyme Assays
Polyphenol oxidase (PPO) (EC 1.10.3.2) and peroxidase (EC 1.11.1.7) activities were measured spectrophotometrically using pyrogallol (PG) as a substrate (Allison and Jastrow 2006). β–glucosidase activity was assayed by the method of Eivazi and Tabatabai (1988), using the substrate analogue para–nitrophenyl–β–D–glucopyran– oside (pNPG). Invertase (EC 3.2.1.26) activity was assayed by incubating 1.00 g sieved (~2 mm) fresh soil in a 100 mL Erlenmeyer flask mixed with 15 mL 2 M acetate buffer and 15 mL 1.2% sucrose substrate solution for 50 °C for 3 h. A modified assay for urease (EC 3.5.1.5) activity based on that of Kandeler and Gerber (1988) was used. The activities of L–asparaginase were determined using the method previously reported with a few modifications: fresh soil (1.0 g) was placed in a 50 mL volumetric flask was treated with 0.2 mL toluene and 9 mL TRIS buffer (0.1 M, pH 10.0). The 50 mL flask was swirled for a few seconds to mix the contents, 1 mL 0.05 M L–asparagine solution was added, and the flask was swirled again for a few seconds. Then the flask was stopped and placed in an incubator at 37 °C for 2 h. Protease activity was determined as reported by Ladd and Butler (1972). All enzymes activities were analyzed used colorimetric method by spectrophotometer. Measuring wavelengths were: Polyphenol oxidase–490 nm, Peroxidase–294 nm, β–glucosidase–400 nm, Invertase–508 nm, Urease–578 nm, L–asparagine–500 nm and Protease–500 nm.
PLFAs Extraction and Separation
PLFA lipids were extracted from lyophilized soil biomass using a single-phase Bligh and Dyer method (White et al. 1979) with minor modifications. Briefly, 5 g (n = 4) mixed soil sample was extracted twice with one phase mixture of chloroform– methanol–phosphate buffer (1:2:0.8, v/v/v, pH 7.4) for a minimum 2 h by vigorously shaking. The solutions were split into two phases by adding one volume of water and chloroform. The organic phase was recovered and then fractionated into neutral lipids, glycolipids and phospholipids on a silica acid (Unisil, Clarkson ChemicalCo., Williamsport, Pa.) column by consecutive elution with chloroform, acetone and methanol, respectively. The phospholipids were subjected to a mild alkaline methanolysis, which yields the methylesters of ester–linked fatty acids (FAMEs), and then PLFAs samples were preserved in −20 °C until analysis. FAMEs were analysised by gas chromatography (GC) and identified using the Sherlock Microbial Identification System. It consisted of a 6890A Series GC (Agilent Technologies, Palo Alto, CA), a flame ionization detector equipped with an Ultra 2 column (30 m, 0.2 m ID, 0.33 um film) and a computer and associated software (Sherlock Pattern Recognitition Software, MIDI, Newark, DE).
Statistical Analysis
All samples were measured in quadruplicate. One-way analysis of variance (ANOVA) was used to assess differences in soil factors and microbial properties along the attitude; LSD Multiple Range Test was performed on significant results using the SPSS software. Differences in PLFA profiles, soil enzyme activities and physicochemical characteristics of soils along the altitude gradient were analyzed using Canonical Correspondence Analysis (CCA) in the CANOCO 4.5 program, which is a constrained ordination method, for which ordination axes are constrained to be linear combinations of environmental factors. All statistical analyses were statistically significant at P = 0.05 level.
Results
Soil Physicochemical Properties with Altitudinal Changes
Soil physicochemical properties mainly displayed a decreasing trend with increasing altitude (Table 1). From 4267 to 4956 ma.s.l, WSOC and WSON decreased sharply, from 555.41 mg kg−1 to 146.58 mg kg−1 and from 50.83 mg kg−1 to 13.57 mg kg−1, respectively. Soil TOC and TN were highest at 4352 ma.s.l (TOC = 112.4 g kg−1 soil and TN = 6.9 g kg−1 soil, respectively) and, in general, declined with increasing elevation. A similar decreasing trend was found for nitrate, nitrite and ammonium with increasing altitude. The pH significantly decreased from 7.88 to 6.87 with increasing elevation. All sample pH values were near neutral. Total phosphorus, moisture and sulphate showed no significant change along the altitudinal gradient.
Enzyme Activities along the Altitudinal Gradient
Most enzyme activities also showed a trend similar to that of soil physicochemical properties, which decreased with increasing altitude (Fig. 2). Peroxidase, phenol oxidase and alkaline phosphatase had the highest activities at 4267 m, which were 1598.24 mg PG g−1 h−1 for peroxidase, 296.35 μg PG g−1 h−1 for phenol oxidase and 840.81 mg pNP g−1 h−1 for alkaline phosphatase. Peroxidase, phenol oxidase and alkaline phosphatase activities decreased sharply to 423.44 mg PG g−1 h−1, 180.26 μg PG g−1 h−1 and 166.38 mg p NP g−1 h−1 at the highest altitude of 4956 m, respectively. L–asparaginase, urease and protease had their highest activities at 4352 m, which were 0.67 μg NH4 +–N g−1 h−1 for L-asparaginase, 31.03 μg NH4 +–N g−1 h−1 for urease and 463.43 μg Tyrosine g−1 h−1 for protease. L-asparaginase, urease and protease activities also quickly decreased to 0.33 μg NH4 +–N g−1 h−1, 18.33 μg NH4 +–N g−1 h−1 and 190.36 μg Tyrosine g−1 h−1 at the highest altitude of 4956 m. All enzyme activities had a significant decreasing trend with increasing altitude except for β–glucosidase and invertase. Both of their highest activities were at 4737 m. The β–glucosidase level was 1.38 mg PNP g−1 h−1, and invertase was 5.65 mg glucose g−1 h−1. The activities of β–glucosidase and invertase had no significant changes at other altitude gradients other than 4737 m.
Community Composition (PLFAs) and Biomass along the Altitudinal Gradient
The microbial biomass, expressed as total PLFAs, significantly decreased with increasing altitude (Fig. 3). Along the altitude gradient from 4267 m to 4956 m, total microbial biomass per gram of soil was highest in stands with the lowest altitude of 4267 m and was lowest in stands with the highest altitude of 4956 m. Overall, the biomass of bacteria (i14:0, i15:0, a15:0, C15:1, i16:0, 16:1w9c, 16:w7c, 16:1w5c, 16:1w3c, i17:0, a17:0, C17:1, cy17:0, C18:1, i19:0, cy19:0, (Federle et al. 1986; Frostegard et al. 1993; Tunlid et al. 1989)), actinomycetes (10Me16:0, 10Me17:0, 10Me18:0, (Zelles 1997)), Gram-positive bacteria (i14:0, i15:0, a15:0, i16:0, i17:0, a17:0, i19:0, 10Me16:0, 10Me17:0, 10Me18:0, (Zogg et al. 1997)), Gram-negative bacteria (cy17:0, cy19:0, 15:1, 16:1w9, 16:1w7, 16:1w5, 18:1w7, 19:1, (Mutabaruka et al. 2007)) and fungi (C18:2, 18:1w9c, (Federle et al. 1986)) all decreased along the altitude gradient. Form low altitude to high altitude, bacteria biomass quickly fell from 91.99 nmol g−1 to 35.22 nmol g−1 and fungal biomass decreased from 9.78 nmol g−1 to 0.90 nmol g−1, respectively. Actinomycetes, Gram-positive bacteria and Gram-negative bacteria decreased 50%, 50% and 85% from 4267 ma.s.l. to 4956 ma.s.l., respectively.
Soil microbial community structure and community-relation ratios change along the altitude gradients. G+–gram-positive bacteria, G−–gram negative bacteria, F/B–fungi/bacteria, G+/G−–gram positive bacteria/g negative bacteria, cy/pre–cyclopropyl/precursor. Soil (a) bacteria, (b) fungi, (c) actinomycete, (d) gram positive bacteria, (e) gram negative bacteria, (f) fungi/bacteria ratio, (g) gram positive bacteria/g negative bacteria ratio, (H) cyclopropyl/precursor ratio. Values are means ± SD
The microbial community structure also showed significant changes with increasing altitude. The fungus to bacteria ratio (F/B) was used as a broad taxonomic microbial grouping, and the ratio of Gram-positive bacteria to Gram-negative bacteria (G+/G−) was used in conjunction with the ratio of the sum of cyclopropyl PLFAs to the sum of their monoenoic precursors (cy17:0 + cy19:0)/(16:1w7c + 18:1w7c; abbreviated as cy/pre) as indicators of environmental stress in bacteria communities (Kieft et al. 1997). The ratio of fungi/bacteria, the ratio of Gram-positive bacteria/Gram-negative bacteria and the ratio of cyclopropyl/precursor increased with altitude, indicating that environmental stress was increasing and that the soil microbial community was changing with increasing altitude. With increasing altitude, the cy/pre ratio ranged from 0.2 to 2.3. Likewise, the F/B ratio and the G+/G− ratio ranged from 0.1 to 0.13 and 0.6 to 2.1, respectively.
Multivariate Analysis of Soil Physicochemical and Microbial Properties
In mountain areas, temperature decreases with increasing altitude. Meanwhile, soil physicochemical and microbial properties can change along altitude gradients. Pearson correlation showed a significantly positive relationship between MAT and many soil physicochemical variables, and MAT might be the key factor that affects soil physicochemical properties (Table 2). WSON and WSON were significantly related to MAT (r = 0.738, P < 0.001; r = 0.673, P < 0.001, respectively). In addition, pH was positively correlated with MAT (r = 0.764, P < 0.001). A similar positive relationship was found between MAT/ TOC and TN (r = 0.443, P < 0.01; r = 0.442, P < 0.01).
Soil physicochemical properties showed a significant correlation with MAT, and soil microbial variables also showed the same trend. Phenol oxidase (r = 0.386, P < 0.05, n = 28), peroxidase (r = 0.629, P < 0.001, n = 28), urease (r = 0.539, P < 0.01, n = 28), protease (r = 0.588, P < 0.001, n = 28) and alkaline phosphatase (r = 0.759, P < 0.001, n = 28) had a significant positive correlation with MAT (Table 3). MAT was significantly correlated with bacteria biomass (r = 0.438, P < 0.01, n = 28), fungal biomass (r = 0.338, P < 0.05, n = 28), actinomycete biomass (r = 0.467, P < 0.01, n = 28), Gram-positive bacteria biomass (r = 0.338, P < 0.05, n = 28) and Gram-negative bacteria biomass (r = 0.537, P < 0.01, n = 28) (Table 4). There were many soil physicochemical properties affected by soil microbial variables besides MAT, including moisture, pH, TOC, TN, WSOC and WSON. This is why all of the variables significantly decreased with altitude. In particular, phenol oxidase activity was affected by MAT, WSOC, WSON, Cl− and nitrate. Peroxidase, urease, protease and alkaline phosphatase activities were affected by MAT, moisture, pH, TOC, TN, WSOC, WSON, Cl−, nitrate and sulphate. Invertase and glucosidase activities were affected by moisture, pH, TOC, TN and TP. Asparaginase activity was only affected by moisture and TN. Additionally, bacteria, Gram-positive bacteria and Gram-negative bacteria biomass were affected by MAT, moisture, pH, TOC, TN, WSOC and WSON. Similarly, actinomycete biomass was affected by MAT, pH, TN, WSOC, WSON, Cl− and nitrate. Fungal biomass was affected by MAT, TOC, TN and Cl−.
Using a Canonical Correspondence Analysis, we retained four components. The first two components (CCA1 and CCA2) explained 67.2% and 11.6% of the total variance, respectively, while the other two components explained 5.4% and 4.6%, respectively. CCA1 and CCA2 were chosen to draw a biplot (Fig. 4), as they together explained 78.8% of the total variance. Notably, CCA 1 was mainly weighted by physicochemical characteristics (sulphate, TOC, TN, moisture, Cl−, WSOC and WSON) and biological (invertase, L–asparaginase, protease and urease) variables together with mean annual temperature (MAT). TOC, pH, L–asparaginase, TN, moisture, urease, protease, chloride ion, WSOC and WSON exhibited strong positive correlations with MAT. The relationships between MAT and soil parameters indicated that soil enzyme activities and soil physicochemical properties corresponded to variation in MAT. In comparison with CCA1, CCA2 was weighted by only a few variables, and β-glucosidase and phenol oxidase exhibited a more powerful influence on discrimination of soil samples. Additionally, pH, peroxidase, nitrate, WSON, TP and WSOC also showed an important influence on the second axis. The samples at 4267 and 4352 m were apparently discriminated from other altitude samples, as more than half of the variables clustered near the low-altitude samples.
Discussion
In this study, we examined soil microbial community structure and enzyme activities along an altitude gradient in the Nyainqentanglha Mountains on the Tibetan Plateau. We found that most soil microbial biomass and enzyme activities decreased with an increase in altitude. In contrast, Djukic et al. (2010) and Wagai et al. (2011) observed that soil microbial communities did not display a consistent altitudinal change along altitude gradients. One possible explanation for the apparent disparity lies in the difference in site selection. For example, Djukic et al. (2010) and Wagai et al. (2011) collected samples from forests and shrubland, whereas we analysed microbial communities in a wetland ecosystem. Compared with forests, wetland ecosystems are more sensitive to temperature (Erwin 2009), in terms of changes in associated vegetation and soil physicochemical properties with changes in temperature.
Higher-altitude regions are characterized by very cold temperature and frequent frost and ice events. Overall, temperature represents a key factor that is closely correlated to environmental harshness, i.e., cold climate, low levels of nutrients and sparse vegetation, which in turn influence microbial activities and community structure, as shown in this study. Low–altitude soils had higher levels of total nitrogen, soil organic matter and clay than high-altitude soils, which might explain the difference in enzyme activities and microbial biomass between low–altitude soils and high–altitude soils.
Lovell et al. (1995) suggested that differences in soil microbial biomass were associated with changes in community structure, such as a change in the relative proportion of bacteria and fungi. In our study, we interpreted a difference in soil microbial biomass (bacteria, actinomycetes, fungi, Gram-positive bacteria and Gram–negative bacteria) and community structure along the altitude gradient as a result of soil physicochemical properties including nutrients that were affected by temperature decreases. There was a significant decrease in bacteria biomass, as well as a decrease of actinomycete and Gram-negative bacteria biomass in high-altitude soils compared with low-altitude soils, whereas the ratio of Gram-positive to Gram-negative bacteria increased with the altitude gradient. Gram-negative bacteria appeared to be more competitive in low-altitude soils (with suitable temperature and high nutrient content) because they grow under substrate–rich conditions, while Gram-positive bacteria are dominant in resource-limited areas (Atlas and Bartha 1997. However, the fraction of Gram-positive bacteria also decreased with increasing altitude, mainly caused by low nutrient content. However, these organisms were more adapted to harsh conditions than Gram-negative bacteria, which could help explain why the ratio of Gram-positive to Gram-negative bacteria increased with altitude. The fraction of fungal biomass and the ratio of bacteria to fungi showed no significant change along the altitude gradient. In cold environments, fungi are characterized by lower optimum and maximum temperatures for growth compared with bacteria (Margesin et al. 2003) and are thus well adapted to cold climates. The ratio of cyclopropyl/precursor could be used to indicate the level of environmental harshness. Cyclopropyl fatty acid increases when environmental harshness increases, which explains why the ratio of Cy/Pre increased with increasing altitude.
Soil enzyme activities reveal microbial metabolic requirements and available soil nutrients (Caldwell 2005). Bacteria, fungi and plant roots can synthesize enzymes and release them into soils. Soil enzyme activities are very stable and can retain their functions for more than a year (Alkorta et al. 2003). Bacteria can be a major source of peroxidase, alkaline phosphatase and protease (Bach and Munch 2000; Kamimura and Hayano 2000), whereas plants can be a major source of urease and β–glucosidases. Fungi (Halaouli et al. 2006), bacteria (Faccio et al. 2012) and plants (Mayer 2006) are sources of phenol oxidase, which is a “latch” on a global carbon store (Freeman et al. 2001). The tested β-glucosidase, invertase, phenol oxidase, peroxidase, L-asparaginase, protease, urease and alkaline phosphatase in the present study play an essential role associated with C, N, and P cycles in soils.
In mountain areas, temperature decreases with increasing altitude; elevation gradients thus represent powerful natural experiments in which soil enzyme activities respond to variation in temperature. Most soil enzyme activities clearly decreased with increased elevation. Similar results were found by Margesin et al. (2009), who showed that soil enzyme activities decreased with increasing elevation in alpine and subalpine soils. Invertase, β-glucosidases, phenol oxidase and peroxidase are closely related to carbon cycling in soil. Invertase is an important enzyme in the carbon conversion cycle via the hydrolysis of carbohydrates. The inhibition of most β-glucosidases by glucose significantly contributes to the need for high cellulase loads (Qing et al. 2010). Both invertase and β-glucosidases display similar patterns along an altitude gradient, with the highest activities detected at 4737 ma.s.l. The activities of invertase and β-glucosidases were generally recognized to be related to SOC, consistent with our results. We speculate that temperature might indirectly influence these two enzymes by influencing SOC and TP in soils. Phenol oxidase can promote phenol oxidation into 1,2–benzoquinone and 1,4–benzoquinone in aerobic conditions. Quinones can inhibit microbial infection for self-protection. Moreover, phenol oxidase can degrade lignin and soluble phenolic compounds, thereby reducing carbon storage (Fenner et al. 2005). Peroxidase can oxidize substrates using hydrogen peroxide as the electron acceptor. The activities of phenol oxidase and peroxidase significantly decreased with an increase in altitude, suggesting that an increasingly anaerobic environment accompanies an increase in altitude, which led to decreased enzyme activity (Fenner et al. 2005; Freeman et al. 2001). Thus, wetland ecosystems at lower elevation in the Tibet Plateau play a more important role in global carbon cycles. L-asparaginase, protease and urease were correlated with the nitrogen cycle. All of these enzymes are hydrolytic enzymes. In particular, urease is a key enzyme for soil nitrogen transformation that can decompose urea into ammonia and carbon dioxide. Therefore, urease activity is a very important indicator of soil nitrogen status, which is closely related to TN, DON and ammonium. Urease activity significantly decreased with an increase in altitude, which coincides with the change in nitrogen content along the altitude gradient. The activities of the other two enzymes showed consistent changes along the altitude gradient. Enzyme activities also decreased with increasing altitude, which suggests that decomposition of soil organic matter might decrease with an increase in altitude, and lower elevations surely play larger roles in the global nitrogen cycle. Uchima et al. (2011) also observed a decrease in enzyme activities with an increase in altitude (i.e., a decrease in temperature). Alkaline phosphatase is responsible for phosphorus cycling in wetland soils, and alkaline phosphatase also decreased with a decrease in temperature, indicating that low temperature is not favourable for phosphorus absorption from soil minerals.
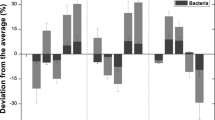
An understanding of the patterns and mechanisms of soil enzyme activities along an altitudinal gradient would greatly enhance our understanding of the influence of elevation on microbial distribution, especially for soil enzymes that are mainly derived from soil microbes. Peroxidase, alkaline phosphatase and protease activities showed consistent changing patterns along the altitude gradient (Fig. 5). It is obvious that soil enzyme activities decreased faster than bacterial biomass with increasing altitude, which suggests that decreasing soil enzyme activities were not only caused by decreased bacterial biomass but were also was affected by decreased temperature. In other words, the decreasing activities of peroxidase, protease and alkaline phosphatase were mainly caused by a decline in enzyme capacity with temperature, with the latter mainly affected by temperature (r = 0.629, P < 0.001; r = 0.588, P = 0.001, r = 0.759, P < 0.001, respectively).
In conclusion, our results showed that temperature affects microbial community structure and soil enzyme activities. With an increase in altitude, i.e., a decrease in temperature, soil physicochemical properties changed along the altitude gradient. Soil enzyme activities (phenol oxidase, peroxidase, L–asparaginase, protease, urease and alkaline phosphatase) and microbial biomass all decreased with increasing altitude. The declines in peroxidase, alkaline phosphatase and protease activities, all derived from soil microbes, were mainly caused by decreasing enzyme capacity with temperature rather than a decrease in microbial biomass.
References
Alkorta I, Aizpurua A, Riga P, Albizu I, Amezaga I, Garbisu C (2003) Soil enzyme activities as biological indicators of soil health. Reviews on Environmental Health 18:65–73
Allison SD, Jastrow JD (2006) Activities of extracellular enzymes in physically isolated fractions of restored grassland soils. Soil Biology and Biochemistry 38:3245–3256
Amador JA, Glucksman AM, Lyons JB, Gorres JH (1997) Spatial distribution of soil phosphatase activity within a riparian forest. Soil Science 162:808–825
Atlas RM, Bartha R (1997) Microbial ecology: fundamentals and applications. 4th ed. Benjamin/ Cummings Science Publishing, New york; pp: 341, 348–350
Bach HJ, Munch JC (2000) Identification of bacterial sources of soil peptidases. Biology and Fertility of Soils 31:219–224
Boerner REJ, Decker KLM, Sutherland EK (2000) Prescribed burning effects on soil enzyme activity in a southern Ohio hardwood forest: a landscape-scale analysis. Soil Biology and Biochemistry 32:899–908
Bolton H, Smith JL, Link SO (1993) Soil microbial biomass and activity of a disturbed and undisturbed shrub-steppe ecosystem. Soil Biology and Biochemistry 25:545–552
Caldwell BA (2005) Enzyme activities as a component of soil biodiversity: a review. Pedobiologia 49:637–644
Djukic I, Zehetner F, Mentler A, Gerzabek MH (2010) Microbial community composition and activity in different alpine vegetation zones. Soil Biology and Biochemistry 42:155–161
Eivazi F, Tabatabai MA (1988) Glucosidases and galactosidases in soils. Soil Biology and Biochemistry 20:601–606
Erwin KL (2009) Wetlands and global climate change: the role of wetland resoration in a changing world. Wetlands Ecology and Management 17:71–84
Faccio G, Kruus K, Saloheimo M, Thony-Meyer L (2012) Bacterial tyrosinases and their applications. Process Biochemistry 47:1749–1760
Federle TW, Dobbins DC, Thorntonmanning JR, Jones DD (1986) Microbial biomass, activity, and community structure in subsurface soils. Ground Water 24:365–374
Fenner N, Freeman C, Reynolds B (2005) Hydrological effects on the diversity of phenolic degrading bacteria in a peatland: implications fro carbon cycling. Soil Biology and Biochemistry 37:1277–1287
Fierer N, Jackson RB (2006) The diversity and biogeography of soil bacterial communities. Proceedings of the National Academy of Sciences of the United States of America 103:626–631
Freeman C, Ostle NJ, Kang H (2001) An enzyme 'latch' on a global carbon store. Nature 409:149
Frostegard A, Baath E, Tunlid A (1993) Shifts in the structure of soil microbial communities in limed forests as revealed by phospholipid fatty-acid analysis. Soil Biology and Biochemistry 25:723–730
Garcia C, Hernandez T (1997) Biological and biochemical indicators in derelict soils subject to erosion. Soil Biology and Biochemistry 29:171–177
Giri DD, Shukla PN, Kashyap S, Singh P, Kashyap AK, Pandey KD (2007) Variation in methanotrophic bacterial population along an altitude gradient at two slopes in tropical dry deciduous forest. Soil Biology and Biochemistry 39:2424–2426
Griffiths RI, Thomson BC, James P, Bell T, Bailey M, Whiteley AS (2011) The bacterial biogeography of British soils. Environmental Microbiology 13:1642–1654
Guckert JB, Hood MA, White DC (1986) Phospholipid ester-linked fatty-acid profile changes during nutrient deprivation of vibrio-cholerae-increases in the trans cis ratio and proportions of cyclopropyl fatty–acids. Applied and Environmental Microbiology 52:794–801
Halaouli S, Asther M, Sigoillot JC, Hamdi M, Lomascolo A (2006) Fungal tyrosinases: new prospects in molecular characteristics, bioengineering and biotechnological applications. Journal of Applied Microbiology 100:219–232
Jones DL, Willett VB (2006) Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biology and Biochemistry 38:991–999
Kamimura Y, Hayano K (2000) Properties of protease extracted from tea-field soil. Biology and Fertility of Soils 30:351–355
Kandeler E, Gerber H (1988) Short-term assay of soil urease activity using colorimetric determination of ammonium. Biology and Fertility of Soils 6:68–72
Kieft TL, Wilch E, O'Connor K, Ringelberg DB, White DC (1997) Survival and phospholipid fatty acid profiles of surface and subsurface bacteria in natural sediment microcosms. Applied and Environmental Microbiology 63:1531–1542
Kourtev PS, Ehrenfeld JG, Haggblom M (2002) Exotic plant species alter the microbial community structure and function in the soil. Ecology 83:3152–3166
Ladd J, Butler J (1972) Short–term assays of soil proteolytic enzyme activities using proteins and dipeptide derivatives as substrates. Soil Biology and Biochemistry 4:19–30
Limpens L, Berendse F, Blodau C, Canadell JG, Freeman C, Holden J, Roulet N, Rydin H, Schaepman-Strub G (2008) Peatlands and the carbon cycle: from local processes to global implications–a synthesis. Biogeosciences 5:1475–1491
Lomolino MV (2001) Elevation gradients of species-density: historical and prospective views. Global Ecology and Biogeography 10:3–13
Lovell RD, Jarvis SC, Bardgett RD (1995) Soil microbial biomass and activity in long–term grassland– effects of management changes. Soil Biology and Biochemistry 27:969–975
Ma XJ, Chen T, Zhang GS, Wang R (2004) Microbial community structure along an altitude gradient in three different localities. Folia Microbiologica 49:105–111
Ma K, Zhang Y, Tang SX, Liu J (2016) Spatial distribution of soil organic carbon in the Zoige alpine wetland, northeastern Qinghai–Tibet plateau. Catena 144:102–108
Mannisto MK, Tiirola M, Haggblom MM (2007) Bacterial communities in Arctic fjelds of Finnish Lapland are stable but highly pH–dependent. FEMS Microbiology Ecology 59:452–465
Margesin R, Gander S, Zacke G, Gounot AM, Schinner F (2003) Hydrocarbon degradation and enzyme activities of cold-adapted bacteria and yeasts. Extremophiles 7(6):451–458
Margesin R, Jud M, Tscherko D, Schinner F (2009) Microbial communities and activities in alpine and subalpine soils. FEMS Microbiology Ecology 67:208–218
Mayer AM (2006) Polyphenol oxidases in plants and fungi: going places? A review. Phytochemistry 67:2318–2331
Mutabaruka R, Hairiah K, Cadisch G (2007) Microbial degradation of hydrolysable and condensed tannin polyphenol-protein complexes in soils from different land-use histories. Soil Biology and Biochemistry 39:1479–1492
Qing Q, Yang B, Wyman CE (2010) Xylooligomers are strong inhibitors of cellulose hydrolysis by enzymes. Bioresource Technology 101:9624–9630
Rahbek C (2005) The role of spatial scale and the perception of large-scale species-richness patterns. Ecology Letters 8:224–239
Rousk J, Baath E, Brookes PC, Lauber CL, Lozupone C, Caporaso JG, Knight R, Fierer N (2010) Soil bacterial and fungal communities across a pH gradient in an arable soil. The ISME Journal 4:1340–1351
Schinner F, Gstraunthaler G (1981) Adaptation of microbial activities to the environmental-conditions in alpine soils. Oecologia 50:113–116
Shen C, Xiong J, Zhang H, Feng Y, Lin X, Li X, Liang W, Chu H (2013) Soil pH drives the spatial distribution of bacterial communities along elevation on Changbai Mountain. Soil Biology and Biochemistry 57:204–211
Sinsabaugh RL, Carreiro MM, Repert DA (2002) Allocation of extracellular enzymatic activity in relation to litter composition, N deposition, and mass loss. Biogeochemistry 60:1–24
Tscherko D, Rustemeier J, Richter A, Wanek W, Kandeler E (2003) Functional diversity of the soil microflora in primary succession across two glacier forelands in the central alps. European Journal of Soil Science 54:685–696
Tunlid A, Hoitink HAJ, Low C, White DC (1989) Characterization of bacteria that suppress rhizoctonia damping–off in bark compost media by analysis of fatty–acid biomarkers. Applied and Environmental Microbiology 55:1368–1374
Uchima CA, Tokuda G, Watanabe H, Kitamoto K, Arioka M (2011) Heterologous expression and characterization of a glucose-stimulated β-glucosidase from the termite Neotermes Koshunensis in aspergillus oryzae. Applied Microbiology and Biotechnology 89:1761–1771
Wagai R, Kitayama K, Satomura T, Fujinuma R, Balser T (2011) Interactive influences of climate and parent material on soil microbial community structure in Bornean tropical forest ecosystems. Ecological Research 26:627–636
Waldrop MP, Balser TC, Firestone MK (2000) Linking microbial community composition to function in a tropical soil. Soil Biology and Biochemistry 32:1837–1846
Wang Z, Luo T, Li R, Tang Y, Du M (2013) Causes for the unimodal pattern of biomass and productivity in alpine grasslands along a large altitudinal gradient in semi–arid regions. Journal of Vegetation Science 24:189–201
White D, Davis W, Nickels J, King J, Bobbie R (1979) Determination of the sedimentary microbial biomass by extractible lipid phosphate. Oecologia 40:51–62
Yao TD, Liu XD, Wang NL, Shi YF (2000) Amplitude of climatic changes in Qinghai–Tibetan plateau. Chinese Science Bulletin 45:1236–1243
Zelles L (1997) Phospholipid fatty acid profiles in selected members of soil microbial communities. Chemosphere 35:275–294
Zogg GP, Zak DR, Ringelberg DB, MacDonald NW, Pregitzer KS, White DC (1997) Compositional and functional shifts in microbial communities due to soil warming. Soil Science Society of America Journal 61:475–481
Acknowledgements
This work was funded by 973 Project from Science & Technology Department of China (2010CB951304–3), the Knowledge Innovation Program of the Chinese Academy of Sciences (KZCX2–EW–112), and the Program of western light of the Chinese Academy of Sciences (Y508RC1SGC).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tianzhu, L., Guicai, S., Jian, W. et al. Microbial Communities and Associated Enzyme Activities in Alpine Wetlands with Increasing Altitude on the Tibetan Plateau. Wetlands 37, 401–412 (2017). https://doi.org/10.1007/s13157-017-0876-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-017-0876-6