Abstract
We assessed the role of vegetation and hydrology in the Si cycle in the Okavango Delta. Our results show a large storage of biogenic Si (BSi) in vegetation and the sediments. The biological storage is among the highest observed so far for any ecosystem worldwide. Floodplain vegetation accumulates similar amounts of BSi in both the temporary floodplains and the permanent floodplains, with most values observed between 20 and 100 g Si m−2. This vegetation Si, after litterfall, contributes to a large biogenic Si storage in the sediments. In temporary floodplains, sediments contain less BSi (375–1950 g Si m−2 in the top 5 cm) than in the permanent floodplains (1950–3600 g Si m−2 in the top 5 cm). BSi concentrations in the floodplain sediments decline exponentially indicating rapid dissolution. In the occasional and seasonal floodplains, unidirectional solute transfer from floodplains to the islands will remove Si from the riverine systems. Our work clearly emphasizes the crucial role of floodplains and wetlands in Si transport through tropical rivers, and the potential interference of hydrology with this role.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Research on Si cycling in continental ecosystems has received increasing attention in the past decade. While it is clear that large amounts of weathered Si are cycling through ecosystems (Conley 2002; Carey and Fulweiler 2012), the quantification of biogenic Si in ecosystem soils and sediments (Barao et al. 2014), how this is related to biogenic Si (BSi) accumulation in vegetation and how this influences Si transport in rivers is still largely enigmatic (Struyf and Conley 2012). Current studies clearly point to the role of wetlands as Si-accumulators in the global biogeochemical Si cycle. Wetlands, situated at the interface between terrestrial and aquatic ecosystems, can act as strong filters on the transport of Si from the continents to the coastal environment (Struyf and Conley 2009), especially because large amounts of Si accumulating grasses often dominate the vegetation. More and more evidence points to the accumulation of Si in wetlands grasses being a functional trait, where uptake of Si is dependent on the environmental stressors experienced by the vegetation, e.g. herbivory or flooding dynamics (Schoelynck et al. 2014; Carey and Fulweiler 2014). As such, wetlands are essential ecosystems to study in the emerging research field of biological control on the continental Si mobilization (Derry et al. 2005). In temperate and subarctic climates, both riparian (e.g. Struyf et al. 2009, 2010) and tidal wetlands (e.g. Struyf et al. 2006; Müller et al. 2013) have been studied, but tropical wetlands have not yet received attention.
The Okavango Delta (Botswana) is one of the world’s largest tropical wetland systems, and hence an interesting study area in this regard. It consists of a mosaic of permanent and temporary floodplains, intersected with tree-covered islands (McCarthy et al. 2012). The Okavango Delta’s water balance is dominated by evapotranspiration: 98 % of all water is lost to the atmosphere before reaching the outlets. The islands play an unusual and essential ecological role in the Okavango Delta as they act as permanent sinks for a major part of the solutes (ca. 360,000 tons a−1) that enter the system (Ramberg and Wolski 2008). Various biological mechanisms result in unidirectional solute transfer from the floodplains to the islands that allow the Delta to remain a productive freshwater wetland ecosystem (Ramberg and Wolski 2008). Evapotranspiration by island trees induces a local lowering of the water table below the islands, creating large hydraulic gradients and inducing lateral groundwater flow. As a result, water infiltrates into the floodplains and flows belowground from the floodplains to the islands bringing dissolved salts with water flow.
The concentration of dissolved Si (DSi) increases below the islands due to evaporative enrichment, causing precipitation of base-cation rich clays, carbonates and amorphous Si (McCarthy et al. 1998). This causes volumetric expansion, which is a key mechanism of island growth. More soluble components reach the center of the islands (e.g. sodium bicarbonate), where they are lost to deeper groundwater via a density fingering process (Bauer et al. 2006; Zimmermann et al. 2006).
The role of biota in the formation of islands has been recognized. Islands in the Delta initiate around a termite mound, gradually building from this basis (McCarthy et al. 1998). Large trees—which are especially dense on the island fringes (McCarthy et al. 2012) - play an essential role in maintaining the hydraulic pressure gradient through transpiration. However, while the role of islands in sequestering Si is known, we have little understanding of the role of vegetation in depositing and sequestering Si in the floodplains, though they actually cover a much greater surface area. We here hypothesize that floodplain vegetation plays an essential role in the assimilation of dissolved Si from the water and, after dissolution of plant material, its delivery to the island sub surfaces. The selective removal of dissolved Si from the Okavango Delta surface waters has been identified, based on its relative enrichment in concentration below that expected from evaporation and rain water input (Sawula and Martins 1991). However, Si precipitation has not been linked earlier to floodplain vegetation associated processes.
Vegetation in the Okavango Delta floodplains mainly consists of sedges and grasses, with key species including Miscanthus junceus, Cyperus papyrus and Phragmites australis. These species are well known Si accumulators, with elemental Si concentrations in P. australis reaching 5 % of dry biomass (e.g. Struyf et al. 2007). Si in vegetation occurs as BSi mainly in structures called phytoliths, deposited at sites of transpiration. Plant BSi plays a role in stress resistance and intra/interspecific competition, e.g. increasing resistance against flow shear stress and salt stress (e.g. Cooke and Leishman 2011). In aquatic macrophytes, BSi has a role similar to lignin and cellulose, increasing the capability of vegetation to withstand high flows and to maintain an upright stature, important for sustained photosynthesis in flooded environments (Schoelynck et al. 2010). Apart from vegetation, diatoms are also prominent in the Delta floodplains (e.g. Mackay et al. 2012; Davidson et al. 2012) and have an obligate requirement for dissolved Si for building their frustules. Thus, there is a large potential for biologically mediated Si accumulation in the floodplains of the Okavango Delta, corroborated by previous research which suggests the substantial occurrence of plant phytoliths in the peat of the permanent floodplains (McCarthy et al. 1989).
We hypothesize that biogenic Si accumulation in the vegetation functions as a trapping mechanism for most of the DSi entering and flowing through the Delta. After vegetation senescence, the BSi contributes to the accumulation of biogenic Si in floodplain sediments, where it is available for dissolution. After dissolution, DSi may be taken up again by the vegetation or transported further down the Delta. Crucially, a significant part may also be transported along with lateral water flow to the island sub-surfaces.
We sampled the uppermost sediments on both islands and floodplains and the associated vegetation, along gradients from island centers to the channels bordering the floodplains at both a temporary and a permanent floodplain and their adjacent islands. We make conservative estimates of the storage of BSi in the vegetation and compare it to annual inputs of Si into the Delta, then relate this to Si storage patterns across hydrological gradients within floodplain sediments. We hypothesize that the highest storage of biogenic Si in the sediments will occur in areas with permanent inundation, while the highest dissolution potential of accumulated BSi exists in the temporal floodplains, where strong hydraulic pressure from floodplains to islands causes more frequent water refreshing, enhancing porewater leaching of DSi (recycled from BSi).
Study Site and Transect Sampling
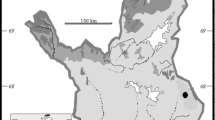
The Okavango Delta (Fig. 1) is in fact not a delta but a low gradient (1:3400) alluvial fan (McCarthy et al. 1998). The inundated area of the Delta fluctuates from 6000 km2 during low flow seasons to over 15,000 km2 during high flow seasons (Gumbricht et al. 2004). The annual flood pulse that inundates the Okavango Delta originates as precipitation in the highlands of Angola and reaches the Mohembo inlet (Botswana) by February through June, peaking in April. The mean annual discharge into the Delta is approximately 9.0 × 109 m3 (McCarthy et al. 2003). It takes about four months for the flood water to reach the most downstream distal reaches of the Delta near Maun, Botswana about 260 km from the inlet at Mohembo (Wolski et al. 2006). Direct rainfall into the Delta contributes about 42 % (about 6 × 109 m3 per annum) to the mean flow, mostly by summer rains out of phase with the riverine flood-pulse (Ramberg and Wolski 2008). The Okavango Delta annually receives between 0.0024 and 0.0036 TMol (×1012 mol) of DSi from the Okavango River which flows into the Delta through a narrow conduit called the Panhandle (Ramberg and Wolski 2008, calculated using water input and measured Si concentrations in the Panhandle).
The Okavango Delta has been divided into physiographic regions: (i) the Panhandle, the confined entry channel; (ii) the permanent swamp; (iii) the seasonal and occasional swamps. The Okavango River (~100 m width) flows into the Panhandle (~25 km wide) where it meanders through a permanently-flooded Cyperus papyrus dominated landscape. From the Panhandle the Okavango River enters the permanent swamps where it divides into three main distributary channels. The permanent swamp is intersected by channels, lagoons and lakes, dominated by emergent C. papyrus. Water movement in the permanent swamps is slow due to the low gradient and the submerged and emergent vegetation, and dry land surfaces are relatively small. The seasonal swamp and occasional swamps (hereafter referred to as temporary swamps) have more prominent dry land areas. In the temporary swamp, flood waters flow mainly through channels, bordered by grasses and reeds, and overflow onto shallow, grassy floodplains. Most islands are thus found in the temporary floodplains in the distal ends of the delta, where they cover over 50 % of the total surface area. The exact proportion is variable and depends on the actual extent of the flooding (Gumbricht et al. 2004).
Subsurface geology of the 165,000 km2 catchment is dominated by Precambrian/Palaeozoic continental cratons and is nearly entirely overlain by highly weathered Kalahari sands of Cenozoic age, in places >300 m deep, whose influence dominates the Okavango River dissolved and suspended loads. Clay mineral neoformation occurs throughout the Delta—when the geochemistry permits (Frings et al. 2014): however, the majority of element sequestration occurs beneath the islands (Frings et al. 2014).
We selected two island-floodplain gradients in the Okavango Delta: one in the permanently flooded wetlands (“Guma”) and one in the temporary wetlands (“Nxaraga”) (Fig. 1). At both locations, we sampled sediment and vegetation along full hydrological gradients, from the center of the islands to the edges of the channel bordering floodplain. These gradients were subdivided into 9 (Guma) and 12 (Nxaraga) sampling locations respectively, representing the visual variability in vegetation cover (or absence thereof). Vegetation and surface sediment samples (25 cm) were taken at each of these sampling sites, as described below. Additionally, at Nxaraga, deeper (max 8 m) boreholes were drilled in five island sampling sites and one floodplain sampling site.
Materials and Methods
Sampling
Aboveground vegetation and litter samples were taken from two random quadrats (0.25 m2) at each selected sampling site (no trees were sampled, only herbaceous/grass vegetation and litter). Standing biomass is at its peak at the time of sampling. Vegetation species composition was determined during cutting (Table 1). At each selected sampling point along the transects, three sediment cores (25 cm length and 28 mm ø) were sampled using a hammer auger with a removable plastic lining (Eijkelkamp 04.15.SA Foil sampler). The sediment cores were each subsampled at 6 different depths and pooled per depth per sampling spot. The litter layer was included in the vegetation samples and not the sediment core. Depths at which samples were taken were not exactly the same at all sampling spots, as we took into account clearly visible horizons of organic and inorganic sediment. In general, the first 10 cm was sliced into three parts and the next 15 cm also. The samples were wet-weighed, immediately frozen and stored until analysis. Along an overlapping transect at Nxaraga (Fig. 1), six boreholes were drilled by large-diameter hand-auger (Eijkelkamp) along the island-floodplain gradient to a maximum depth of 8 m using hole casing and a bailer where necessary. Samples of ~40 g were taken every 25 cm from the visually undisturbed/uncontaminated centre of the auger, and stored cool in zip-lock bags until freeze-drying.
Si Analysis
Vegetation samples (litter + aboveground vegetation) were thoroughly rinsed and cleaned to remove sediments, epiphytic algae and macro-invertebrates. Subsequently, they were oven dried at 75 °C for three days (72 hours). The dried samples were weighed immediately after removing from the oven and then ground and homogenized (< 0.5 mm) in bulk. The total BSi content of the bulk vegetation from every quadrat was analysed: 25 mg biomass was digested with 25 ml 0.094 M Na2CO3 for 6 h at 80 °C (e.g. Struyf et al. 2005). For the most abundant species, individuals were selected in the area directly next to the sampling spots, for analysis of BSi in these individual species. Individual species analyses refer to the whole aboveground biomass: no organ-specific extraction was performed.
Sediment samples were dried using an IMA-Telstar Cryodos-50 freeze dryer, sieved at 500 μm to remove macroscopic plant and shell material before analysis, and weighed for bulk density. Subsequently, they were digested in 30 ml of 0.094 M Na2CO3 in a shaking water bath at 80 °C (e.g. Struyf et al. 2009, 2010). After 4, 5 and 6 h, subsamples were taken and analyzed with an ICP-OES (iCAP 6300 Duo, © Thermo Scientific) or by the molybdate blue spectrophotometric method (for the borehole samples only) for extracted DSi (all Si concentrations expressed as elemental Si further on, and not SiO2). To correct for the amount of Si resulting from mineral dissolution, the Si content of the subsamples was plotted against dissolution time. All biogenic Si dissolves within the first few hours: the y-axis intercept of the linear least-squares regression line through the measured values represents the estimated BSi content (DeMaster 1981). Recent research has clearly shown that non-biogenic Si fractions can interfere with the extraction of BSi (Struyf and Conley 2012), e.g. other amorphous Si fractions like adsorbed Si. The term ASi (amorphous Si) has therefore been adopted by other studies. The analysis is currently undergoing rapid methodological development (Barao et al. 2014). Recent insights show that extraction using 0.094 M Na2CO3 could also lead to an underestimation of the total BSi content, as some fractions of phytoliths only dissolve in stronger alkaline solutions (e.g. 0.5 M NaOH) (e.g. Saccone et al. 2007; Meunier et al. 2014). However, these stronger solutions also more readily extract mineral silicates. Due to the abundance of quartz and kaolinite in Okavango sediments (e.g. McCarthy et al. 1989), we used 0.094 M Na2CO3. This also facilitates comparison to most other studies on soil BSi storage, which have typically applied Na2CO3 to digest BSi (e.g. Clymans et al. 2011; Carey and Fulweiler 2013). In this manuscript, we decide to adopt the term BSi for alkaline extracted Si in both surface sediments and vegetation. In sediment, the mainly biogenic origin of the extracted Si was confirmed by microscopical analyses (Frings et al. 2014). However, for the deep cores we adopted the term ASi, as earlier research has confirmed that large non-biogenic amorphous Si precipitates here constitute most of the amorphous Si. Si concentrations in vegetation and litter are expressed as elemental Si per total dry biomass (%). Sediment BSi or ASi (for the deep cores) is expressed as elemental Si relative to the total weight of dry sediment (%). Si stock calculations were calculated from the multiplication of biomass per quadrat and relative BSi content of the biomass. Sediment Si stocks are calculated from the total sediment weight on a square meter basis, for either 5 or 25 cm depth, based on the bulk density, and the relative elemental BSi (or ASi) content of the sediment.
Results
Vegetation BSi
Vegetation samples included only herbaceous vegetation, grass and leaf litter, and only leaf litter at the forest site (GumDr5). The dominant species cover at each sample site is shown in Table 1. Vegetation generally contained a higher stock of BSi in the Guma floodplain compared to the Guma island (except for the riparian forest site). At Guma, floodplain vegetation contained between 20 and 80 g BSi m−2, while island vegetation contained between 1 and 30 g m−2. The tendency for lower vegetation BSi stock on the island was also clear in Nxaraga (2–40 g m−2), whereas floodplains contained about 20–160 g BSi m−2 in the vegetation (Fig. 2). Vegetation BSi concentrations at islands and floodplains showed no clear island/floodplain distinction. At Guma, island vegetation contained (for complete sampled plots) on average 1.7 % of BSi (range 1.2–2.8 %) and floodplain vegetation contained 2.5 % BSi on average (range 1.0–3.4 %). At Nxaraga, island vegetation contained (for complete sampled plots) on average 2.3 % of BSi (range 1.6–2.6 %) and floodplain vegetation contained 1.9 % BSi on average (range 0.9–2.9 %). Differences in stocks were fully attributable to the high biomasses occurring in the floodplains.
Vegetation BSi content (top panels) and sediment BSi content (lower panels, for top 5 cm of the sediment profile) in Guma (left) and Nxaraga (right), expressed as Si per m2. Sediment samples are pooled samples of 3 cores per location per depth. Right of the line are the floodplain sites, to the left island sites. The line itself indicates the last island site. Note the different scale of the vegetation BSi axis for both sampling sites (top panels)
Sediment BSi
Permanent Floodplains (Guma)
At the Guma site, BSi in the island sediment was both low in concentration (Fig. 3) and in total stock (Fig. 2). In the driest parts of the island, sediment BSi concentrations were between 0 and 0.5 %. In the riparian forest (GumDr5), sediment BSi concentrations were slightly higher than at the other island sites (Fig. 3). In the permanent floodplains, BSi exhibited different behaviour. Surface BSi concentrations ranged between 1 and 5 % BSi, then gradually decreased with depth to <0.5 % in all cores (Fig. 3). Stocks of BSi in the permanent floodplains (top 5 cm) were between 100 and 550 g BSi m−2 at sites on the islands, and between 1950 and 3600 g m−2 in the floodplains themselves (Fig. 2). Storage over the full 25 cm depth ranged between 1300 and 4600 g BSi m−2 on Guma island and between 5200 and 10,500 g BSi m−2 in the Guma floodplains.
Temporary Floodplains (Nxaraga)
At the driest island sites in the temporary floodplain region, BSi concentrations were low throughout the cores and generally below 0.5 % BSi (Fig. 3). At the two riparian forest sites (NXADr4 and NXADr5), concentrations were higher averaging 1 % with slightly higher values in the deeper section (20–25 cm). Overall, variability in BSi from the island cores was low. In the Nxaraga floodplain cores, BSi concentration decreased with depth in the 5 cores closest to the channel. Maximum concentrations at the surface between 0.6 and 1.7 % BSi was always lower than in the permanent floodplains at Guma (Fig. 3). In the two floodplain cores closest to the island, surface BSi concentrations were always 0.4 % BSi or lower. In all floodplain cores, BSi concentrations at depth (20–25 cm) were lower than 0.4 %. BSi stocks in the top 5 cm of sediment were similar across the entire transect in cores from Nxaraga island and its floodplains, ranging between 175 and 1300 g m−2 (island cores) and 375–1950 g m−2 (floodplain cores). This is in stark contrast to the clear separation between floodplains and islands in the permanently flooded transect at Guma (Fig. 2). BSi stocks (25 cm profile) ranged between 800 and 7200 g m−2 in Nxaraga island and between 1300 and 3900 g m−2 in the floodplain with the highest BSi accumulation in the riparian forest sites on the island.
ASi concentrations at Nxaraga were also explored further in deep cores up to 8 m depth (Fig. 4). The floodplain coring here clearly shows that negligible concentrations of ASi (<0.3 %) occur through the deeper layers up to 8 m depth. In the island cores, a clear accumulation peak of ASi can be observed at about 3–3.5 m in the centre of the island and the riparian forest, with concentrations up to 4.5 %. Concentrations in the other island cores generally ranged between 0.5 and 2 % throughout the depth profile.
ASi in the deep cores from Nxaraga (weigth percent in percent ASi per sediment dry mass), in sample sites from the center of the island, through intermediate samplings and the riparian forest to the floodplain. The exact location of the deep cores can be found in Fig. 1 (Deep 1–6). We have specifically indicated the island center core, the riparian forest core and the floodplain core, as they are specifically referred to in the results section
Discussion
Our results indicate that in the Okavango Delta, the trapping of DSi by vegetation is an important step in the retention of Si. Floodplain vegetation stores similar amounts of BSi in both the temporary floodplains and the permanent floodplains, with most values observed between 20 and 100 g Si m−2. For sediment BSi storage, most of the BSi occurs in the upper layers. The temporary floodplains (375–1950 g BSi m−2 in the top 5 cm) contain less BSi than the permanent floodplains (1950–3600 g ASi m−2 in the top 5 cm). BSi concentrations in the floodplain sediment decline exponentially indicating rapid dissolution of BSi with most dissolution occurring within the first 5 cm. The top sediment layers represent a reservoir of biologically accumulated Si that rapidly recycles.
Sediment accumulation rates in the Okavango Delta can reach up to 5 cm per year in certain settings (McCarthy et al. 1989). If we assume a recent estimate of ~0.25 cm a−1 is more representative of average accumulation (Bernal and Mitsch 2013), this implies between 100 and 175 g of BSi is recycled annually to DSi per m2 in the top 5 cm in the permanent floodplain, based on the decrease in BSi concentrations in the sediment. Rapid rates of recycling from marsh grass litter have been shown in tidal wetlands and under experimental conditions (Struyf et al. 2007; Schaller and Struyf 2013), but are unavailable currently for tropical wetlands. Future studies on dissolution rates of Si from tropical wetland litter are necessary to quantify recycling rates in more detail. Most of the “recycled” DSi will end up in the vegetation again, as annual biomass production is approximately double the standing vegetation stock in the permanent floodplains (McCarthy et al. 1989), requiring annual uptake of 40–200 g DSi m−2 a−1. Spatially distributed riverine annual input from the Okavango river is ca. 20 g m−2 in the permanent floodplains (assuming 6000 km2 of permanent floodplain and an annual input of 0.0030 Tmole of Si). Our calculations require efficient recycling to support the majority of BSi production. Some fraction of the dissolved BSi will not be taken up again by vegetation, and will be exported as DSi from the floodplain sediment, either infiltrating deeper into the sediment or transported further downstream into the Delta. This can be replenished by the spatially distributed annual riverine input into the permanent floodplain.
In the temporary floodplains, abundant islands create a larger hydraulic head between surface water and island groundwater. This probably is an important cause for both lower BSi concentrations and stocks in the temporary floodplain relative to the permanent floodplain. Despite approximately equal amounts of BSi in vegetation in the two regions, the lowest values for sediment BSi storage in the permanent floodplains were greater than the highest values in the seasonal floodplains. In contrast to the permanent floodplains, a strong unidirectional transport of water from floodplains to island interiors is present in the temporary inundated areas. Given the large surface area of islands in the temporary floodplains, the vast majority of Si accumulated is in the interior of the islands, in contrast to the permanent floodplain where island surface area is limited (Gumbricht et al. 2004). This is confirmed in the ASi data in deep cores. It is not surprising that the islands only accumulate small amounts of ASi in the top layers: most Si accumulates underneath the islands in the deeper sediment horizons, especially below the riparian forest. Earlier and our data show the greatest accumulation of Si and other elements in the islands at the sites of strongest evapotranspiration, i.e. the forested island fringes (Bauer et al. 2004). The importance of amorphous Si precipitates in the build-up of islands has been pointed to in earlier research (e.g. Ramberg and Wolski 2008; McCarthy et al. 2012).
Tropical Wetlands and Si Cycling
Our results clearly show a large surficial storage of biogenic Si in the Okavango Delta. We used estimates of the areas of islands, permanent and temporary floodplains from Gumbricht et al. (2004) to assess total storage of BSi in the top 25 cm only of the Okavango Delta sediment, not taking into account deeper storage. Taking the lowest BSi storage amounts observed for the different delta sections, we can conservatively estimate total surficial BSi storage of 0.4 Tmole of Si in the permanent floodplains, 0.2 Tmole in the temporary floodplains and 0.01 Tmole in the islands, leading to a minimum estimate of 0.6 Tmole of BSi storage in the Delta. Alternatively, taking the upper-end estimates of our data results in storage values of 0.8 Tmole, 0.4 Tmole and 0.07 Tmole respectively, implying total Delta storage of 1.3 Tmole of Si in the top 25 cm. This surface storage represents about 200–400 years of DSi input into the Delta, as annual (modern day) input equals ~0.003 Tmole. Based on a vegetation BSi turnover (see earlier) of 100 g Si m−2 a−1, vegetation uptake in the floodplains represent about 0.020 Tmole a−1, compared to an annual input of 0.003 TMole of Si into the Delta.
The BSi storage in both the permanent and temporary floodplains is among the highest observed so far for any ecosystem worldwide (Struyf and Conley 2012), at 110–220 × 103 kg SiO2 ha−1 and 42–80 103 kg SiO2 ha−1 respectively. BSi pools over a full soil profile of between 30 and 104 × 103 kg SiO2 ha−1 were observed in soils from the Great Plains grasslands and equatorial rain forests (Blecker et al. 2006; Alexandre et al. 1997). The floodplains of the Okavango Delta also greatly exceed values observed in temperate tidal marshes and subarctic peat bogs (between 12 and 32 × 103 kg SiO2 ha−1) (Struyf et al. 2005, 2010). It should be emphasised that our estimates are conservative, particularly since we used a weak base extraction (0.094 M Na2CO3) and we are only integrating the top 25 cm of the sediment horizon whereas the other estimates are typically based on 1–2 m horizons. Some aged phytolith material as well as sponges may not have completely dissolved after only a few hours in 0.094 M Na2CO3 (Meunier et al. 2014). However, given the rapid recycling of BSi in sediments, we assume that aged diagenetically altered phytoliths are unlikely to represent a major fraction of BSi in our sediments. Part of the BSi we analysed likely derives from sponges (unpub. data), base-cation rich clays, reactive secondary silicate minerals and from non-biogenic amorphous reactive Si phases, including Si adsorbed to aluminum- or iron oxy-hydroxides (McKeague and Cline 1963). Fractionation between these non-biogenic and biogenic Si phases should be a research priority in the future.
Recently, Carey and Fulweiler (2013) estimated annual BSi uptake in global vegetation of about 85 Tmole. Of this, they calculated that the total surface of wetlands worldwide contributes approximately 4.16 Tmole a−1. Our conservative vegetation BSi production estimate of 0.02 Tmole of Si a−1 would represent about 0.4 % of total annual uptake in wetland vegetation worldwide within the Okavango Delta alone, despite it covering only about 0.1 % of global wetland area (Mitsch et al. 2013). Although data for other tropical wetlands are limited, Cary et al. (2005) observed 2–4 % of BSi in swamp soils in the Nyong river basin in Cameroon, in the same range as concentrations in the Okavango. Tropical rivers deliver about 70–80 % of the global DSi load into the ocean (Jennerjahn et al. 2006), implying it is crucial to assess environmental factors that can influence its transport. Our data and the limited available literature data available clearly show that wetlands and floodplains are an important yet understudied component. Other studies have indicated the potential influence of Si uptake by giant grasses and sedges (e.g. Ding et al. 2008) through Si-isotope biogeochemistry in tropical rivers.
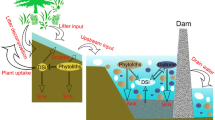
The hydrology of many tropical wetlands is undergoing major changes due to human alteration of river morphology and watersheds. Model predictions also project substantial future changes in hydrology and climate (Hamilton 2010; Wolski et al. 2014). This will have implications for flooding extent and seasonality, factors that induce changes in Si storage in the Okavango Delta. While vegetation is an important trap for Si in the permanently inundated wetlands, permanent long-term burial in sediments is likely low, as indicated by near-negligible BSi concentrations in the deeper layers. These conditions imply that reactive Si stores in the permanent floodplains are still intimately connected to flooding water, and thus a significant part of recycled Si is leaching back into “river” water after BSi dissolution. However, in the occasional and seasonal floodplains, unidirectional solute transfer from floodplains to the islands will remove reactive Si from the riverine systems (Fig. 5).
Conceptual figure of Si cycling in permanent and seasonal swamps. While temporary BSi storage is higher in the permanent swamps, recycling to the water column here is near complete. In contrast, floodplain storage is low in the seasonal swamps, and a strong unidirectional Si transfer to the islands dominates recycling to the water column
Outlook
Our results show that wetland vegetation in the Okavango Delta plays a key role in the annual Si budget of the Okavango Delta. Most of the DSi entering the permanent floodplains cycles through vegetation before transport further downstream. There is a clear distinction in the amount of BSi sequestered between permanent and temporary floodplains. While BSi stores are actively recycled in the sediments of the permanent floodplains (implying strong potential for leaching to surface waters), unidirectional transport of Si leads to the loss of recycled BSi into the tree covered islands in the temporary floodplains. Our work clearly emphasizes the crucial role of floodplains and wetlands in Si transport through tropical rivers.
Although our results are conservative estimates, our analysis indicates that the sediments of the Okavango Delta represent the one of the largest stores of sediment or soil BSi per m−2 yet identified worldwide. This emphasizes the necessity to construct an estimate of worldwide BSi storage and accretion or depletion rates in soils and sediments, which has still only been quantified in a limited number of ecosystems and with a restricted global distribution of sites studied (Struyf and Conley 2012), and to initiate studies on how the storage is affected by environmental conditions, e.g. flooding frequency, sediment and porewater pH and salinity in wetlands. Future studies in wetlands should also investigate the exact nature of stored Si through microscopical studies, and better constrain dissolution/retention fluxes through e.g. the detailed study of porewater profiles and potentially isotope fractionation. A recent study by Frings et al. (2014) shows this potentially provides added insight on processing of the large stocks of Si present in the Okavango delta, and wetlands in general. This will further allow a more nuanced understanding of whether the global Si cycle operates in steady state, or is instead characterised by time-lagged responses to anthropogenic or natural changes.
Based on high rates of vegetation accumulation we observed in the Okavango, the most recent estimate of annual uptake of vegetation BSi production (Carey and Fulweiler 2013) of ~84 Tmole Si year−1 could be underestimated. In our dataset, storage in floodplain sediments is 45 times greater than annual vegetation uptake. In Phragmites australis dominated tidal temperate wetlands, where daily tidal immersion/emersion cycles induce strong recycling potential, the uptake/storage ratio was about 40 (Struyf et al. 2005), while for bamboo, deciduous and coniferous forests with perennial vegetation, ratios of over 1000 are proposed (Conley 2002). This represents massive soil storage relative to annual continental Si fluxes (7.2 Tmole Si a−1; Tréguer and De La Rocha 2013) and annual weathering fluxes (10–40 Tmole Si a−1, Hilley and Porder 2008), showing the clear need for a better quantification of sediment BSi accumulation rates.
References
Alexandre A, Meunier J-D, Colin F, Koud J-M (1997) Plant impact on the biogeochemical cycle of silicon and related weathering processes. Geochimica et Cosmochimica Acta 61:677–682
Barao L, Clymans W, Vandevenne FI, Meire P, Conley DJ, Struyf E (2014) Pedogenic and biogenic alkaline extracted Si distributions along a temperate land-use gradient. European Journal of Soil Science, in press.
Bauer P, Thabeng G, Stauffer F, Kinzelbach W (2004) Estimation of the evapotranspiration rate from diurnal groundwater level fluctuations in the Okavango Delta, Botswana. Journal of Hydrology 288:344–355
Bauer P, Supper R, Zimmermann S, Kinzelbach W (2006) Geoelectrical imaging of groundwater salinization in the Okavango Delta, Botswana. Journal of Applied Geophysics 60:126–141
Bernal B, Mitsch W (2013) Carbon sequestration in freshwater wetlands in Costa Rica and Botswana. Biogeochemistry 115:77–93
Blecker SW, McCulley RL, Chadwick OA, Kelly EF (2006) Biologic cycling of silica across a grassland bioclimosequence. Global Biogeochemical Cycles 20:GB3023
Carey JC, Fulweiler RW (2012) The terrestrial silica pump. PLoS ONE 7:e52932
Carey JC, Fulweiler RW (2013) Nitrogen enrichment increases net silica accumulation in a temperate salt marsh. Limnology and Oceanography 58(1):99–111
Carey JC & Fulweiler RW (2014) Silica uptake by Spartina – evidence of multiple modes of accumulation from salt marshes around the world. Frontiers in Plant Science: 5
Cary L, Alexandre A, Meunier J-D, Boeglin J-L, Braun J-J (2005) Contribution of phytoliths to the suspended load of biogenic silica in the Nyong basin rivers (Cameroon). Biogeochemistry 74:101–114
Clymans W, Struyf E, Govers G, Vandevenne F, Conley DJ (2011) Anthropogenic impact on amorphous silica pools in temperate soils. Biogeosciences 8:2281–2293. doi:10.5194/bg-8-2281-2011
Conley DJ (2002) Terrestrial ecosystems and the global biogeochemical silica cycle. Global Biogeochemical Cycles 16:1121
Cooke J, Leishman MR (2011) Is plant ecology more siliceous than we realise? Trends in Plant Science 16:61–68
Davidson TA, Mackay AW, Wolski P, Mazebedi R, Murray-Hudson M, Todd M (2012) Seasonal and spatial hydrological variability drives aquatic biodiversity in a flood-pulsed, sub-tropical wetland. Freshwater Biology 57:1253–1265
Demaster DJ (1981) The supply and accumulation of silica in the marine-environment. Geochimica et Cosmochimica Acta 45:1715–1732
Derry LA, Kurtz AC, Ziegler K, Chadwick OA (2005) Biological control of terrestrial silica cycling and export fluxes to watersheds. Nature 433:728–731
Ding TP, Zhou JX, Wan DF, Chen ZY, Wang CY, Zhang F (2008) Silicon isotope fractionation in bamboo and its significance to the biogeochemical cycle of silicon. Geochimica et Cosmochimica Acta 72:1381–1395
Frings P, Clymans W, Jeppesen E, Lauridsen T, Struyf E, Conley DJ (2014) Lack of steady-state in the global biogeochemical Si cycle: emerging evidence from lake Si sequestration. Biogeochemistry 117:255–277
Gumbricht T, McCarthy J, McCarthy TS (2004) Channels, wetlands and islands in the Okavango Delta, Botswana, and their relation to hydrological and sedimentological processes. Earth Surface Processes and Landforms 29:15–29
Hamilton S (2010) Biogeochemical implications of climate change for tropical rivers and floodplains. Hydrobiologia 657:19–35
Hilley GE, Porder S (2008) A framework for predicting global silicate weathering and CO2 drawdown rates over geologic time-scales. Proceedings of the National Academy of Sciences of the United States of America 105:16855–16859
Jennerjahn TC, Knoppers BA, de Souze WFL, Brunskill GJ, Silva EIL, Adi S et al (2006) Factors controlling dissolved silica in tropical rivers. In: Ittekot V (ed) The silicon cycle. Island Press, Washington, D. C, pp 29–51
Mackay AW, Davidson T, Wolski P, Woodward S, Mazebedi R, Masamba WRL, Todd M (2012) Diatom sensitivity to hydrological and nutrient variability in a subtropical, flood-pulse wetland. Ecohydrology 5:491–502
McCarthy TS, McIver JR, Cairncross B, Ellery WN, Ellery K (1989) The inorganic-chemistry of peat from the maunachira channel-swamp system, Okavango Delta, Botswana. Geochimica et Cosmochimica Acta 53:1077–1089
McCarthy TS, Ellery WN, Dangerfield JM (1998) The role of biota in the initiation and growth of islands on the floodplain of the Okavango alluvial fan, Botswana. Earth Surface Processes and Landforms 23:291–316
McCarthy JM, Gumbricht T, McCarthy T, Frost P, Wessels K, Seidel F (2003) Flooding patterns of the Okavango wetland in Botswana between 1972 and 2000. AMBIO: A Journal of the Human Environment 32:453–457
McCarthy TS, Humphries MS, Mahomed I, Le Roux P, Verhagen BT (2012) Island forming processes in the Okavango Delta, Botswana. Geomorphology 179:249–257
McKeague JA, Cline MG (1963) Silica in soil solutions: II. The adsorption of monosilicic acid by soil and by other substances. Canadian Journal of Soil Science 43:83–96
Meunier JD, Keller C, Guntzer F, Riotte J, Braun JJ, Anupama K (2014) Assessment of the 1 % Na2CO3 technique to quantify the phytolith pool. Geoderma 216:30–35
Mitsch W, Bernal B, Nahlik A, Mander Ü, Zhang L, Anderson C, Jørgensen S, Brix H (2013) Wetlands, carbon, and climate change. Landscape Ecology 28:583–597
Müller F, Struyf E, Wanner A, Hartmann J, Jensen K (2013) A comprehensive study of silica pools and fluxes in Wadden Sea salt marshes. Estuaries and Coasts 36:1150–1164
Ramberg L, Wolski P (2008) Growing islands and sinking solutes: processes maintaining the endorheic Okavango Delta as a freshwater system. Plant Ecology 196:215–231
Saccone L, Conley DJ, Koning E, Sauer D, Sommer M, Kaczorek D, Blecker SW, Kelly EF (2007) Assessing the extraction and quantification of amorphous silica in soils of forest and grassland ecosystems. European Journal of Soil Science 58:1446–1459
Sawula G, Martins E (1991) Major ion chemistry of the lower Boro River, Okavango Delta, Botswana. Freshwater Biology 26:481–493
Schaller J, Struyf E (2013) Silicon controls microbial decay and nutrient release of grass litter during aquatic decomposition. Hydrobiologia 709:201–212
Schoelynck J, Bal K, Backx H, Okruszko T, Meire P, Struyf E (2010) Silica uptake in aquatic and wetland macrophytes: a strategic choice between silica, lignin and cellulose? New Phytologist 186:385–391
Schoelynck J, Müller F, Vandevenne F, Bal K, Barão L, Smis A, Opdekamp W, Meire P, Struyf E (2014) Silicon-vegetation interaction in multiple ecosystems: a review. Journal of Vegetation Science 25:301–313
Struyf E, Conley DJ (2009) Silica: an essential nutrient in wetland biogeochemistry. Frontiers in Ecology and the Environment 7(2):88–94
Struyf E, Conley DJ (2012) Emerging understanding of the ecosystem silica filter. Biogeochemistry 107:9–18
Struyf E, Van Damme S, Gribsholt B, Middelburg JJ, Meire P (2005) Biogenic silica in freshwater marsh sediments and vegetation. Marine Ecology Progress Series 303:51–60
Struyf E, Dausse A, Van Damme S, Bal K, Gribsholt B, Boschker HTS, Middelburg JJ, Meire P (2006) Tidal marshes and biogenic silica recycling at the land-sea interface. Limnology and Oceanography 51(2):838–846
Struyf E, Van Damme S, Gribsholt B, Bal K, Beauchard O, Middelburg JJ, Meire P (2007) Phragmites australis and silica cycling in tidal wetlands. Aquatic Botany 87:134–140
Struyf E, Opdekamp W, Backx H, Jacobs S, Conley DJ, Meire P (2009) Vegetation and proximity to the river control amorphous Si storage in a riparian wetland (Bierbza National Park, Poland). Biogeosciences 6:623–631
Struyf E, Mörth M, Humborg C, Conley DJ (2010) An enormous amorphous silica pool in boreal wetlands. Journal of Geophysical Research 115:G04008
Tréguer PJ, De La Rocha CL (2013) The world ocean silica cycle. Annual Review of Marine Science 5:477–501
Wolski P, Savenije HHG, Murray-Hudson M, Gumbricht T (2006) Modelling of the flooding in the Okavango Delta, Botswana, using a hybrid reservoir-GIS model. Journal of Hydrology 331:58–72
Wolski P, Stone D, Tadross M, Wehner M, Hewitson B (2014) Attribution of floods in the Okavango basin, Southern Africa. Journal of Hydrology 511:350–358
Zimmermann S, Bauer P, Held R, Kinzelbach W, Walther JH (2006) Salt transport on islands in the Okavango Delta: numerical investigations. Advances in Water Resources 29:11–29
Acknowledgments
We would like to thank several funding agencies for their funding contributions: University of Botswana, EU Marie Curie Program (Hobits), National Geographic Explorer Grant, the Swedish National Science Foundation (VR) and the Knut and Alice Wallenberg Foundation. We would like to thank BELSPO for funding the project SOGLO. This research was carried out under Permit EWT 8/36/4 XVI(6) from the Government of Botswana. Part of the funding was provided by the University of Botswana Office of Research and Development.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Struyf, E., Mosimane, K., Van Pelt, D. et al. The Role of Vegetation in the Okavango Delta Silica Sink. Wetlands 35, 171–181 (2015). https://doi.org/10.1007/s13157-014-0607-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-014-0607-1