Abstract
As the largest country in Africa, Algeria is rich in substantial mineral reserves. This mineral potential continues to be revealed day by day; but unfortunately, they are generally underutilized. The detailed mechanisms of marine sedimentary phosphates origin are still imperfectly known. Our study aims to reconstitute the depositional environment of phosphorites of El Kouif locality NE of Algeria. Our method used an extensive sequential analysis, Microscopic Petrography, X-ray diffractometry, and geochemical analysis. Cross-sections carried out on the outcrops of the El Kouif city, have shown a sub-tabular formation containing almost eight layers of phosphorites. The sequential analysis revealed that the phosphatic series have taken place in a reduced negative mega-sequence. Petrographic observations have shown phosphatic intraclasts bearer of the organic matter at various rates. XRD of whole-rock powder has confirmed the presence of apatite minerals, carbonates quartz, clays, sulfides, and gypsum. The geochemical trend has indicated a high fluctuating content of P2O5. The dispersion pattern, correlation coefficient, and mutual relationship of significant major oxides, represented by plotted diagrams; showed that SiO2, CO2, MgO, are antipathetically related to P2O5. The geochemical behavior between P2O5 contents and of CaO, H2O, Na2O, SO3 contents have shown a positive relationship. The richness of the phosphatic rocks in Apatite is autonomous of the depth in the Tebessa basin. The results of the chemical analysis from purified grains were used to calculate the structural formulas for apatite. This non-stoichiometric apatite presented significant anionic substitutions affecting up to 24% of the sites. The higher values of CO2 have obviously resulted in the richness of organic substance, the notable presence of sulfides minerals, indicated through the high concentration of SO3, and the lower concentration of Na suggested that the reducing conditions of the basin were prevailing at the time of phosphatic formation in a shallow marine environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phosphorite is a prevalent mineral matter in the Middle East and North Africa (MENA) region, especially in Maghrebian country (Nouioua et al. 2015; Kechiched et al. 2020; Boumaza et al. 2021). Their genesis depended on the evolution of the Neo-Tethys Ocean (Flandrin 1948). The upwelling seawater currents of the Cretaceous–Eocene trans-global circulation (TCC) have favored the formation of this substance (Föllmi 1996; Abed et al. 2016). Cenozoic phosphates are located at relatively low latitudes, (below 40°), which indicates their affinity with warm water (Slansky 1986). Commonly, the phosphates are deposited in very shallow (Kechiched et al. 2018), near-shore marine or low-energy environments. These favorable environments are the supratidal zones, littoral or intertidal zones, and estuarine zones (Notholt 1991). In the Paleo-Eocene, marine phosphorites arise in the passage from the platform to the shelf. Phosphorite deposits occur then as a fine-grained rock, formed at low temperatures underwater from phosphorus-bearing organic material (Filippelli 2011; Nelson et al. 2010). While the Amorphous calcium phosphate precipitates and crystallizes into Francolite (Arning et al. 2009). The geochemical tracing of the apatite structure substitution is an important tool to reconstitute the paleo marine chemistry and therefore the depositional environment of phosphorites. The substitution elements indicate the compositions of interstitial waters and the paleo salinity. Several scientists (McConnell 1973; Manheim and Gulbrandsen 2018) discussed the substitutions of cations/anions in Apatite samples.
El Kouif province is one of the most important phosphatic deposits hosted in Paleocene–Eocene sedimentary rocks. In the Algerian–Tunisian border, the Tunisian side was largely studied by several researches, (Fourine 1980; Belayouni 1983; Chaabani 1995; Beji-Sassi 1999; Ounis 2011). Whereas the research on the Algerian side is still insufficient despite the contributions of Boulemia (2015), Kechiched et al. (2016, 2018, 2020) and Kechiched (2017).
Our purposes consists to: (i) identify the mineralogical and geochemical composition of phosphatic rocks; (ii) define the genesis of minerals in phosphorites; (iii) assess the quality of the deposit and (iv) to plot a conceptual genetic model for all similar geologic and environmental setting.
In our study, we operated new rock and powder geochemical data of El Kouif’s phosphorites which allowed us to determine the abundance, distribution pattern, and inter-element relationship of phosphorites, ultimately, to interpret their impact on phosphatic mineralization.
A particular interest will be devoted to purified grains (pellets, coprolites, and granules) which will be used for the calculation of mineralogical formulas. Therefore, the present investigation would contribute to clarifying the mechanisms and conditions (depositional environment) that prevailed for morphogenesis in the El Kouif locality.
Most of the studies that deal with the same research axis in the same deposit conditions follow approaches that are based either on mineralogy, or on petrography, or on geochemistry, or sedimentology, giving results with sensitive uncertainties. While consulting the geoscientific literature, little pieces of information on phosphorites of the El Kouif region is available. We can only evoke the biostratigraphic study of Flandrin (1948), the petro-mineralogical contribution of Boulemia et al. (2015), and the geochemical approach based on the behaviour of REEs by Kechiched et al. (2018, 2020).
Our multidisciplinary study deals with a more global aspect of the phosphorites to overcome the uncertainties problem in this study area. It traces the chemical elements as constituents of a mineral. A rock is made up of an assemblage of different minerals, and this rock gives rise to a geological formation. Our methodology combines these different aspects from the geochemical analysis of the total rock and purified grains, to the sequential analysis of sediment formation, including sedimentology, mineralogy, gitology and petrography. This permits the deduction of the depositional paleo-environment of phosphorites of the El Kouif locality.
Environmental relevance of phosphate studies
Besides, to the economic concerns associated with the identification and the characterization of phosphorus levels along the sedimentary sequence, these deposits have revealed increasing environmental importance during the last decades (Gadri et al. 2015; Raïs et al. 2017; Khelifi et al. 2021). In fact, phosphorus-based technologies for recovery from wastewater and heavy metal pollution has been widely discussed. The published researches indicated positive evaluation and encouraging results in terms of a decrease in dependency on global phosphate rock cumulative energy demand, acidification potential and global warming potential, fertilizer efficiency (Boudiaf et al. 2020; Driouech et al. 2020).
Amann et al. (2018) have assessed according to a large spectrum of indicators overall environmental performance of P-based recovery approaches highlighted that the results expose certain tradeoff for the different used technologies. The increasing importance accompanied with the use of P-practices conceivably leading to various environmental concerns while the increasing awareness of the concentrated phosphate rock economic value is a handful of countries worldwide. The increased circular economy relying on these new P-based technologies reducing simultaneously environmental impacts from phosphate industries and mines lead to the development of a broad of technologies on the basis of the intensive research and innovation studies (Binder et al. 2009; Egle et al. 2015). To provide a more comprehensive picture for stakeholders dealing with specific drawbacks and (or) advantages of these approaches with respect to the variable energy demand and pollutant emissions namely greenhouse gases. The environmental performance of these recoveries relies principally on the different characterization of the investigated sedimentary units.
The studies of phosphorus has received increasing attention as an important tool to project and monitor climate change. In fact, many scientists are currently tracking phosphate to understand global warming and weather variability pattern (Ajayi and Ilori 2020; Almazroui et al. 2020). Indeed, the continuous climate changes require heavy investigation and analyses of variability trend. Establishing sensitive effective management systems requires the development of control tools and measure devices to point out the predicted changes via accurate control of different environmental components. Phosphate loading variable concentrations has been used by several studies to evaluate climatic variability impacts on natural cycles and to project numerous evolution scenarios to highlight the feasible alternatives to deal with weather variability issues and the potential repercussions in different sectors (Vincent et al. 2015; Ogega et al. 2020; Ajayi and Ilori 2020). These models have been developed in accordance with the principal of the changes in the factors governing the concentrations of a different permissions will be detected via changes in natural element cycles.
The sensitive detection and explanation of these changes instead requires accurate knowledge about phosphate deposition, characterization. Variability in response to different environmental conditions. Thus, the relevance of environmental performance of P-indicators relies on the detailed knowledge of its cycles and paleo-evolution to retrace its evolution for a preliminary diagnostic of its further variability.
Given these important roles in climate variability; project models and pollution decontamination and remedial measures, countries strive to study phosphate-based sensitive approaches especially for the hit dry regions such as North Africa. Where future increase of mean temperature may reach, 2–3 °C with important seasonal cycle variability has been reported (Ilori and Ajayi 2020). To deal with rainfall evolution and intensification frequency of extreme events pointed out by previous researchers and their likely repercussions on social, economic and ecological dimensions. Significant statistical association of climate variability with chemical cycle evolution and their projected changes to represent adequately rehabilitation measures reducing uncertainties in the climate variable patterns and controlling key issues related to the weather conditions and pollution is needed. The climate impact on water resources in Algerian–Tunisian border have been largely discussed by several researchers as Hadji et al. (2013, 2014), Hamed et al. (2014, 2017, 2018), Mokadem et al. (2016), Ncibi et al. (2020a, 2021; b), Besser et al. (2018, 2021), Dahoua et al. (2017a, b), and Gulbrandsen (1966).
General setting
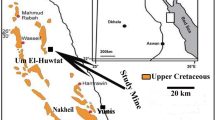
Situated at 25 km East of the chief town of Tebessa province; the study area belongs to the Eastern Saharian Atlas; as a part of the Algerian–Tunisian transboundary basin. It includes several outcrops of phosphate ores (Hamad et al. 2018a, b; Tamani et al. 2019) (Fig. 1a, b). These substances, of marine origin, were formed during the Thanetian at Jebel El Kouif, Dyr, Tazbant. phosphate ores consist of grains of phosphatized coprolites and bioclasts. The host is thickly interspersed with thin levels of phosphate ore. The main geological formations outcropped in the study area are of Cenozoic age (Fleury 1969) represented by Dano-Montian marl (“e2-3”), a Thanetian phosphate (“e4”), and Ypresian–Lutetian limestone (“e5-6”) and Miocene (“mc”) continental formation (Kerbati et al. 2020; Brahmi et al. 2021) (Fig. 1c–e). The anticlines geomorphologies resulted from a Neogene compression. The unconformity between the marine Miocene and the continental Eocene reveals a major phase that affects the region during this period (Villa 1980).
Material and methods
We conducted the sampling of whole-rock phosphatic layers on representative outcrops along with two preferred directions. We have done a chemical and mineralogical analysis of the Seventeen samples. We used the friable phosphatic sediments (layer 1 and 2) for extracting purified grains (teeth and coprolite).
We carried out the preparation for mineralogical protocol and XRD in the University of Tebessa’s Geosciences Laboratory Algeria; and the Faculty of Sciences Laboratory in Tunis, Tunisia, respectively. Whereas we carried, out the geochemical analysis for whole rock and purified grains at SOMIPHOS Company laboratory, Algeria.
For XRD diffractometry, we analyzed multiphase powder using the PANalytical XPert PRO rays device. We compared the obtained X-ray diffractogram, with a records database, using a “High Scoreplus” program.
For Petrographic Analysis; we confectioned thin sections at the geoscience laboratory, university of Tebessa. For this task, we used a Leica polarizing microscope DMLP specially adapted for all petrographic observations. Otherwise, for chemical analyzes; we measured the “major elements” by Perkin Elmer flame atomic absorption spectrometry. First, the samples are dissolved after melting with strontium metaborate, and then dissolved in 2% nitric acid. The dosage of trace elements (Zn, Cu, and Cd) was carried out, also, by atomic absorption spectrometer after dissolving the samples, by perchloric—hydrofluoric attack, and recovery of the residue with nitric acid. The fluorine was measured by a potentiometer after the mobilization of this element with concentrated hydrochloric acid. The device used is a Metrohm equipped with a specific fluorine electrode. The loss on ignition was obtained by calcination at 1000 °C. We used the major and trace element chemical data of the purified grains (Table 1), to determine the chemical formulas of the Apatite component. Among various calculation methods, we retained the structural formula, which minimizes the valance of the charges between cations and anions. We performed a calculation based on 16 with 6 mandatory anions, with the possibility of introducing a correction factor. So that the electrical equilibrium is respected, following Pauling’s rules.
Results and discussion
Cross-sections carried out on the outcrops of El Kouif locality, showed sub-tabular layers. A lithostratigraphic column established a formation revealed eight layers of phosphorites (Fig. 2a). These sediments are relatively friable of decimetric order, alternating with layers of limestone and marl-limestone. It should be noted that, apart from the thicknesses of the layers, which differ, a lithostratigraphy of El Kouif locality presents almost the same facies in phosphatic layers.
The sequential analysis of the sediments composing the Paleo-Eocene profile of the perched syncline of El Kouif (Fig. 2b) revealed the existence of an asymmetrical sedimentary cycle composed of a large positive mega sequence (SMS1) at the bottom, surmounted by a relatively reduced negative mega- sequence (RMS1) of around 63 m thick. Through this profile, we can deduce that the sedimentary basin knew increasing subsidence for the lower part with particularly supple of Dano-Montian formation. However, this subsidence appearance of the basin began to reduce during the phosphatic Thanetian (RS1) with marly limestone, passing in the Eocene to increasingly, shallow sedimentary environments where the biomicrites always tend to be replaced by biosparites of environments more energetic (Boulemia 2015).
Megascopic study revealed that the brecciated phosphorites are light beige to very dark brown in color and medium to coarser fractions embedded in a groundmass of clay minerals (friable rock) to carbonates (hard and compact rock) and sometimes partially, siliceous or collophanic.
Microscopic observation of phosphate samples indicated primordially, phosphatic pellets rich in organic matter, devoid of a nucleus, and others nucleated. In sum, it should be noted that phosphatic intraclasts (coprolites and granules) of varying sizes and vertebrate skeletal fragments (bones and teeth) are bearer of organic matter at various rates, which gives them colors ranging from light yellow to very dark or even opaque brown (Fig. 3). The distribution sometimes follows the periphery of the nucleus.
Microscopics illustrations of phosphatic intraclasts. [1-Opaque punctuation of organic matter surrounding a pallet. 2-Organic matter gives hues ranging from light yellow to very dark brown. 3-Lateral view of a tooth (T) in a packstone textured phosphorite. 4-Coprolite of cylindrical shape, granule (G) and pellets (P) in an anisogranular phosphorite, 5-The fibrous microstructure of a bone fragment (F) of elongated shape in bio-pel-phosphorites]
The richness in organic matter is an anoxic sign for conditions associated with the genesis of phosphorites. Phosphatic intraclasts are present with carbonates as groundmass to produce sedimentary wackestones (Fig. 3). High-grade is represented by packstones to grainstones phosphorites (Boulemia et al. 2015). Most probably, the explanation for this phenomenon is the major episodes of phosphorite deposition were tied to climate and sea-level changes.
XRD analysis (whole rocks) has confirmed the presence of apatitic minerals (Hydroxy-apatite, Fluor-apatite, francolite, and Dahlite), carbonates (calcite, magnesium calcite, ankerite, dolomite), the quartz, opal-CT, and even sulfides and gypsum. Besides, we have to note that the existence of pyrite (iron-sulfur) and chalcopyrite (copper sulfur) in some phosphatic rocks (Fig. 4) indicates highly reducing conditions of their formation. In fact, due to the presence of sulfate (SO3) ions in the water, the pyrite could be precipitated at the time of phosphatization. In addition, when Gypsum is associated with phosphorites, it may correspond to a late phase linked to meteoric water circulation. It is therefore of secondary origin (Sassi 1974).
Furthermore, X-ray diffractometric analysis performed on various forms of coprolites isolated from friable phosphorites of the beam El Kouif formation showed that these “fossil” of fecal origin, consist of hydroxylapatite with fluorapatite and sometimes associated with dahllite. In other words, the coprolites are single-phase and only the Apatitic components are recorded there (Fig. 5).
The results of the chemical analyzes of the 17 samples of multi-phase phosphate facies (total rock) collected from eight mineralized layers constituting the phosphate bundle of El Kouif locality are reported in Table 2.
The vertical spectra of variation of chemical analyzes results, across the eight layers, shows a fluctuating and not monotonic behavior with the depth. The distribution of P2O5 is characteristic of phosphatic-rich layers. It reaches its highest concentration (30.81%) in the middle part of layer II. This indicates more concentration of apatite constituent (collophane). It shows a minor peak (14.60%) in layer VII. There is no gradual increase within the eight phosphatic beds of El Kouif locality. The main reason for the vertical variation of the major oxides across the phosphatic layers consists of the content of the chemical element (mainly, abundant phosphorus) in seawater, good communication of the sea, and high organic productivity. This resulted in the synsedimentary deposition of phosphorites by the combination in reducing conditions in a shallow oceanic environment.
The distribution pattern of CaO is parallel to that of P2O5 (Fig. 6). It reaches its lowest concentration (24.6%) at layer V. Its climax (47.11%) is recorded in the lower part of layer III. It seems that phosphatic components (pellets, coprolites, and fish debris) are the most important phases controlling the distribution of CaO and P2O5 at El Kouif phosphorites. P and Ca are the two major elements making the framework of the CFA mineral. However, the presence of a substantial amount of calcium also is in the carbonates as calcite and dolomite. Apparently, Ca-sulfates play a minor role.
The distribution of MgO is contrary to that of P2O5 and CaO. MgO content shows the highest peak in layer II and shows a minor peak within the middle part of layer II. The lower MgO content indicates that little dolomitization has occurred, and can be attributed to the formation of the phosphatic grains in low MgO seawater (Baioumy 2007). As magnesium forms dolomite lattice, it participates in chert (silex) and may be hosted in clay minerals. The distribution pattern of CO2 is similar to that of MgO, its contents are very irregular and reach its maximum concentration. (36%). at the layer V. Carbon characterizes both carbonates and organic matter.
The contents of the siliceous phase (SiO2) are remarkable and oscillate between 2.6 and 12.5%. It reaches its maximum value at the middle of layer II. The samples with high silica contents in the chemical analysis are due to the presence of the biogenic quartz and the partial or complete silicification (chert) of some levels. In addition, SiO2 may indicate the presence of phyllosilicates in phosphatic rocks of El Kouif locality.
The concentration of Al2O3 is lower than 0.83% in the studied section this is may be due to minor occurrences of the clay mineral. Aluminum is the lead element usually taken as indicative of the clay fraction within the sedimentary rocks (Krauskopf and Bird 1967). In addition, the low content of alumina in the samples of phosphorites indicates the arid to humid climatic conditions at the time of precipitation and phosphatization of these sediments in the Tebessa area. Finally, the sulfur contents are relatively high and fluctuate between 2.6 and 10.6%. They are similar to those of the phosphate deposits in northern Tunisia. This is a sign of a more confined environment (Sassi 1974).
In sum, the vertical variation of the main major oxides across the eight phosphatic layers of El Kouif locality indicates that CaO and in degrees less SO3 profile are similar to P2O5, however, those of MgO, CO2, and SiO2 are antipathetically related with P2O5. The geochemical behavior is autonomous of the depth of the basin.
The relationships between the dosed chemical elements are known by calculating the correlation coefficients for each couple. These sometimes show good mutual inter-elementary relationships.
CaO correlates strongly with P2O5 (r = 0.77) as shown in Table 3. This coefficient value provides information on a positive and significant bivariate relationship. If the P2O5 contents represent only apatite, Ca is an element constituting both phosphates and carbonates (calcite dolomite). Analysis of the behavior of this couple provides information on the importance of carbonates in the phosphate rocks analyzed. The correlation diagram of the El Kouif locality (Fig. 7a, b) shows two small groups of points separated and drawing an alignment of type P2O5 = a Ca0 + b. The separation between the two lots indicates two types of phosphate facies, the first more mineralized and the other less rich in P2O5. Few points provide information on low-content P2O5 facies with a more abundant carbonate Exo-gangue.
MgO contents represent the dolomitic Exo gangue. Its percentage does not exhibit any high values in the P2O5-rich stratigraphic levels. Indeed, the correlation coefficient is of the order of − 0.90, which indicates an antagonism between apatite and Magnesian minerals. Furthermore, an inverse relationship between MgO and P2O5 in phosphorites may indicate a continuous replacement of MgO by P2O5 during diagenesis. The MgO diagrams faithfully translate this negative correlation to a downward slope.
The P2O5/SO3 correlation for the total rock is less significant 0.54 despite the sulfated nature of the El Kouif phosphatic components intraclasts. It is explained by the notable presence of sulfated minerals (gypsum) and sulfides in this region, confirmed by X-ray diffractometric analysis.
The H2O molecule is involved in the formation of clay minerals and gypsum but very actively to characterize hydroxyapatite. Correlation plots show two separate groups but tend to align positively. The dispersion of a few points probably provides information on the remarkable Exo gangue in phyllo silicates.
A good positive correlation between P2O5/Na2O, of the order of 0.90 is showed in plot scatter. It provides information on the clear geochemical affinity between sodium and phosphorus for the phosphates of El Kouif. The work of several authors such Gulbrandsen (1969) has confirmed this relationship. Moreover, the slight dispersion could be explained by the distribution of Na between the phase apatite (mainly the Dahllite mineral) and other sodium minerals such as the dawsonite.
It is very clear, that CaO (as a constituent of apatite) correlates negatively with MgO and SiO2 representing siliceous phase and clay minerals. In other words, if the phosphate rock is more mineralized, the clayey, siliceous, or dolomitic matrix is less developed. Thus, generally, calcite gives way to dolomite. Thus, the correlation diagrams clearly express this CaO/MgO and CaO/SiO2 antagonism. That suggesting a dilution of the clay’s minerals by carbonates or by a test of organisms and anhydrite or gypsum.
The processing of the analysis results shows the very close association between Al2O3 and K2O. The highly significant correlation (0.99) and the perfect alignment on the scatter diagram, indicates a structural affinity of these majors chemicals, contained in clay minerals (Al and K are majors elements constituent of the phyllites).
Since, CO2 and MgO essentially, form dolomite and only enter as substituents for PO3 and Ca in the apatite framework. Therefore, very logical that the CO2/P2O5 correlation coefficient becomes negative and the one that qualifies the CO2/MgO pair is very positively individualized. It should be noted that the point clouds of the diagrams (CO2) sometimes do not line up. This may be an indication of a random dispersion of organic matter within the phosphorites of the El Kouif locality.
The chemical analysis results of purified grains extracted from phosphorites of layers I and II at El Kouif locality are listed in Table 1. An example of the abundance trend of chemical elements from the phosphatic peloid sample is presented in Fig. 8. It seems that concentration trends of certain major oxides generally, indicate contents of CaO, P2O5, CO2, SO3, and Fluor more enriched, with a few percent than of SiO2, Al2O3, Fe2O3, MgO, Na2O, and K2O. The concentration trends of trace elements reveal that these intraclasts are relative, enriched in Cd, Zn, and Cu. The P2O5 values are around 33%, otherwise, the CaO contents also vary between 50 and 52%. CaO/P2O5 ratios recorded from treated purified grains are more than about 1.55. Presumably, this value approaches that of carbonate Fluorapatite (CFA).
The results of the elemental analyzes in Table 1 were used to calculate the structural formulas for apatite at El Kouif locality (Paleogene basin of Algerian–Tunisian border area). The established average formulas are written as follow: (Ca9.09Na0.36Mg0.13) (PO4)4.54(SO3)0.35 (CO3)1.16(F, OH)2.13.
This non-stoichiometric apatite presents significant anionic substitutions (CO3 and SO3 partially replaced the PO4) affecting up to 24% of the sites; they also contain excess Fluor. However, cationic replacements remain relatively minor, covering approximately 4–6% of the sites. Furthermore, the Cu, Zn and Cd cations can replace the Ca ions in the apatite network (Jarvis et al. 1994) or are adsorbed in humic acids from organic matter (Belayouni 1983).
We can deduce that the high CO3 replacement rates of PO4 indicate an environment favorable to the replacement of phosphorus by carbon; the environment in which this group is abundant corresponds to the neritic section favorable to the formation of carbonates and the profusion of marine organisms. It is therefore not surprising to note that CFA formed within the carbonate base. The high SO3 contents are indicators of paleo-salinity for an anoxic environment. As for cationic sites, their occupation rate with Na+ and Mg2+ is of the order of 5–7%. The number of Na moles is significant, higher than that of Mg, approximately in a ratio of 5. The variation of Na constitutes a witness to anoxic deposition and digenesis environment. Indeed, its low concentration could be due to good communication with the open sea, unlike the lagoon environment. Otherwise, the contents of some trace elements (Cd, Cu, and Zn) from purified grains samples shows high values compared to the average recorded in the sedimentary phosphorites established by Altschuler (1980). Indeed, the divalent; Cd2+, Cu2+, Zn2+ can replace Ca2+ in the structure of apatite. The trapping of heavy metals by organic matter seems to play an important role in this distribution. This indicates that planktonic organic matter may be the prime source of these elements in the Tebessa phosphate deposits.
The weakness point of our study that we intend to consider in the next investigations is the lack of high-tech scanning technologies and analysis procedure.
Our study did not take into consideration the analytical methods of Scanning Electron Microscopy (SEM), the geochemical study of some trace elements that were not taken into account during this study such as Uranium, and rare metals. This could give more insight and precision on the phosphogenesis in the Algerian Tunisian border Paleocene–Eocene basin.
Conclusion
A lithostratigraphic column established from the Tebessa series have shown a sub-tabular formation host of eight phosphatic layers. The sequential analysis of the sediments composing the Paleo-Eocene profile of the perched syncline of El Kouif revealed a subsidence of the basin during Dano-Montien, which to be reduced at the phosphatic Thanetian.
The microscopic observation of phosphate samples showed phosphatic intraclasts rich in organic matter, this richness is an anoxic sign for genesis conditions. Bone debris accumulated due to the occasional mass mortality of fish and other animals occurring in upwelling zones. Allochems are present mainly, with carbonates as groundmass, to produce wackestones, packstones, or grainstones phosphorites. This indicates leading episodes for sedimentary deposition, tied to climate and sea-level changes.
X-ray diffractometric analysis of the purified grains encountered in sedimentary phosphorites showed that these intraclasts were made only, of apatite components. The phosphate-rich sediments are mainly composed of coprolites, granules, pellets, and fish debris and this existence of sulfides in some phosphatic rocks indicates highly reducing conditions of their formation. However, when Gypsum is associated with phosphorites, it may correspond to a late phase linked to meteoric water circulation.
The chemical analysis of the samples coming from the different phosphate layers constituting the phosphate bundle showed high and fluctuating contents of P2O5. The dispersion pattern, correlation coefficient, and mutual relationship of significant major oxides, represented by plotted diagrams, indicate that SiO2, CO2, MgO are antipathetically related to P2O5, this relationship suggests a gradual replacement among these oxides during diagenesis and the poor correlation between MgO and P2O5 in phosphorites of the area indicates that a little dolomitization has occurred. Mg, Al, K, and Si, are recorded with variable concentrations. The lack of correlation between these elements and P2O5 suggests different origins for the apatite. Indeed, these elements can be associated with clays, carbonates, or zeolites.
The geochemical behavior between the contents of P2O5 with the contents of CaO, H2O, Na2O, and SO3 shows a positive correlation and strong correlation coefficients, respectively. The latter result from the fact that these elements belong to the apatite framework, the high values of P2O5 and CaO in the phosphorites indicate more concentration of apatite constituent. The sulfur contents are thus, linked to the secondary gypsum. Finally, the richness of the phosphatic rocks in Apatite is autonomous of the depth in the Tebessa basin.
The difference in geochemical behavior of CaO and MgO may be also, due to ionic substitution of Ca2+ by Mg2+ in the apatite crystal lattice during the reducing environment of the basin. The low concentration of alumina may be due to minor occurrences of clay minerals that constitute the cementing material of the phosphatic rocks. The presence of low Al2O3 in phosphorites indicates the humid to low temperature climatic conditions at the time of precipitation and phosphatization of these sediments at El Kouif locality.
The results of the chemical analysis from purified grains were used to calculate the structural formulas for apatite at El Kouif locality. This non-stoichiometric apatite presents significant anionic substitutions affecting up to 24% of the sites. However, cationic replacements remain relatively minor, covering approximately 4–6% of the site. The high CO3 replacement rates of PO4 indicate a neritic section favorable to the formation of carbonates and the profusion of marine organisms.
The chemical trend indicates that; the variation in major and trace elements abundance is attributed to a change in the environmental condition of deposition (contents of seawater). The obvious richness of organic matter, higher values of CO2, notable presence of sulfides minerals, high concentration of SO3, and lower concentration of Na suggested that the reducing conditions of the basin were prevailing at the time of phosphatic formation in shallow marine environments. Besides, high contents of (Cd, Cu, and Zn) indicate that planktonic organic matter may be the prime source of these elements in the Tebessa phosphate deposits.
This phosphatic series as those of El Kouif locality (southern Tethyan margin) during a reduced negative sequence, shallow sedimentary environments, good communication of the sea, abundant phosphorus in seawater, and high organic productivity were considered essential for the formation of phosphorites from areas of historical upwelling with nutrient-rich water or high sedimentation rates (anoxic sediments).
References
Abed AM, Jaber O, Alkuisi M, Sadaqah R (2016) Rare earth elements and uranium geochemistry in the Al-Kora phosphorite province, Late Cretaceous, northwestern Jordan. Arab J Geosci 9(3):187
Ajayi VO, Ilori OW (2020) Projected drought events over West Africa using RCA4 regional climate model. Earth Syst Environ 4(2):329–348
Almazroui M, Saeed F, Saeed S, Islam MN, Ismail M, Klutse NAB, Siddiqui MH (2020) Projected change in temperature and precipitation over Africa from CMIP6. Earth Syst Environ 4(3):455–475
Altschuler ZS (1980) The geochemistry of trace elements in marine phosphorites: part I. Characteristic abundances and enrichment. SEPM Spec Publ 29:19–30
Amann A, Zoboli O, Krampe J, Rechberger H, Zessner M, Egle L (2018) Environmental impacts of phosphorus recovery from municipal wastewater. Resour Conserv Recycl 130:127–139
Arning ET, Birgel D, Brunner B, Peckmann J (2009) Bacterial formation of phosphatic laminites off Peru. Geobiology 7(3):295–307
Baioumy HM (2007) Iron–phosphorus relationship in the iron and phosphorite ores of Egypt. Geochemistry 67(3):229–239
Beji-Sassi A (1999) Phosphates in the Paleogene basins of the southern part of the North-South axis (Tunisia). Doctoral thesis, Department of Geology, University of Tunis II, Tunisia
Belayouni H (1983) Study of organic matter in the phosphate series of the Gafsa–Métlaoui basin (Tunisia) application to the understanding of the mechanisms of phosphatogenesis. Doctoral thesis, University of D’Orlans, France
Besser H, Mokadem N, Redhaounia B, Hadji R, Hamad A, Hamed Y (2018) Groundwater mixing and geochemical assessment of low-enthalpy resources in the geothermal field of southwestern Tunisia. Euro Mediterr J Environ Integr 3(1):16
Besser H, Dhaouadi L, Hadji R, Hamed Y, Jemmali H (2021) Ecologic and economic perspectives for sustainable irrigated agriculture under arid climate conditions: an analysis based on environmental indicators for southern Tunisia. J Afr Earth Sci 177:104134
Binder CR, De Baan L, Wittmer D (2009) Phosphorflüsse in der Schweiz: stand, Risiken und Handlungsoptionen. Abschlussbericht. Umwelt-Wissen (0928)
Boudiaf B, Dabanli I, Boutaghane H, Şen Z (2020) Temperature and precipitation risk assessment under climate change effect in northeast Algeria. Earth Syst Environ 4:1–14
Boulemia S (2015) Analysis of marine phosphates; case of the locality of Dj. Dyr and El Kouf (Algerian–Tunisian borders). Doctoral thesis, University of Tébessa, Algeria
Boulemia S, Hamimed M, Bouhlel S, Bejaoui J (2015) Petro-mineralogical analysis of sedimentary phosphate of marine origin, case of the locality of El Kouif (Algerian–Tunisian confines). Open J Geol 5(03):156
Boumaza B, Kechiched R, Chekushina TV (2021) Trace metal elements in phosphate rock wastes from the Djebel Onk mining area (Tébessa, eastern Algeria): a geochemical study and environmental implications. Appl Geochem 127:104910
Brahmi S, Baali F, Hadji R, Brahmi S, Hamad A, Rahal O et al (2021) Assessment of groundwater and soil pollution by leachate using electrical resistivity and induced polarization imaging survey, case of Tebessa municipal landfill, NE Algeria. Arab J Geosci 14(4):1–13
Chaabani F (1995) Dynamics of the eastern part of the Gafsa basin in the Cretaceous and Paleogene: mineralogical and geochemical study of the Eocene phosphate series, southern Tunisia. Doctoral thesis, University of Tunis II, Tunisia
Dahoua L, Yakovitch SV, Hadji R, Farid Z (2017a) Landslide susceptibility mapping using analytic hierarchy process method in BBA-Bouira Region, case study of East-West Highway, NE Algeria. In: Euro-Mediterranean conference for environmental integration. Springer, Cham, pp 1837–1840
Dahoua L, Yakovitch SV, Hadji RH (2017b) GIS-based technic for roadside-slope stability assessment: an bivariate approach for A1 East-west highway, North Algeria. Min Sci 24:117–127
Driouech F, ElRhaz K, Moufouma-Okia W, Arjdal K, Balhane S (2020) Assessing future changes of climate extreme events in the CORDEX-MENA region using regional climate model ALADIN-climate. Earth Syst Environ 4(3):477–492
Egle L, Rechberger H, Zessner M (2015) Overview and description of technologies for recovering phosphorus from municipal wastewater. Resour Conserv Recycl 105:325–346
Filippelli GM (2011) Phosphate rock formation and marine phosphorus geochemistry: the deep time perspective. Chemosphere 84(6):759–766
Flandrin J (1948) Contribution to the stratigraphic study of the Algerian Nummulitic. Publ Geol Surv Alger 19(2)
Fleury J (1969) Stratigraphy of the Cretaceous and Eocene (Aptian to Lutetian) of the Morsot map, n ° 178. Publ Alger Geol Surv 39:145–157
Föllmi KB (1996) The phosphorus cycle, phosphogenesis and marine phosphate-rich deposits. Earth Sci Rev 40(1–2):55–124
Fourine D (1980) Phosphates et pétrole en Tunisie, Mém. BRGM N.04, pp 30–34
Gadri L, Hadji R, Zahri F, Benghazi Z, Boumezbeur A, Laid BM, Raїs K (2015) The quarries edges stability in opencast mines: a case study of the Jebel Onk phosphate mine, NE Algeria. Arab J Geosci 8(11):8987–8997
Gulbrandsen RA (1966) Chemical composition of the phosphorites of the Phosphoria Formation. Geochim Cosmochim Acta 30:769–778
Gulbrandsen RA (1969) Physical and chemical factors in the formation of marine apatite. Econ Geol 64(4):365–382
Hadji R, Boumazbeur A, Limani Y, Baghem M, Chouabi A (2013) Geologic, topographic and climatic controls in landslide hazard assessment using GIS modeling: a case study of Souk Ahras region, NE Algeria. Q Int 302:224–237
Hadji R, Limani Y, Boumazbeur A, DemdoumA ZK, Zahri F, Chouabi A (2014) Climate change and their influence on shrinkage—swelling clays susceptibility in a semi—arid zone: a case study of Souk Ahras municipality, NE-Algeria. Desalin Water Treat 52(10–12):2057–2072
Hamad A, Baali F, Hadji R, Zerrouki H, Besser H, Mokadem N et al (2018a) Hydrogeochemical characterization of water mineralization in Tebessa-Kasserine karst system (Tuniso-Algerian Transboundry basin). Euro Mediterr J Environ Integr 3(1):7
Hamad A, Hadji R, Bâali F, Houda B, Redhaounia B, Zighmi K et al (2018b) Conceptual model for karstic aquifers by combined analysis of GIS, chemical, thermal, and isotopic tools in Tuniso-Algerian transboundary basin. Arab J Geosci 11(15):409
Hamed Y, Ahmadi R, Hadji R, Mokadem N, Ben DH, Ali W (2014) Groundwater evolution of the Continental Intercalaire aquifer of Southern Tunisia and a part of Southern Algeria: use of geochemical and isotopic indicators. Desalin Water Treat 52(10–12):1990–1996
Hamed Y, Redhaounia B, Ben Sâad A, Hadji R, Zahri F (2017) Groundwater inrush caused by the fault reactivation and the climate impact in the mining Gafsa basin (southwestern Tunisia). J Tethys 5(2):154–164
Hamed Y, Hadji R, Redhaounia B, Zighmi K, Bâali F, El Gayar A (2018) Climate impact on surface and groundwater in North Africa: a global synthesis of findings and recommendations. Euro Mediterr J Environ Integr 3(1):25
Ilori OW, Ajayi VO (2020) Change detection and trend analysis of future temperature and rainfall over West Africa. Earth Syst Environ 4:493–512
Jarvis I, Burnett WC, Nathan Y, Almbaydin FSM, Attia AKM, Castro LN et al (1994) Phosphorite geochemistry: state-of-the-art and environmental concerns. Eclogae Geol Helv 87(3):643–700
Kechiched R (2017) Phosphates from the north of Tébessa (Dyr and El-Kouif): sedimentological, gitological and geochemical study. Doctoral thesis, Badji Mokhtar University, Annaba, Algeria
Kechiched R, Laouar R, Bruguier O, Laouar-Salmi S, Ameur-Zaimeche O, Foufou A (2016) Preliminary data of REE in Algerian phosphorites: a comparative study and paleo-redox insights. Procedia Eng 138:19–29
Kechiched R, Laouar R, Bruguier O, Salmi-Laouar S, Kocsis L, Bosch D et al (2018) Glauconite-bearing sedimentary phosphorites from the Tébessa region (eastern Algeria): evidence of REE enrichment and geochemical constraints on their origin. J Afr Earth Sci 145:190–200
Kechiched R, Laouar R, Bruguier O, Kocsis L, Salmi-Laouar S, Bosch D et al (2020) Comprehensive REE+ Y and sensitive redox trace elements of Algerian phosphorites (Tébessa, eastern Algeria): a geochemical study and depositional environments tracking. J Geochem Explor 208:106396
Kerbati NR, Gadri L, Hadji R, Hamad A, Boukelloul ML (2020) Graphical and numerical methods for stability analysis in surrounding rock of underground excavations, example of Boukhadra iron mine NE Algeria. Geotech Geol Eng 38:2725–2733
Khelifi F, Caporale AG, Hamed Y, Adamo P (2021) Bioaccessibility of potentially toxic metals in soil, sediments and tailings from a north Africa phosphate-mining area: insight into human health risk assessment. J Environ Manag 279:111634
Krauskopf KB, Bird DK (1967) Introduction to geochemistry, vol 721. McGraw-Hill, New York
Manheim FT, Gulbrandsen RA (2018) Marine phosphorites. In: Marine minerals. De Gruyter, pp 151–174
McConnell D (1973) Apatite: its crystal chemistry, mineralogy utilization and geologic and biologic occurrences. In: Applied mineralogy, vol 5. Springer, Vienna, New York
Mokadem N, Demdoum A, Hamed Y, Bouri S, Hadji R, Boyce A, Laouar R, Saad A (2016) Hydrogeochemical and stable isotope data of groundwater of a multi-aquifer system: Northern Gafsa basin e Central Tunisia. J Afr Earth Sci 114:174–191
Ncibi K, Chaar H, Hadji R, Baccari N, Sebei A, Khelifi F et al (2020a) A GIS-based statistical model for assessing groundwater susceptibility index in shallow aquifer in Central Tunisia (Sidi Bouzid basin). Arab J Geosci 13(2):98
Ncibi K, Hadji R, Hamdi M, Mokadem N, Abbes M, Khelifi F et al (2020b) Application of the analytic hierarchy process to weight the criteria used to determine the Water Quality Index of groundwater in the northeastern basin of the Sidi Bouzid region, Central Tunisia. Euro Mediterr J Environ Integr 5:1–15
Ncibi K, Hadji R, Hajji S, Besser H, Hajlaoui H, Hamad A et al (2021) Spatial variation of groundwater vulnerability to nitrate pollution under excessive fertilization using index overlay method in central Tunisia (Sidi Bouzid basin). Irrig Drain. https://doi.org/10.1002/ird.2599
Nelson GJ, Pufahl PK, Hiatt EE (2010) Paleoceanographic constraints on Precambrian phosphorite accumulation, Baraga Group, Michigan, USA. Sediment Geol 226(1–4):9–21
Notholt AJ (1991) African phosphate geology and resources: a bibliography, 1979–1988. J Afr Earth Sci (middle East) 13(3–4):543–552
Nouioua I, Fehdi C, Boubaya D, Serhane B, Djellali A (2015) Mapping underground cracks using 2D electrical resistivity tomography: the case of the landslide of Kef Essenoun phosphate deposit, Djebel Onk (northeast of Algeria). Arab J Geosci 8(10):7731–7738
Ogega OM, Koske J, Kung’u JB, Scoccimarro E, Endris HS, Mistry MN (2020) Heavy precipitation events over East Africa in a changing climate: results from CORDEX RCMs. Clim Dyn 55:993–1009
Ounis A (2011) Contribution of rare earth geochemistry and isotopes for understanding the mechanisms of phosphatogenesis: example of the western part of the Gafsa-Métlaoui basin. PhD thesis, University of Tunis el Manar, Tunisia
Raïs K, Kara M, Gadri L, Hadji R, Khochman L (2017) Original approach for the drilling process optimization in open cast mines; case study of Kef Essenoun open pit mine Northeast of Algeria. Min Sci 24:147–159
Sassi S (1974) Paleocene phosphate sedimentation in southern and central western Tunisia. Doctoral thesis, Univ Paris-Sud Orsay, France
Slansky M (1986) Geology of sedimentary phosphates. North Oxford Academic Publisher Ltd, London, pp 19–35
Tamani F, Hadji R, Hamad A, Hamed Y (2019) Integrating remotely sensed and GIS data for the detailed geological mapping in semi-arid regions: case of Youks les Bains Area, Tebessa Province, NE Algeria. Geotech Geol Eng 37(4):2903–2913
Villa JM (1980) The alpine chain of northeastern Algeria and the Algerian–Tunisian borders. Doctoral thesis, Univ. P. and M. Curie, Paris IV, France
Vincent LA, Peterson TC, Barros VR, Marino MB, Rusticucci M, Carrasco G, Ramirez E, Alves LM, Ambrizzi T, Berlato MA (2015) Observed trends in indices of daily temperature extremes in South America 1960–2000. J Clim 18:011–5024
Acknowledgements
We would like to thank Mrs. Selmania and Bedhmaoui, for helping with fieldwork. We are grateful for the help received from Hemaidia in his technical assistance in the laboratory of the Department of Earth Sciences and the Universe, Tebessa University. We warmly thank the head of the analysis laboratory, Somiphos Company. Finally, the facilities of Mr. Bouhlal the responsible for the mineralogy laboratory, Tunis University is also much appreciated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Boulemia, S., Hadji, R. & Hamimed, M. Depositional environment of phosphorites in a semiarid climate region, case of El Kouif area (Algerian–Tunisian border). Carbonates Evaporites 36, 53 (2021). https://doi.org/10.1007/s13146-021-00719-4
Accepted:
Published:
DOI: https://doi.org/10.1007/s13146-021-00719-4