Abstract
Pores in the Seroe Domi Formation on Curacao contain large quantities of clay minerals, organic material, and protodolomite or very high-magnesium calcite (VHMC) crystals. Transmission electron microscopy (TEM) applied to bio-sectioned rock samples showed the in situ relationship between the organic material, clay minerals, and VHMC. Dumbbells, consisting of two globular bodies connected by a narrow waist, ~20 mm in length and 5–8 mm wide, characterize the organic masses. The dumbbells are coated by clay minerals. VHMC crystals grew from nucleation points within microbial films and sheaths that surround the dumbbells. DNA extraction for 16 s rRNA gene analysis revealed the presence of sulfate- and sulfur-reducing bacteria, a variety of marine cyanophytes, bacteridetes, and proteobacteria, plus freshwater cyanophytes within the rock samples. This study provides evidence from a new field locality for the microbial nucleation and growth of VHMC associated with clay minerals, and the in situ appearance of microbial dumbbells associated with dolomite. Additionally, this study is the first to reveal the internal structure of these dumbbell features indicating that they are organic in origin with crystalline material in the surrounding sheath.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Geologic setting
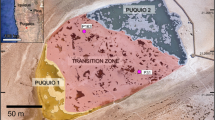
Curacao (located at 12°07′N, 68°56′W), a small island (472 km2) with about 1/3 carbonate rock cover, is located in the southern Caribbean approximately 80 km north of Venezuela (Fig. 1). The island is oblong in shape: approximately 61 km long by 14 km wide. The carbonate landscape of northern Curacao is relatively flat with slopes that are horizontal or slightly dipping (Beets 1972).
Maps showing the location of Curacao, the outcrops of the Seroe Domi Formation as reported by Fouke et al. (1996) on Curacao, and details of the sampled site
The geology of Curacao can be broken into three main units, from oldest to youngest: (1) Cretaceous and Danian Sequence, (2) Eocene limestones, marls, sandstones, and clays, and (3) Carbonates of Neogene and Quaternary age (Beets 1972; De Buisonje 1974). The Quaternary carbonates are elevated marine terraces broken into four main units: the Lower Terrace (youngest), Middle Terrace, Higher Terrace, and Highest Terrace (oldest) located primarily along the island’s north shore. The Neogene Seroe Domi Formation (Fig. 1) is located primarily along the south shore (Beets 1972; De Buisonje 1974). The Cretaceous and Danian Sequence is composed of basalts of the Curacao Lava Formation, volcaniclastic sediments of the Knip Group, and turbidites of the Midden-Curacao Formation (Beets 1972).
The Seroe Domi Formation is composed of limestone and dolomitized limestone and is located mainly on the leeward side of the island as carbonate capped slopes. Steep scarps, usually ranging from 10 to 15 m, form on the landward side of these slopes. The dipping slopes, usually between 5° and 25°, were interpreted as not being a result of tectonic tilting, but rather a result of depositional slumping (De Buisonje 1974) later uplifted. The formation is detrital in nature, mainly composed of detritus of reef deposits (calcareous algae and corals), with minor constituents of eroded non-carbonate clasts. Some units also contain detrital clasts of the underlying Curacao Lava Formation. The basaltic diabase inclusions of the Curacao Lava Formation weather to clay.
Dolomitization of the Seroe Domi Formation
The dolomitization of the Seroe Domi Formation occurred under mixing zone conditions (Sibley 1980; Fouke et al. 1996). The primary evidence for the mixing zone model was isotopic and trace element composition data that were interpreted as being consistent with precipitation of dolomite from mixing seawater and freshwater. While the geochemical data indicated that the dolomitzation mechanism was mixing of freshwater and seawater, the updip stratiform distribution of some of the dolomite units (instead of a patchy, lenticular, or pervasive bed cross-cutting distribution) implies that brine reflux dolomitization also may have occurred (Fouke et al. 1996). The dolomite was divided into three units based on the extent of dolomitization (Fouke et al. 1996). Dating by 87Sr/86Sr indicates the oldest dolomite to be Middle Miocene (~12 mya), and the youngest dolomite to be Pliocene (~4 mya; Fouke et al. 1996).
Microbial carbonates
The metabolic activities of microorganisms have been shown to significantly alter the chemistry of their surroundings by exchanging ions with a solution, thereby producing various by-products that can cause changes in pH or redox conditions, and may chemically degrade minerals (Simkiss and Wilbur 1989; Chafetz and Buczinski 1992; Fortin et al. 1997; Sánchez-Navas et al. 1998; Leveille et al. 2000; Gerasimenko and Mikhodyuk 2009; Spadafora et al. 2010). Microbial activity can create localized supersaturation conditions that may lead to precipitation of carbonate minerals, especially in oxic conditions (Casanova et al. 1999; Sánchez-Román et al. 2011). In particular, cyanophyte photosynthesis has been shown to promote precipitation of CaCO3 in both laboratory experiments and natural environments (Dupraz et al. 2009). The extracellular polymeric substances (EPS) and cellular walls have been suggested as being effective at binding ionic species from solutions (Leveille et al. 2000) and are frequently cited as being the sites of carbonate nucleation (e.g. Rivadeneyra et al. 1996; Dupraz et al. 2004; Krause et al. 2012; Bontognali et al. 2014).
The observed relationships shown in natural environments between microbial cells and carbonate minerals suggest that microbes directly precipitate carbonates by nucleation (Vasconcelos et al. 1995; Vasconcelos and McKenzie 1997; van Lith et al. 2003a; Sánchez-Román 2006; Sánchez-Román et al. 2008; Gerasimenko and Mikhodyuk 2009; Spadafora et al. 2010; Sánchez-Román et al. 2011; Krause et al. 2012; Bontognali et al. 2014). Evidence supporting microbial nucleation of carbonates has been provided by laboratory experiments (Vasconcelos and McKenzie 1997; Warthmann et al. 2000; Bosak and Newman 2003; Sánchez-Román et al. 2007; Bontognali et al. 2008; Sánchez-Román et al. 2008; Sanchez-Román et al. 2011); however, the nature of these carbonates remains in question with respect to the crystallographic nature of the Ca–Mg carbonates identified (Gregg et al. 2015; Machel et al. 2015; Kaczmarek et al. 2017). Microbial nucleation of carbonate minerals on cell material has been hypothesized to be the dominant mode of microbial carbonate formation throughout the geologic record (Aloisi et al. 2006).
There is ample evidence that microorganisms can be involved in the precipitation of Mg–Ca carbonate minerals. Recent laboratory experiments have suggested that microbes are able to precipitate dolomite (e.g. Vasconcelos et al. 1995; Warthmann et al. 2000; Roberts et al. 2004; Wright and Wacey 2005; Sánchez-Román 2006; Sánchez-Román et al. 2008; Sánchez-Román et al. 2011); however, more recent reevaluation of the X-ray data presented in these studies indicates that protodolomite (very high-magnesium calcite or VHMC), not dolomite, was synthesized (Gregg et al. 2015; Machel et al. 2015; Kaczmarek et al. 2017). The term ‘protodolomite’ has previously been used to describe Ca–Mg carbonates (40–50 mol% MgCO3) that are metastable and deviate from dolomite stoichiometry by displaying weak or incomplete ordering (see Graf and Goldsmith 1956; Gaines 1977; Land 1980; Zhang et al. 2010; Gregg et al. 2015 for complete review of the usage of protodolomite). For the purposes of this study, very high-magnesium calcite (VHMC) will refer to Ca–Mg carbonates with near-dolomite stoichiometry but lacking ordering as suggest by Gregg et al. (2015).
Several studies have proposed bacterial mediation for the formation of dolomite in microbial mats (Vasconcelos et al. 1995; van Lith et al. 2003a; Visscher and Stolz 2005; Wright and Wacey 2005). Again, these studies have been reevaluated and interpreted to have synthesized VHMC instead of dolomite (Zhang et al. 2012; Gregg et al. 2015; Machel et al. 2015). Nucleation around bacterial cells within EPS in modern stromatolites has been attributed to the mineralization process of sulfate-reducing bacteria (van Lith et al. 2003b). Nucleation of Mg–Ca carbonates on cell walls of microorganisms that colonize on basalt has been previously documented in experiments (Roberts et al. 2004).
There still remains a gap in the understanding of the role of microorganisms in the precipitation of dolomite. If low-temperature microbial VHMC is analogous to high-temperature experiments, then microbial mediation may play a role in dolomitization (Gregg et al. 2015). It is uncertain that if given enough time whether VHMC will develop cation ordering through a dissolution–re-precipitation reaction (Gregg et al. 2015).
This study presents new field evidence of VHMC formed in association with microbial dumbbells (two globular bodies connected by a narrow waist, ~20 mm in length and 5–8 mm wide), which has only previously been documented in controlled settings (Vasconcelos et al. 1995; Warthmann et al. 2000; van Lith et al. 2003a; Vasconcelos et al. 2005; Sánchez-Román 2006; Sánchez-Román et al. 2008) and in sediments from Lagoa Vermelha, Brazil (Vasconcelos and Mckenzie 1997; Warthmann et al. 2000). Furthermore, this study reports active microbial communities forming dumbbell structures associated with clay minerals within pore spaces of carbonate rocks. The internal structure of the dumbbells is revealed for the first time, indicating an organic origin of these features.
Materials and methods
Field sites
A total of 18 dolomite rock samples were collected along the southeastern coast of Curacao at Sites 1 and 2 (Figs. 1, 2; GPS 12°4.260′N, 68°52.396′W and 12°4.703′N, 68°52.105′W, respectively). Site 1 is located along the southern edge of the western shore of Caracas Baii at a small erosional inlet (Fig. 2), while Site 2 is located along the western shore of Caracas Baii along cliffs near the small jetty (Fig. 2).
Sample preparation and analyses
Bulk rock samples (0.5–2 kg) were collected for analysis. Samples were examined at a variety of scales ranging from standard petrographic thin sections through scanning electron (SEM) to transmission electron (TEM) microscopy, which produces high magnification and high-resolution imagery of individual microbial structures. For TEM analysis, three rock samples (~75 to 100 g) from Site 1 were osmicated, rinsed with distilled water, incrementally dehydrated with acetone, embedded with incremental mixtures of acetone and Spurr’s Resin, and finally added to resin block molds to cure at 70 °C overnight (Bozzola and Russell 1998). Thick sections (2 μm) and ultra-thin sections (85 nm) were then cut using an ultra-microtome (model Reichert Jung Ultracut E) equipped with a diamond knife. Ultra-thin sections were collected on a copper grid, allowed to dry, and then stained with uranyl acetate for 20 min and with lead citrate for 5 min. A few unstained sections were used as a control to determine if staining had resulted in precipitate formation on the section and grid. This method is commonly used in biological science (Bozzola and Russell 1998); however, it is rarely used on geological materials. The TEM sections were analyzed using a JEOL JEM-100CXII and JEOL JEM-2100F instrunments.
Scanning electron microscope (SEM) observations of 16 fresh rock surfaces from four samples at Site 1 and two fresh rock surfaces from one sample at Site 2 were made using a Zeiss EVO-40XVP Environmental SEM equipped with an energy dispersive spectrometer (EDS). Samples were carefully fractured to expose fresh, broken surfaces, which were mounted on aluminum stubs using either hot glue or carbon tape, and Pt-coated. SEM observations focused on the features occupying pores in the host dolomite rocks as well as imaging the replacive dolomite rhombs.
Ten samples (eight from Site 1 and two from Site 2; Fig. 2) were made into standard petrographic thin sections (27 mm × 46 mm). The original rocks were trimmed, then vacuum embedded with blue-dyed epoxy, mounted on glass slides, and polished by Spectrum Petrographics, Inc. (Vancouver, WA, USA). The sections were stained with Alizarin Red-S to distinguish calcite and dolomite.
A single sample (~250 g in size) was sent to Research and Testing Laboratory (Lubbock, TX, USA) for bacterial DNA extraction and 16S rRNA gene analysis. The sample was fractured and swabbed on fresh broken surfaces and within pore spaces. Samples were amplified for pyrosequencing using a forward and reverse fusion primer. Amplifications were performed in 25 μl reactions with Qiagen HotStar Taq master mix (Qiagen Inc, Valencia, California, USA), 1 μl of each 5 μM primer, and 1 μl of template. Reactions were performed on ABI Veriti thermocyclers (Applied Biosytems, Carlsbad, California, USA) under the following thermal profile: 95 °C for 5 min, then 35 cycles of 94 °C for 30 s; 54 °C for 40 s, 72 °C for 1 min, followed by one cycle of 72 °C for 10 min and 4 °C hold. Amplification products were visualized with eGels (Life Technologies, Grand Island, New York, USA). Products were then pooled equimolar and each pool was cleaned with Diffinity RapidTip (Diffinity Genomics, West Henrietta, New York, USA), and size selected using Agencourt AMPure XP (BeckmanCoulter, Indianapolis, Indiana, USA) following Roche 454 protocols (454 Life Sciences, Branford, Connecticut). Size selected pools were then quantified and 150 ng of DNA was hybridized to Dynabeads M-270 (Life Technologies) to create single-stranded DNA following Roche 454 protocols (454 Life Sciences). Single-stranded DNA was diluted and used in emPCR reactions, which were performed and subsequently enriched. Sequencing followed established manufacturer protocols (454 Life Sciences).
Results
SEM and TEM micrographs reveal large (20 μm) and small (400 nm) rhombohedra crystals associated with clay minerals (Figs. 3, 4). EDS of these rhombs indicate mol% MgCO3 values of ~40 to 48% for the small crystals. In addition, microbes surrounded by clay minerals were identified within pore spaces (Fig. 4). EDS analysis of these clay minerals revealed Mg, Fe, Al, and Si, indicating that these clays are Mg–Fe aluminum silicates.
TEM micrograph and four EDS maps showing crystals associated with organics and clay minerals. White color in EDS maps represents the higher abundance of each element. A white circle is in the same location on all five images circling a very high-magnesium calcite (VHMC) crystal. The cellular material at the bottom of the TEM micrograph can be seen as lacking the elements shown in the four elemental maps. The darker material surrounding the cellular material in the TEM micrograph is interpreted as clay material that is rich in Si, Al, and Mg. This material is shown to have Si, Al, and Mg in the corresponding EDS maps
The TEM investigation also revealed dumbbells ~20 μm in length and 5–8 μm wide that had a “fuzzy” external texture (Figs. 4, 5b). Dumbbell morphologies have been seen in many other carbonate sediments (Folk et al. 1985; Buczynski and Chafetz 1991; Vasconcelos and McKenzie 1997 and references therein); however, these dumbbells are identical in shape and size to dumbbells interpreted as being microbial in origin forming in association with sulfate-reducing bacteria in algal mats (Warthmann et al. 2000). Additional sectioning found more dumbbell structures that were not “fuzzy” (Fig. 5a, c). Silicate clay minerals were found surrounding the dumbbell and the Ca–Mg carbonate minerals were precipitated within the cell walls, sheaths, and within the biofilm surrounding the dumbbell (Fig. 5).
TEM micrographs of dumbbell structures. a TEM micrograph of sectioned dumbbells showing internal cellular structure. Micrograph (a) clearly displays the cellular nature of the dumbbells. b Micrograph (b) is cut from the same block as micrograph (a), displaying a microbial dumbbell with small crystals nucleating (white circles) within the dumbbell. c Clay material surrounds the sheath of each dumbbell and very high-magnesium calcite (VHMC) was identified within the sheath and surrounding the dumbbell. The two cellular structured “ends” lack precipitates and clay minerals. The black circle is the approximate area that was elementally mapped using EDS in Fig. 4. d Cartoon showing the difference in outer texture and inner structure of the dumbbells. Outer sections show the texture of the organic sheath and surrounding minerals. Inner sections represent the internal structure after several more sections are cut from the sample
SEM micrographs indicate that all samples from Sites 1 and 2 contained a second type of rhombic, carbonate that may be protodolomite or VHMC. These crystals appear in association with both clay minerals and organic textures (Figs. 3, 6). EDS analysis indicates Mg content of ~25 to ~45 mol%, which suggests these are at least a VHMC composition (Fig. 7). Whether the crystals exhibit cation ordering or not is unknown; thus this material is referred to hereinafter as VHMC. It is important to mention that all samples examined from Sites 1 and 2 contained clay minerals. Microbial textures such as filaments and biofilms were found in most samples from Sites 1 and 2 (Figs. 6, 7). In addition, frambiodal pyrite was identified in one sample from Site 1 (Fig. 8).
TEM images and EDS maps showing the circled area surrounding the dumbbell of Fig. 5c. The white color in EDS images represents the higher abundance of each element. Abundant Mg–Fe aluminum silicate (white square) and Mg–Ca carbonate (white oval) surround the dumbbell. Silicate clay minerals can be seen surrounding the dumbbell and the Ca–Mg carbonate mineral (very high-magnesium calcite, VHMC) were precipitated within the cell walls, sheaths, and within the biofilm surrounding the dumbbell as in Fig. 4
In petrographic thin sections, partially fabric-retentive, fine to very finely crystalline dolomite (100 to 50 μm) is the most common dolomite found at Sites 1 and 2 (Figs. 9, 10). This is the replacive dolomite described by Fouke et al. (1996). Euhedral dolomite spar with cloudy centers and clear rims commonly fills intraparticle porosity. Staining with Alizarine Red-S indicates that the centers of these crystals are more calcitic. These euhedral dolomite cements are in turn overlain by masses and thin coatings of brown-colored material, although details as to that material’s composition are unclear at the scale of a petrographic thin section (Figs. 9, 10). In addition to the dolomite, abundant weathered basalt fragments can be observed in the petrographic thin section micrographs (Fig. 10). These weathered rock fragments are pervasive throughout the outcrops at both sites.
Photomicrographs of petrographic thin sections from Sites 1 and 2. a Plain polarized light showing replacive euhedral dolomite crystals of the Seroe Domi Formation, pore space in a partially dissolved red algal allochem (blue-dyed epoxy), small clasts of altered volcanic rock fragments (brown and greenish brown grains). Euhedral dolomite cement crystals line the pore space and those crystals are overlain by a microporous mass of brown material. That last generation of material is the focus of this study. b Cross polarized light showing partial fabric retentive nature of a dolomitized foraminifera. Dark brown and yellowish brown grain in top right is an altered volcanic rock fragment
DNA results indicate that Cyanophyta dominate the microbial community with species of Symploca as the most abundant. Sulfate-reducing bacteria (Thermodesulforhabdus norvegicus and Desulfoomonile tiedjei) and sulfur-reducing bacteria (Desulfuromonas acetoxidans) are present in the sample. Multiple marine species of Cyanohyta, Bacteroidetes, and Proteobacteria are present (e.g. Microcoleus chthonoplastes, Nodularia spumigena, Gracilimonas tropica, Gramella forsetii) as well as freshwater Cyanophyte species (e.g. Microcystis aeruginosa, Leptolyngbya antartica, Microcystis viridis).
Discussion
The texture and size of the replacement dolomite of the matrix of the Seroe Domi Formation is fine-crystalline in thin section and SEM micrographs as described by Fouke et al. (1996). The Ca–Mg carbonate crystals (VHMC) in the overlying organic material are interpreted to be precipitated in association with the clay minerals and cyanophyte communities within pore spaces of the rock. The Ca and Mg in the VHMC could have been derived from at least one of several potential sources: (1) basalt clasts chemically broken down into clay; (2) seawater; (3) Mg-rich allochems, (4) replacive dolomite. The first three are viable if the VHMC formed below the water table in either seawater or freshwater fluids prior to uplift: weathering of basalt clasts, sea-spray, and dissolution of the replacive dolomites would be possible if the VHMC formed in the vadose zone after uplift of the host dolomites.
The proposed origin of the VHMC is microbial precipitation and nucleation in association with clay minerals. These VHMC crystals are found in association with organic textures, clay minerals, and cyanophyte communities filling pore spaces of the rock (Figs. 3, 4, 5, 6). Nucleation of VHMC is observed in the EPS surrounding the cellular dumbbells as small crystals (Figs. 5, 7). We suggest that nucleation of clay minerals and the VHMC begins within the EPS of the biofilm generated by the microbial community, and crystal growth continues in the geochemical environment facilitated by the microbial community.
VHMC precipitation is likely induced by microbial metabolic activities related to several processes: (1) decomposition of organic material (e.g. Berner 1968; Ehrlich and Newman 2008); (2) sulfate reduction of saline pore waters (e.g. Braissant et al. 2007); (3) weathering of inclusions of basalt clasts (Leveille et al. 2000); and (4) cation exchange from charged clay minerals (e.g. Kahle 1965). Organic material originates from at least one of the three possible sources: (1) syndeposition with the rock; (2) collection at density interfaces (e.g. the halocline or water table of a freshwater lens; Bottrell et al. 1991, 1993); (3) modern collection associated with percolation of meteoric and marine water.
The “fuzzy” dumbbell found in section (Fig. 5b) differs in appearance from the cellular dumbbell (Fig. 5a, c). While TEM shows internal structure quite well, it does not usually show surface texture. However, the “fuzzy” dumbbell and the cellular dumbbells are likely the same feature. The dumbbells shown in Fig. 5b, c were cut from the same resin block. The “fuzzy” dumbbell was cut from “outer” sections, and it likely represents the outer surface of a dumbbell. Furthermore, the cellular dumbbell was cut from “inner” sections, and it likely represents the inner structure of a dumbbell (Fig. 5d). The “fuzzy” dumbbell represents the sectioning of the extreme outer surface of a dumbbell, and it appears “fuzzy” because clay minerals surround the sheath of the cyanophyte community. The cellular dumbbell represents the internal structure of a dumbbell, and it also indicates that these dumbbells are single-cellular cyanophytes connected by sheaths. Clay minerals and VHMC crystals surround the dumbbells and are also found within the sheaths (Figs. 5, 7).
The similarity of the dumbbells found in TEM of rocks in outcrop to that reported by previous studies (e.g. Vasconcelos and McKenzie 1997; Warthmann et al. 2000) of algal mats further demonstrates microbial mediation of VHMC formation (Fig. 5). Cyanophytes play a role in the formation of clay minerals and VHMC in the Seroe Domi Formation on Curacao. It is possible that microbes may also have played a role in the formation of VHMC, dolomite, and clay minerals in other studies (e.g. Vaconcelos and McKenzie 1997; Warthmann et al. 2000) and other settings. Dumbbells found by previous in situ studies (e.g. Vaconcelos and McKenzie 1997; Warthmann et al. 2000) had a similar origin as the dumbbells found in this study.
The microbial assemblage detected by gene sequencing is composed of a diverse set of marine, brackish, and freshwater microbes as well as photosynthesizers, sulfur reducers, and sulfate reducers. The swab sampling technique likely contributes to this large biodiversity. Marine organisms are possibly remnants from deposition or surface communities related to sea spray. Sulfate and sulfur reducers likely represent pore water communities. Frambiodal pyrite was identified within a pore in one sample from Site 1, further suggesting biological sulfate and/or sulfur reduction occurring (Fig. 8). Freshwater communities are related to meteoric waters infiltrating into the outcrop. It is uncertain which communities are contributing to VHMC formation or if these communities are associated with basalt clast weathering. It is likely that the marine pore water communities are those associated with VHMC formation as imaged by TEM analysis. Further analysis is required to determine the nature of the biogeochemical reactions occurring and the principle contributors of this microbial assemblage to VHMC formation.
Conclusions
The Ca–Mg carbonate crystals (VHMC) found in association with microbial communities and clay minerals from samples of the Seroe Domi Formation on Curacao are precipitated within the pore spaces of these rocks. These observations provide further evidence of a microbial VHMC formation in a natural setting, a process that has been identified by previous studies (Vasconcelos et al. 1995; Vasconcelos and McKenzie 1997; Warthmann et al. 2000; van Lith et al. 2003a; Vasconcelos et al. 2005; Sánchez-Román 2006; Sánchez-Román et al. 2008; Gregg et al. 2015). The observations of precipitation within the internal structure of the biofilm provide visible evidence that Ca–Mg carbonate-crystal (VHMC) dumbbells are formed in association with microbial communities. These images show that the microbial community creates biofilms where VHMC and clay minerals nucleate and grow, are organic in origin, and the microbial community is capable of precipitating VHMC. All cellular structures (dumbbells) identified in TEM were surrounded by clay minerals and contained very small VHMC crystals. Furthermore, DNA analysis indicates the presence of many microbial species including Cyanophyta and sulfate-reducing bacteria that likely are promoting the precipitation of VHMC within the Seroe Domi Formation on Curacao.
References
Aloisi G, Gloter A, Kruger M, Wallmann K, Guyot F, Zuddas P (2006) Nucleation of calcium carbonate on bacterial nanoglobules. Geology 34:1017–1020. doi:10.1130/G22986A.1
Beets DJ (1972) Lithology and stratigraphy of the cretaceous and danian succession of Curacao (Ph.D. thesis): University of Amsterdam, The Netherlands, 153 p
Berner RA (1968) Calcium carbonate concretions formed by the decomposition of organic matter. Science 159(3811):195–197
Bontognali TRR, Vasconcelos C, Warthmann RJ, Dupraz C, Bernasconi SM, McKenzie JA (2008) Microbes produce nanobacteria-like structures, avoiding cell entombment. Geology 36:663–666. doi:10.1130/G24755A.1
Bontognali TR, McKenzie JA, Warthmann RJ, Vasconcelos C (2014) Microbially influenced formation of Mg-calcite and Ca-dolomite in the presence of exopolymeric substances produced by sulphate-reducing bacteria. Terra Nova 26(1):72–77
Bosak T, Newman DK (2003) Microbial nucleation of calcium carbonate in the Precambrian. Geology 31:557–580. doi:10.1130/0091-7613(2003)031<0577:MNOCCI>2.0.CO;2
Bottrell SH, Smart PL, Whitaker F, Raiswell R (1991) Geochemistry and isotope systematics of sulphur in the mixing zone of Bahamian blue holes. Appl Geochem 6(1):97–103
Bottrell SH, Carew JL, Mylroie JE (1993) Bacterial sulphate reduction in flank margin environments: evidence from sulphur isotopes. In: Proceedings of the 6th symposium on the geology of the Bahamas, Port Charlotte, Florida, Bahamian Field Station, pp 17–21
Bozzola JJ, Russell LD (1998) Electron Microscopy. Principles and techniques for biologists. Jones and Bartlett Publishers, Sudbury
Braissant O, Decho AW, Dupraz C, Glunk C, Przekop KM, Visscher PT (2007) Exopolymeric substances of sulfate-reducing bacteria: interactions with calcium at alkaline pH and implication for formation of carbonate minerals. Geobiology 5(4):401–411
Buczynski C, Chafetz HS (1991) Habit of bacterially induced precipitates of calcium carbonate and the influence of medium viscosity on mineralogy. J Sediment Petrol 61:226–233
Casanova J, Bodenan F, Negrel P, Azaroual M (1999) Microbial control on the precipitation of modern ferrihydrite and carbonate deposits from the Cezallier hydrothermal springs (Massif Central, France). Sed Geol 126:125–145. doi:10.1016/S0037-0738(99)00036-6
Chafetz HS, Buczinski C (1992) Bacterially induced lithification of microbial mats. Palaios 7:277–293. doi:10.2307/3514973
De Buisonje PH (1974) Neogene and quaternary geology of Aruba, Curacao, and Bonaire (Ph.D. thesis): University of Utrecht, The Netherlands, p 293
Dupraz C, Visscher PT, Baumgartner LK, Reid RP (2004) Microbe-mineral interactions: early carbonate precipitation in a hypersaline lake (Eleuthra Island, Bahamas). Sed Geol 51:745–765
Dupraz C, Reid PR, Braissant O, Decho AW, Norman RS, Visscher PT (2009) Processes of carbonate precipitation in modern microbial mats. Earth Sci Rev 96:141–162. doi:10.1016/j.earscirev.2008.10.005
Ehrlich HL, Newman DK (eds) (2008) Geomicrobiology, 5th edn. Boca Raton, FL, p 628
Folk RL, Chafetz HS, Tiezzi PA (1985) Bizarre forms of depositional and diagenetic calcite in hot-spring travertines, central Italy. SEPM Special Publication 36, Carbonate Sediments, pp 349–369
Fortin D, Ferris FG, Beveridge TJ (1997) Surface-mediated mineral development by bacteria. In: Banfield JF, Nealson KH (eds), Geomicrobiology: interactions between microbes and minerals: reviews in mineralogy: Washington, D.C., Mineralogical Society of America, vol. 35, pp 161–180
Fouke BW, Beets DJ, Meyers WJ, Hanson GN, Melillo AJ (1996) 87Sr/86Sr chronostratigraphy and dolomitization history of the Seroe Domi Formation, Curacao (Netherlands Antilles). Facies 35:293–320. doi:10.1007/BF02536966
Gaines AM (1977) Protodolomite redefined. J Sediment Res 47(2):543–546
Gerasimenko LM, Mikhodyuk OS (2009) Halophilic algal-bacterial and cyanobacterial communities and their role in carbonate precipitation. Paleontol J 43(8):940–957
Graf DL, Goldsmith JR (1956) Some hydrothermal syntheses of dolomite and protodolomite. J Geol 64(2):173–186
Gregg JM, Bish DL, Kaczmarek SE, Machel HG (2015) Mineralogy, nucleation and growth of dolomite in the laboratory and sedimentary environment: a review. Sedimentology 62(6):1749–1769
Kaczmarek SE, Gregg JM, Bish DL, Machel HG, Fouke BW (2017) Dolomite, very high-magnesium calcite, and microbes—implications for the microbial model of dolomitization. SEPM Special Publication No. 109
Kahle CF (1965) Possible roles of clay minerals in the formation of dolomite. J Sediment Res 35(2):448–453
Krause S, Liebetrau V, Gorb S, Sánchez-Román M, McKenzie JA, Treude T (2012) Microbial nucleation of Mg-rich dolomite in exopolymeric substances under anoxic modern seawater salinity: new insight into an old enigma. Geology 40(7):587–590
Land LS (1980) The isotopic and trace element geochemistry of dolomite: the state of the art. SEPM Special Publication No. 28, pp 87–110
Leveille RJ, Fyfe WS, Longstaffe FJ (2000) Geomicrobiology of carbonate-silicate microbialites from Hawaiian basaltic sea caves. Chem Geol 169:339–355. doi:10.1016/s0009-2541(00)00213-8
Machel HG, Gregg JM, Bish DL, Kaczmarek S (2015) Microbial dolomite that isn’t dolomite. 15th Bathurst Meeting, 13–16th July, 2015, University of Edinburgh, U.K. Technical Program with Abstracts, p 80
Rivadeneyra MA, Ramos-Cormenzana A, Delgado G, Delgado R (1996) Process of carbonate precipitation by Deleya halophile. Curr Microbiol 32:308–313. doi:10.1007/s002849900055
Roberts J, Bennett PC, González LA, Macpherson GL, Miliken KL (2004) Microbial precipitation of dolomite in methanogenic groundwater. Geology 32:277–280. doi:10.1130/G20246.2
Sánchez-Navas A, Martín-Algarra A, Nieto F (1998) Bacterially-mediated authigenesis of clays in phosphate stromatolites. Sedimentology 45:519–533. doi:10.1046/j.1365-3091.1998.00157.x
Sánchez-Román M (2006) Calibration of microbial and geochemical signals related to dolomite formation by moderately halophilic aerobic bacteria: Significance and implication of dolomite in the geologic record (Ph.D. thesis): Switzerland, ETH Zurich (Swiss Federal Institute of Technology), p 134
Sánchez-Román M, Rivadeneyra M, Vasconcelos C, Mckenzie JA (2007) Biomineralization of carbonate and phosphate by halophilic bacteria: influence of Ca2+ and Mg2+ ions. FEMS Microbiol Ecol 61:279–284
Sánchez-Román M, Vasconcelos C, Schmid T, Dittrich M, McKenzie JA, Zenobi R, Rivadeneyra MA (2008) Aerobic microbial dolomite at the nanometer scale: implications for the geologic record. Geology 36:879–882. doi:10.1130/G25013A.1
Sánchez-Román M, Romanek CS, Fernández-Remolar DC, Sánchez-Navas A, McKenzie JA, Pibernat RA, Vasconcelos C (2011) Aerobic biomineralization of Mg-rich carbonates: implications for natural environments. Chem Geol 281(3):143–150
Sibley DF (1980) Climatic control of dolomitization, Seroe Domi Formation (Pliocene). Bonaire, NA
Simkiss K, Wilbur KM (1989) Biomineralization: cell biology and mineral deposition. Academic Press, San Diego
Spadafora A, Perri E, McKenzie JA, Vasconcelos C (2010) Microbial biomineralization processes forming modern Ca: Mg carbonate stromatolites. Sedimentology 57(1):27–40
van Lith Y, Wartmann R, Vasconcelos C, McKenzie JA (2003a) Microbial fossilization in carbonates sediments: a result of the bacterial surface involvement in dolomite precipitation. Sedimentology 50:237–245. doi:10.1046/j.1365-3091.2003.00550.x
van Lith Y, Warthmann R, Vasconcelos C, McKenzie JA (2003b) Sulfate-reducing bacteria induce low-temperature Ca-dolomite and high Mg-calcite formation. Geobiology 1:71–79. doi:10.1046/j.1472-4669.2003.00003.x
Vasconcelos C, McKenzie JA (1997) Microbial mediation of modern dolomite precipitation and diagenesis under anoxic conditions (Lago Vermelha, Rio de Janeiro, Brazil). J Sediment Res 67:378–390
Vasconcelos C, McKenzie JA, Bernasconi S, Grujic D, Tien AJ (1995) Microbial mediation as a possible mechanism for natural dolomite formation at low temperatures. Nature 377:220–222. doi:10.1038/377220a0
Vasconcelos C, McKenzie JA, Warthmann R, Bernasconi SM (2005) Calibration of the δ18O paleothermometer for dolomite precipitated in microbial cultures and natural environments. Geology 33:317–320. doi:10.1130/G20992.1
Visscher J, Stolz JF (2005) Microbial mats as bioreactors: populations, process, and products. Palaeogeogr Palaeoclimatol Palaeoecol 219:87–100. doi:10.1016/j.palaeo.2004.10.016
Warthmann R, van Lith Y, Vasconcelos C, McKenzie JA, Karpoff AM (2000) Bacterially induced dolomite precipitation in anoxic culture experiments. Geology 28:1091–1094. doi:10.1130/0091-7613(2000)28<1091:BIDPIA>2.0.CO;2
Wright DT, Wacey D (2005) Precipitation of dolomite using sulphate-reducing bacteria from the Coorong region, South Australia: significance and implications. Sedimentology 52:987–1008. doi:10.1111/j.1365-3091.2005.00732.x
Zhang F, Xu H, Konishi H, Roden EE (2010) A relationship between d104 value and composition in the calcite-disordered dolomite solid–solution series. Am Miner 95(11–12):1650–1656
Zhang F, Xu H, Konishi H, Shelobolina ES, Roden E (2012) Polysaccharide-catalyzed nucleation and growth of disordered dolomite: a potential precursor of sedimentary dolomite. Am Miner 97(4):556–567
Acknowledgements
The authors would like to thank David Budd and all of the anonymous reviewers that helped shape this manuscript with their insightful comments and suggestions. Special thanks to Brenda Kirkland for insight and guidance during the pilot portion of this project. The Carmabi Research Institute provided logistical assistance for field reconnaissance. Mr. R. Sledge Simmons provided funding for lodging. A Grant from the National Speleological Society provided funds for equipment use. Mississippi State University Department of Geosciences assisted with field and analytical expenses. Special thanks for laboratory assistance during TEM imaging/analysis to Amanda Lawrence and Rooban Venkatesh K. G. Thirumalai of I2AT at Mississippi State University. Sam Houston State University Department of Geography and Geology assisted with TEM analytical expenses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sumrall, J.B., Larson, E.B. & Mylroie, J.E. Very high magnesium calcite formation and microbial communities found in porosity of the Seroe Domi Formation of Curacao, Netherland Antilles. Carbonates Evaporites 32, 123–133 (2017). https://doi.org/10.1007/s13146-017-0352-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13146-017-0352-7