Abstract
In southern Tunisia, the major aquifer system is characterized by the superposition of three principal levels: the upper unconfined Quaternary alluvial aquifer; the intermediate semi-confined/confined complex terminal (Segui, Beglia and Zebbag Formations) and the Continental intercalaire (Bou Dinar and Sidi Aïch Formations) which constitutes the deeper confined aquifer. The hydrodynamic functioning of this aquifer system is largely influenced by tectonics (Gafsa and Sehib faults), lithologic variation and recharge conditions. The tectonics has contributed to the discontinuity of the aquifer levels by creating some barriers, which play the role of hydraulic sills. A study of the potentiometric maps shows four principal flow directions. These flow directions converge at the sill zones (Chott El Guettar and Chott Djerid depressions). The total mineralization evolution shows an increase from the periphery of the basin to the discharge area (1.75 ≤ TDS ≤ 14.4 g/l). Dissolution of halite, gypsum and/or anhydrite-bearing rocks is the principal source of the salinity of the groundwater. The isotopic signatures permit to classify the studied groundwaters into two different groups: Non-evaporated groundwaters that are characterized by depleted δ18O and δ2H contents highlight the importance of modern recharge at higher altitude. Evaporated groundwaters with enriched contents reflect the significance infiltration of return flow irrigation waters and the influence of the water dam. Tritium data in the studied groundwaters lend support to the existence of pre-1950 and post-1960 recharge. According to radiocarbon residence time estimates, the deep groundwater is approximately 40 kyears old, and was recharged during a period when the climate was wetter and colder. Based on radiocarbon content, the shallow groundwater is older than 0.5 kyear, and represents modern waters mixed with a limited quality of palaeo-recharge. Moreover, the insulation of certain compartments of the reservoir and inter-communications between the aquifer levels seem to play a principal role in the degradation of quality of groundwater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In most arid and semi-arid regions in the world, the availability of sufficient freshwater has become a limiting factor for development. In North African regions, where water scarcity was always a dominant problem, the interference with the natural hydrologic cycle as a result of groundwater resources overexploitation and changes in land usage have provoked not only the reduction of the available water but also the deterioration of the water quality. During the past decades, groundwater exploitation in the El Guettar area (mining Gafsa basin) has increased dramatically, mainly due to an increase in irrigated agriculture, tourism and industry. To meet the needs of drinking water for future generations, sustainable watershed management is essential for drinking water supply especially in semi-arid areas, and requires a more detailed knowledge about recharge processes.
At the beginning, the oases have been created around the artesian springs, forming assembly points of desert nomads. Based on landform, oases, in southern Tunisia, can be classified as alluvial plain (Chott El Guettar margin), mountains (Gafsa and Northern Chotts range piedmonts) and coastal (Gulf of Gabes) (Coque 1962). Saharan hot strong winds and sands, serious soil salinization and water insufficiency are the natural causes for the disappearance of some ancient oases (Mamou and Kassah 2002). Fast population growth, and expanding agricultural and industrial (Phosphate Company of Gafsa “CPG”) areas have resulted in a rapid increase in water demand from the shallow and the deeper aquifers. The long-term withdrawals have engendered several deleterious problems such as water-level decline, distinction of artesianism, salinization and deterioration of groundwater resources. The objective of this study was the investigation of the hydrogeochemical evolution and the recharge processes of different groundwater bodies as well as the natural and anthropogenic processes that control their mineralization, which will contribute to the sustainable management of groundwater resources in the El Guettar basin. In this study, hydrogeologic, hydrochemical and isotopic information from the aquifer system will be integrated and used to determine the main factors and mechanisms controlling the hydrodynamic and the chemistry of groundwaters in the study area (Southwest Tunisia).
Study area
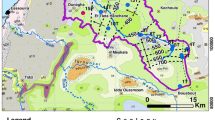
The study area is located between the longitudes 6°30–7°30′ east and the latitudes 34°00–34°30 north. It is limited in the west by the Algerian frontier, in the east by the Gulf of Gabes, by the Gafsa range in the north and by Northern Chott Mont in the south. The El Guettar oasis basin covers an area extent of approximately 450 km2 (Fig. 1). In the study region, oases area increased from single to double in 43 years (1970–2013) by the extension of the old one and the creation of new oases to reduce increasing unemployment, affecting the extreme water and soil resources in the region.
The surface drainage network, which is very dense, is constituted by several non-perennial wadis (Bayeich, El Maleh, Majni, Lortess, As Sad, Berda…). It collects surface runoff from the Algerian territory and from the surrounding hills toward the continental depression of Chott El Guettar (Fig. 1). Pediments in the study district are mainly composed of poorly evolved alluvial soils with a thickness of more than 4 m. These alluvial soils undergo salinization processes downstream. However, topomorphic and lithomorphic vertisols are present in inland plains and are characterized by the presence of swelling clays of dark color. These organic matter-rich soils show cracking during the dry season and are generally suitable for horticultural and orchard cultivation. Some other parts of the study area are marked by the abundance of Mediterranean red soils, which have fine texture and a well-developed polyhedral structure and calcareous accumulations (Elmejdoub and Jedoui 2009).
Climate
Mediterranean areas of both southern Europe and North Africa are subject to dramatic changes that will affect the sustainability, quantity, quality, and management of water resources. Most climate models forecast an increase in temperature and a decrease in precipitation at the end of the twenty-first century. Tunisia is located in the Western Mediterranean, which represents a climatic transition zone open to the influence of the cool North Atlantic air masses and the warm Mediterranean air masses (Rindsberger et al. 1990). Moreover, specific geomorphologic characteristics of Tunisia, i.e. the absence of high mountains and the relatively limited geographic extension allow the integration of Saharan air masses into the atmospheric circulation (Celle-Jeanton et al. 2001a). However, hydrometeorological studies (Bousnina 1997; Celle et al. 2000; Celle-Jeanton et al. 2001b) suggest the existence of two major trajectories for dominant air masses. These are (i) Atlantic air masses that circulate from the west over Northern Africa and (ii) Mediterranean air masses that come from the north (Hamed 2009, 2011; Hamed and Dhahri 2013). Quantitatively, Mediterranean precipitation represents 66 % of the total rainfall. The main part of the regional aquifer recharge is supplied by Mediterranean rain events.
The study area is an ecologically fragile area, which is characterized by an arid type climate with a mean annual precipitation of <250 mm. The mean annual temperature is 20 °C with maximum value of up to 42 °C in summer (August month) and with minimum value of up to −2 °C in winter (January month). Data on evaporation and evapotranspiration are very scarce. Few measurements were recorded by the National Office for Meteorology (ONM 2010). Figures recorded at Gafsa station between 1965 and 1972 revealed a mean inter-annual rate of 1,900 mm/year for evaporation (UNESCO 1972). On the other hand, mean annual evapotranspiration, as estimated by means of empirical formula such as those of Thornthwaite, Turc and Penman, gave results ranging from 1,345 (year 1984) to 1,905 mm (year 2000) (ONM 2010). Potential evapotranspiration was calculated for each grid point at a resolution of 0.5 km2, based on the monthly average temperature and the latitude (Thornthwaite 1948). In southern Algeria, at about 150 km western study area, the evaporative losses from the shallow aquifer through a 6-m thick unsaturated zone were estimated to be 2 mm/year (Guendouz et al. 2003).
The Earth’s average temperature has tended to increase since the nineteenth century, particularly since the 1920s (e.g., Folland et al. 2002; Brohan et al. 2006; Hansen et al. 2006). The Mediterranean basin has been no exception, with an increase in average temperature having been observed during the twentieth century (Bethoux et al. 1998; Repapis and Philastras 2004; Camuffo et al. 2010). All studies for the region indicate a trend toward higher temperatures. Thus, reports of the temperature increase over the entire basin in the last 100 years have ranged from 1 to 4.5 °C (Brunetti et al. 2004; Alpert et al. 2008; Bertin 2008). At a more localized scale, warming temperatures have been reported for the Iberian Peninsula (Sanz-Elorza et al. 2003; Vargas-Yáñez et al. 2008), Italy (Brunetti et al. 2000), Greece (Kostopoulou and Jones 2005), the eastern Mediterranean (Saaroni et al. 2003; Alpert et al. 2004), the central western Mediterranean (Piervitali et al. 1998), the alpine region (Böhm et al. 2001; Beniston and Jungo 2002; Casty et al. 2005) and North Africa (Hassanean and Abdel Basset 2006; El Kenawy et al. 2009). The generalized increase in temperature is particularly evident in summer (Jacobeit 2000; Palutikof and Holt 2004; Ziv et al. 2005; Abaurrea et al. 2007), and in the maximum temperatures attained in the northern Mediterranean basin (Brunetti et al. 2000; Saaroni et al. 2003; Abaurrea et al. 2007). This contrasts with observations in the southern Mediterranean basin, where the main temperature increase in Egypt (Domroes and El-Tantawi 2005), Libya (El Kenawy et al. 2009) and Tunisia (Hamed 2009) has occurred in the minimum temperature, whereas the maximum temperature has remained relatively constant. In addition, an increasing occurrence of warm events during winter has been reported in the Iberian Peninsula (Santos and Corte-Real 2006) and the Alps (Beniston 2005). Figure 2 summarizes the climate evolution in the second half of the twentieth century over the Mediterranean region. The data were obtained by the Climate Research Unit of the University of East Anglia (http://www.cru.uea.ac.uk/~timm/grid/CRU_TS_2_1.html) (José et al. 2011).
Seasonal and annual climate evolution in the Mediterranean region between 1950 and 2002. Colors show the magnitude of changes in precipitation (left) and potential evapotranspiration (right), in mm. Black isolines areas with significant trends (p < 0.05) (José et al. 2011)
Geology and hydrogeology
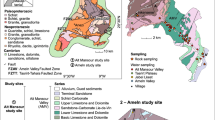
Southern Tunisia is underlain by two major aquifer systems the CI overlain by the CT (Fig. 3). The CI aquifer is one of the largest confined aquifers in the world. This immense multi-layered aquifer is hosted in the continental formations of the Lower Cretaceous (Neocomian, Barremian, Aptian and Albian). The CI aquifer is located within a complex succession of clastic sediments of Mesozoic age. The geological succession is composed of several units of detrital sediments separated by clay strata and gypsum intercalations, giving rise to a degree of heterogeneity of this aquifer. At its maximum, the aquifer thickness exceeds 1,500 m. The geological formations forming the CI aquifer show significant lateral variations in facies and thickness beginning from the Saharan platform reaching the Chott area.
Lithostratigraphic column of the study area (Hamed 2011)
The CI aquifer is shared between Algeria, Tunisia and Libya and covers a surface of more than 1 million km2 of which 700,000 km2 are in Algeria, 80,000 km2 in Tunisia, and 250,000 km2 in Libya (SASS 2002). The aquifer is, however, hydraulically continuous over the whole basin. Given the arid to Saharan climate conditions, these formations are slightly recharged: the system recharge represents about 1 billion m3/year, essentially percolating in the Saharan Atlas piedmont plains in Algeria, the Dahar and the Tunisian Atlas in Tunisia and Djebal Nefoussa in Libya (Fig. 1). However, the system extension and the layer thickness have facilitated the accumulation of considerable reserves over the past centuries (SASS 2002). The general direction of groundwater flow in the study area is from North to South and from West to East (Guendouz et al. 2003; Kamel et al. 2008; Hamed 2009; Mokadem 2012).
The groundwater reservoir of the CT is contained in the Upper Cretaceous and Tertiary formations. The CT formations are relatively heterogeneous and are composed of two main aquifer horizons separated by semi-permeable to impermeable strata: (1) the Cenomanian–Turonian carbonates (Zebbag Formation: main aquifer of the El Guettar region), extending over the whole basin. In the study area the Zebbag formation represented by two distinct members: to the basis, dolomite and chalky dolomite with a thickness varying between 100 and 250 m and to the top by intercalations of marl, clay, gypsum and thin levels of limestone. The thickness of the superior member of this formation varies between 180 and 350 m. The transmissivity of this carbonate formation, obtained from pumping tests, ranges from 1.4 × 10−3 to 2.2 × 10−2 m2 s−1. However, the permeability varies between 7.3 × 10−4 and 15.1 × 10−2 m2 s−1 (obtained by grain-size distribution) in the same formation (DGRE 2010).
The high hydraulic gradient is associated with over-pumping of groundwater, which is extracted mainly for irrigation and for washing the phosphate. These values indicate that this carbonate aquifer has high potentiality and it is considered as the most productive aquifer in the El Guettar basin. This hydrogeological reservoir is formed by a complex conduit system. Moreover, the resources are very hard to exploit, with flow velocities ranging from a few centimeters a day to hundreds of meters an hour (Fig. 4).
Note, however, that this “karst” aquifer is continuously recharged by rainfall, where water seepage is facilitated by the fractured and karstified nature of limestone and also recharged by upwards leakage from the underlying of CI aquifer. The word ‘‘karst’’ refers to both a surface and a subsurface landscape, and by extension to a hydrological system shaped by the flow and dissolution conditions occurring mainly in carbonate rock (Mangin 1994; Bakalowicz 2004). Carbonate rock is soluble in the presence of acid dissolved in water, most commonly resulting from CO2 solution. The process that combines solution of carbonate rock and flow in openings is named ‘‘karstification’’ (Ford and Williams 2007). The resulting karst aquifers are characterized by marked heterogeneity related to the presence of open fractures and drainage systems, underground conduits organized in a hierarchical system (Fig. 4).
The second aquifer, (2) the Tertiary Clayey and sandy formation (Segui and Beglia Formations: in the totality of Gafsa mining basin) (Hamed et al. 2010a, b; Hamed 2011). The Beglia Formation (Mio–Pliocene) is constituted by coarse sand with thin levels of red clay and quartz. The thickness of sand ranges from 40 to 100 m. At the Moulares region in the south, where the Beglia outcrops, it is only 50–400 m thick (Hamed et al. 2008). The Beglia Formation is covered by a thick clayey formation (Segui) whose thickness varies between 40 and 120 m. Most recharge of the Mio–Plio–Quaternary (MPQ) aquifers occurs at outcrops around the margins of the Grand Erg Oriental (southwestern of the study area, in Algeria), although there is some minor recharge by direct infiltration from the Grand Erg Oriental itself. Discharge from the aquifer occurs by vertical percolation at the Chott, the Sebkha or/and the Garâat surfaces, upwelling at natural springs and spring mounds and upwelling from water wells. Most of this discharge is concentrated in Southern Tunisia around Chott Djerid in the South, where natural upwelling occurs at a relatively constant rate throughout the year (Coque 1962).
The principal areas of recharge are in the South Atlas Mountains of Algeria, in Dahar uplands (Tunisia) and Tunisian Atlas. The discharge area is in Chotts area (Fig. 1). Quaternary features consist primarily of alluvial fans, glacis and terraces. Instead of terraces, most of the Quaternary stratigraphic units, wind-eroded outcrops that have been interpreted as eolian, sebkha and lacustrine deposits (Swezey 1996). Clayey Segui Formation is considered as the footwall of Quaternary sequence (Mamou 1989) and has been reached by several wells. Quaternary formation is much thicker at 100 m at the El Guettar region and formed by alternation of sand, clayey sand and sandy clay with thin quartz gravel levels. Gypsum is generally associated as a fine to medium grain to clayey sand horizons.
The Plio–Quaternary (PQ) deposit contains a shallow aquifer (alluvial and conglomerate deposits intercalated with varicolored clay and calcareous sandstone), which exploited especially as irrigation complement in Majni, Lorteus, Nechiou and El Guettar oases. This aquifer is essentially recharged by the excess of irrigation water coming from CI and CT deep aquifers, in oases area. Out of these cultivated zones, the PQ shallow aquifer is recharged during the rare rainfall events. The transmissivity of the PQ shallow aquifer ranges from 2.8 × 10−2 to 27 × 10−2 m2 s−1, and the permeability varies between 7.3 × 10−4 and 15.2 × 10−4 m2 s−1 in Segui Formation (DGRE 2010). These ranges depend essentially on the depth of aquifers, on the heterogeneity of their lithology and on the tectonic of the region. Concerning the variation of the electrical resistivity that varies from one area to another and as a function of depth. For the shallow aquifer the values of the resistivity varies from 0 to 100 Ωm, from 5 to 35 Ωm for the intermediate aquifer and from 5 to 10 Ωm for the deep aquifer (Gouasmia 2008).
The groundwater flow of the CT aquifers (Figs. 5a, b, 6 and 7) converges from all directions to the endorheic depression of Chott Guettar and which constitutes the unique natural discharge area of this reservoir (Farhat and Moumni 1989; Hamed 2011). These flow directions are separated by a groundwater divide “M’Dilla ridge” located in the North of M’Dilla region in the West of El Guettar area. In fact, the piezometric map of the 1980s (Mamou 1990) showed two opposites major E–W (to the discharge depression of El Aguila) and W–E (to the discharge depression of Chott El Guettar) flow directions, which diverge from the ‘‘M’Dilla ridge’’ (Fig. 6). These opposite flow directions indicated that the CT outcrops on both sides of the ‘‘M’Dilla ridge’’ constituted the main recharge area of the CT aquifer in the basin. However, the latest piezometric map, established within the present study at 2012 (Fig. 8), shows several changes in the CT groundwater flow patterns. The two flow directions diverging from the ‘‘M’Dilla ridge’’ have been completely disappeared and replaced by other major flows coming from the southeast and from the northeast towards the El Aguila depression. The groundwater flow direction of the shallow aquifer below that the deep aquifer (Figs. 7, 8). So it is clear the influence of the deep aquifer on the shallow aquifer due to overexploitation of groundwater mainly in the industrial sector of the CPG. These new flow directions highlight the presence of two recharge components in relation with the aquifer outcrops in the Atlas Tunisian (Gafsa and northern Chott ranges). On the other hand, the extinction of the flow directions coming from the ‘‘M’Dilla ridge’’ can be explained by the severe climatic condition that characterized the south of Tunisia during the last two decades. Another potentiometric change appears from the comparison of the two maps. It is the remarkable decline of the piezometric indicating the influence of the long-term over-pumping of the CT groundwater in the mining basin of phosphate. This over-pumping, which is in relation with the increasing number of the CT boreholes in response to the large extension of the agricultural areas, has contributed to several deleterious and irreversible consequences including the drying of all Foggara (Mkayel), springs and the water quality degradation, presumably due to the mixing with the overlying and/or the underling groundwaters (Mokadem et al. 2012). The CT groundwater in the El Guettar basin is recharge by surface water via wadies (drainage network) infiltration, Lalla Dam, canal system seepage and farm land irrigation water seepage and infiltrating meteoric water in the basin. It is discharged mainly via evapotranspiration and artificial abstraction.
Piezometric map of the shallow aquifer in El Guettar basin (DGRE 1993)
Piezometric map of the deep aquifer in El Guettar basin (Farhat and Mamou 1989)
Piezometric map of the shallow aquifer in El Guettar basin (DGRE 2012)
In general, secondary cementation and clays decrease the porosity. Furthermore, the fault systems diminish water movement in some compartments as a result of the fault displacement which induces a placing of clay beds in vertical contact with limestones and sands. The study aquifer indicates similar deposits able to ensure the possible communications between the various formations (Fig. 5a, b). Tectonically, the main folding in the Gafsa–El Guettar basin dates from the Plio–Pleistocene age, probably Villafranchian, and it is still active considering current seismic activities (Dlala and Hfaiedh 1993). These faults are part of a complex called the South Tunisian accident (Castany 1982). The effect of several tectonic phases on the Gafsa area seems to have controlled the production of a large sedimentary basin (Ahmadi et al. 2006; Gabtni et al. 2009; Hamed et al. 2010b). From a tectonic point of view, the El Guettar basin represents a transitional zone between the Tunisian Atlas Mont in the north and the Saharan platform in the south. The regional structural studies highlight the existence of E–W and NW–SE major faults, which contributed to the uplifting of the Gafsa anticlines, (Zargouni 1985; Ahmadi et al. 2006; Hamed et al. 2008). Other geophysical explorations mentioned the presence of N–S geophysical faults extending longitudinally in the region of Gafsa city (Zargouni 1985; Jallouli et al. 1996).
Groundwater sampling and analyses
Samples of groundwater from sites in the study area were collected during high-flow conditions in January–February 2012. A total of 49 groundwater samples were obtained from active water supply boreholes penetrating different lithological units in the El Guettar basin (Fig. 9). In situ parameters, such as pH, temperature, EC, TDS and alkalinity (HCO3) were measured in the field with portable devices (WW350) to ensure the exclusion of atmospheric contamination and to improve measurement stability. These fell into two distinct groups (shallow wells with depths <50 m, and deep wells with depths ranging from 80 to 220 m), and a surface water sample (water Dam of Lalla) from the El Guettar reservoir was also collected for an initial reconnaissance of geochemical parameters. Samples for major chemical analyses were filtered (0.45 mm filter paper) and collected in plastic bottles (1.5 l). The analytical precisions for all cations and anions are 6 %. The reaction error (RE %) was calculated using the formula:
Hydrogen (2H and 3H) and oxygen (18O) isotope analyses (Yermani 2000 and Hamed 2009) were performed in the laboratory of the International Atomic Energy Agency (IAEA) in Vienna, by employing, respectively, the standard CO2 equilibration (Epstein and Mayeda 1953) and the zinc reduction techniques (Coleman et al. 1982), followed by analysis on a mass spectrometer. Oxygen and hydrogen isotopes analyses were reported in ‘d’ notation relative to VSMOW, where δ = [(R S/R SMOW) − 1] × 1,000; R S represents either the 18O/16O or the 2H/1H ratio of the sample, and R SMOW is 18O/16O or the 2H/1H ratio of the SMOW. Typical precisions are ±0.1 and ±1.0 % for oxygen-18 and deuterium, respectively. Tritium (3H) analyses were performed in the laboratory of the IAEA by electrolytic enrichment and liquid scintillation counting method (Thatcher et al. 1977). Tritium contents were reported in TU, in which one TU equals one tritium atom per 1018 hydrogen atoms. Oxygen-18 and deuterium groundwater samples were collected in glass phials. These phials were rinsed using the groundwater sample and were later filled in the flow cell and sealed beneath the water surface using an airtight plastic lid. The stable isotope analysis was performed using a mass spectrometer.
Carbon-14 (14C) analyses were carried out in the Sfax School of Engineers (LRAE-ENIS-Sfax-Tunisia) by using liquid scintillation counting on C6H6 synthesised from BaCO3 stripped in the field from 150 l of water samples. Results of 13C analyses are reported in ‰ versus PDB (American Belemnitella from Pee Dee Belemnite, North California, USA) (Coplen 1996) standard and 14C abundances are expressed as percent modern carbon (pmC) (Stuiver and Polach 1977). The δ 13C was measured at the laboratory of IAEA, by converting the DIC to CO2 with 100 % phosphoric acid (H3PO4). Analytical uncertainties are in the range of ±0.3 for δ13C and between 1 and 1.5 pmc for 14C.
Results and discussion
In situ measurements and major elements geochemistry interpretation
The groundwaters have pH values ranging from 7.21 to 7.90 indicating a near neutral pH. These variations are the result of the combination effects of numerous factors, i.e. the depth to groundwater, the residence time in the flow system and/or the groundwater flow time from the recharge area. Partial pressure of carbon dioxide (pCO2) values, calculated with WATEQ4F program (Ball and Nordstrom 1991), range between 3.78 × 10−2 and 39.27 × 10−2 atm. These values are higher than the atmospheric pCO2 that is equal to 10−3.5 (Van der Weijden and Pacheco 2003), suggesting that groundwaters have gained CO2 from root respiration and the decay of soil organic matter. Subsequently, the increase in pCO2 causes a drop in pH as indicated in Fig. 5 (Rightmire 1978; Adams et al. 2001). The lowest pCO2 values are detected up gradient, while higher values characterize the down gradient parts of the basin. This is likely related to relatively longer aquifer residence times, which would allow for more extensive reactions with aquifer minerals and biologically mediated reactions that produce CO2. The groundwater temperatures are relatively heterogeneous; they range between 14 and 21 °C, reflecting the conjunction of several factors, i.e., the depth to groundwater, the residence time in the flow system and/or the groundwater flow time from the recharge area and the rate of return flow of irrigation water.
The EC and the TDS range from 2.20 to 9.62 mS/cm and from 1.75 to 14.4 g/l, respectively. The groundwater temperatures are relatively heterogeneous; they range between 16 and 23 °C. The values of these parameters are low in the northern part of the study area and there is a gradual increase along the groundwater flow path due to the long residence time of the water in the rock, the ambient temperature and the anthropogenic activities. The behaviors of ion concentrations, in general, are similar with the EC and the TDS values of the groundwater. Higher values of these parameters characterize wells located in the southern parts of the basin (discharge zones), especially in the Chott El Guettar, suggesting both the insufficiency of recharge in this part and the relatively long-term water–rock interaction and the evaporation effect of Lalla dam; also, the anthropogenic activities (agricultural and industrial). Generally, TDS increases from the mountainous regions towards the discharge area (central part) (Fig. 10). The spatial distribution conforms partially with the main groundwater flow direction, indicating that the groundwater salinity is in some way controlled by the residence time in the aquifer. Relatively low TDS values characterize wells located near the drainage network and reveal the dilution of groundwater by the water from linear recharge. However, high values of TDS which are relatively related to the evolution of the mineralization in the direction of the groundwater flow, appear near the Chott El Guettar, which can be related to evaporates rich-minerals cropping out in this region. Furthermore, high salinity was observed also in the oases of the basin, especially in Lorteus and El Guettar oases, which is marked by extensive land use development activities.
Salinity map of the study area (Southwestern Tunisia) (Mokadem 2012)
These values indicate the brackish nature of these groundwaters, which is possibly attributed to the nature of the deposits, which are rich in soluble minerals in addition to agricultural return flow that causes leaching of soluble salts precipitated in the soil zone under the high evaporation rate. Mixing with the underlying dense salt water is another source of salinization of groundwaters from the CT aquifer, mostly caused by over-pumping (Fig. 11).
Moreover, high values bicarbonate and calcium (Ca–HCO3 water type) reflect the extent of water–rock interactions where dissolution of carbonate rocks is an active process in such karstified systems (periphery of the study area). But the Na and Cl concentrations are high in the central part of the study area (Chott El Guettar depression), suggesting both the insufficiency of recharge in these parts and the relatively long-term water–rock interaction (Fig. 11).
Hydrochemical facies
Based on the contents of major cations (Ca2+, Mg2+, Na+ and K+) and anions (Cl−, HCO3 −, NO3 − and SO =4 ), three hydrochemical facies could be identified including facies 1: Ca–Mg–HCO3 (predominant water type in the carbonate-rock aquifers because calcite and dolomite are abundant in this aquifer), facies 2: Ca–Mg–SO4 and facies 3: Na–Cl–NO3. Water types were defined by use of the trilinear plotting technique (Piper 1944); the trilinear diagrams are shown in Fig. 12. The geochemical and hydrologic processes responsible for the various water types in the study area are discussed in the following sections:
Calcium–magnesium–bicarbonate “water type 1”
Ca–Mg–HCO3 water, the predominant type in the carbonate-rock aquifers (Cenomanian–Turonian carbonates “Zebbag Formation”), is generally produced by dissolution of the carbonate minerals (calcite and dolomite) and/or amphibole. The reaction of these minerals with water and carbon dioxide can be written as follows:
Bicarbonate was the dominant anion and represented greater than 65 percent of the anion composition of the samples. Calcium and magnesium were the dominant cations, relative to sodium and potassium, and represented >70 % of the cation composition of these samples. The major ion composition of the water in these samples can be classified as Ca–Mg–HCO3 water.
Calcium–magnesium–sulfate “water type 2”
The predominant water type in the center part of the study area is Ca–Mg–SO4. This water is characterized by high concentrations of dissolved solids, calcium, magnesium, and sulfate. The predominance of sulfate over bicarbonate and the lack of agreement of the Ca–Mg–SO4 water with the simple dolomite dissolution model indicate that other processes are controlling the chemistry of this water type. Two reactions can produce this type of water: (1) dedolomitization, which involves dissolution reactions with carbonate minerals and gypsum, and (2) sulfuric acid neutralization, which involves dissolution of carbonate minerals with sulfuric acid generated by the oxidation of pyrite. The results of investigations of other regional carbonate-rock aquifers show that dedolomitization may be a controlling process (Pearson and Rightmire 1980). Groundwater that moves through the aquifer initially dissolves calcite, dolomite and gypsum (or anhydrite). Once calcite and dolomite reach saturation, gypsum is still undersaturated and continues to dissolve, adding calcium and sulfate to the water. Consequently, calcite becomes oversaturated and, as it precipitates, the bicarbonate concentration decreases. The decrease in bicarbonate concentration causes the water to be undersaturated with respect to dolomite and promotes dissolution of dolomite. Dissolution of dolomite causes increases in the concentration of magnesium in the water. The overall reaction can be written as:
Dedolomitization is not the only process. An alternative model involves dissolution of dolomite by sulfuric acid (H2SO4) produced by the oxidation of pyrite (FeS2) that integrate in the phosphate pellets:
The sulfuric acid reacts with dolomite:
Calcium and magnesium were the dominant cations, relative to sodium and potassium, and represented >70 % of the cation composition. The sulfate is the dominant anion for water samples of this type and represents 49–88 % of the major anion composition. The major ion composition of the samples can be classified as Ca–Mg–SO4 water.
Sodium–chloride–nitrate “water type 3”
Na–Cl–NO3 type water predominates in the discharge zones (Chott El Guettar) reflects the dominance of sodium and chloride; and the influence of land use activities on groundwater mineralization (agricultural regions, where flood irrigation is applied). However, this water type is rare in other parts of the carbonate-rock aquifer. Some groundwater that is enriched in sodium or chloride or both is present in this area. Research CI and CT aquifers in the mining Gafsa basin (Hamed 2009) indicates two potential sources of sodium, chloride and nitrate to these aquifers. In the discharge zones, shallow groundwater (MPQ) in highly permeable outwash valleys contains elevated concentrations of this facies that are related to human activities. The other possible source of these groundwaters includes the dissolution of halite and the influence of the excessive use of Ca(NO3)2 fertilizers.
Nitrate content
Intensive use of land resources in arid and semi-arid regions exerts serious pressures on groundwater resources. In the El Guettar unconfined aquifer (MPQ), about 40 % of samples have nitrate concentration that exceeds the drinking water standards of 50 mg/l (WHO 2006). The average value of nitrate in the whole groundwater samples is 75 mg/l. These high nitrate concentrations provide evidence for the significance of the return flow waters contribution in the recharge of the unconfined aquifer. Indeed, ammonium nitrate, liquid fertilizer and other commercial complex nitrogen fertilizers are used in large scale in the agricultural regions, where flood irrigation is applied in Gafsa basin (Hamed et al. 2013c). The highest values (up to 100 mg/l) are detected in the central part around the Chott, particularly in the Majni, the El Guettar oasis and the Nichiou agricultural regions (anthropogenic pollution), where flood irrigation is intensively applied (Fig. 13). Hence, the NO3 − rich groundwater is derived from the return flow of irrigation water that is modified by the excessive addition of fertilizers (Ca(NO3)2) and pesticides that are utilized in large scale in the cultivated fields. Moreover, in the oasis region of El Guettar, where a large fraction of the irrigation water comes from urban areas, the return flow is probably triggered by household wastewater (septic tank system), which constitutes a potential source (25 %) of nitrate contamination (Fig. 14).
Different sources of nitrates in El Guettar basin (DGRE 2012)
Agricultural practices are concentrated in the north and in the western parts (Majni, Lortess, Nechiou and El Guettar oasis) of the basin (around 45 % of the study area). Excessive use of chemical fertilizers and effluent seepage from some animal farms within the area were the main sources for nitrate contamination, while inefficient/inadequate irrigation practices and the resulting irrigation return flow were the main cause for salinization. The application of liquid fertilizer N-32 % (N32) through fertilization is a common practice during the growing season (May–June–July: second fertilization phase) in the area, which contains 16 % urea, 8 % ammonium, and 8 % nitrate. Several studies have reported that a reduction in the proportion of NO3 − in applied fertilizers can lead to a considerable decrease in the potential for NO3 − leaching (Baali et al. 2007). The annual chemical N fertilizer input is estimated as high as 300–500 kg h m−2. In El Guettar province, the uses of N fertilizers are estimated as high as 300–700 kg N h m−2 year−1, and 100–700 kg N h m−2 year−2 for greenhouse vegetables (information for 2000–2011) depending on the crop and on each farmer’s practices.
Nitrate concentration in groundwater depends on the hydrogeological conditions and the availability of electrons donors for denitrification (Postma et al. 1991). Nitrate movement depends also on soil properties including texture and permeability. In clay soils, water movement is slower and pores lack oxygen which favors denitrification (Strong and Fillery 2002). Although water percolates slowly in clayey soils, cracks and pore abundance of soil horizons change this rule (Oostindie and Bronswijk 1995). Preferential flow along soil cracks and macropores can lead to rapid transport of water and dissolved pollutants to the groundwater. Soil thickness and distance between the root zone and groundwater also determine the vulnerability of an aquifer to pollution. In the confined CT aquifer, the decrease in nitrate with depth can be due to the process of the reduction of nitrate by oxidation of organic matter within the sediments. A highly permeable, alluvial aquifer provides favorable conditions for the vertical transport of oxygen to deeper parts of the aquifer. Moreover, the soils of the study area are mainly fluvial-soils and have predominantly silt loam texture. Consequently, the alluvial aquifer of the El Guettar plain is aerobic throughout its depth, with a mean dissolved oxygen (DO) concentration in groundwater of 6.69 mg/l. Under these conditions, urea and ammonium are readily transformed into nitrate. The nitrification rate of ammonium is very fast under aerobic conditions (15–20 days at 20 °C; Kpomblekou and Killorn 1996). Therefore, infiltration of nitrogen-rich waters into the aerobic groundwater system seems to be the major cause of high nitrate concentrations in groundwater. The elevation of NO3 −–N in the soil is an important reason for the increase in concentration in the groundwater (Kraft et al. 1999). Therefore, more attention should be paid to nitrate involving the soils under rocky desertification in karst regions with poor stability and disturbance resistance. The industry is another potential source of nitrogen to groundwater (atmospheric pollution). Nitrogen compounds are used extensively in industrial processes and also integrated nitrogen molecule into the organic matter of the phosphate rock and the Gafsa mining basin is the typical example in the southern Tunisia and in the word (Fig. 14). Currently, the merchant rock output rises to more that 8 million metric tons/annum, placing Tunisia fifth in the word for phosphate production (CPG-GCT 2010). This production is ensured by the Phosphate Company of Gafsa-CPG, ‘‘la Compagnie des Phosphates de Gafsa” (Figs. 14, 15).
Given the spatial variation in nitrate concentrations and the fact that the nitrate input is not constant with time, it is difficult to determine the exact extent to which nitrate reduction has taken place within the aquifer with the current level of data. The groundwater flow may gradually retard the nitrate in some regions and enhance the accumulation of nitrate in other regions through the translocation of nitrate in the groundwater. However, this phenomenon was unlikely in our study areas due to the gentle hydraulic potential. For this specific region, land use change and irrigation water resource may strongly affect nitrate concentrations in the groundwater, but may not necessarily be the sole factor affecting the variability in groundwater nitrate. Moreover, in areas using sewage irrigation, all other non-irrigation ditches were reserved for wastewater perennially or seasonally, which may pose additional leaching risks.
Concerning the “Fe” concentration, the high values (≈0.01 mg/l) are observed in the NE part of the basin. This is strongly linked to the existence of the mine of iron (Fe) located at J. Chemsi (Fig. 1) and contaminates the water table of the shallow aquifer by the intermediary of Wadi as Sad. In the rest of the basin the values of “Fe” are low (<0.06 mg/l) (Table 1).
El Guettar basin is considered the first basin, which suffers from several types of pollution (air, soil and water). In this perspective there are the proliferations of several types of diseases such as cancer, cardiovascular diseases, respiratory diseases (Mokadem et al. 2013). After the revolution of January 14, 2011, the Tunisian state has found several areas of releases that originate outside Tunisia following exchanges with other countries. The typical example is the watershed of El Hamma–Gabes (southeastern Tunisia) where the radioactive activity is important (IIR 2012); but this finding was not confirmed by the Tunisian experts. So the probability of such discharges in the El Guettar basin exists but remains to be confirmed by detailed studies.
Isotope geochemistry
The environment isotopes of oxygen δ18O–H2O and hydrogen δ2H–H2O are excellent tracers for determining the origin of groundwater and widely used in studying the natural water circulation and groundwater movement. It can offer an evaluation of physical processes that affect water masses, such as evaporation and mixing (Geyh 2000; Ali 2004). Only two isotopic studies (Yermani 2000; Hamed 2009) were made about the stable isotopes in the groundwater in relation to the surface water as well as the modern rainfall in the Gafsa basin (Table 2). Firstly these studies indicate that the groundwater recharge resources mainly source from precipitation. Secondly the groundwater is strongly depleted in both 18O and 2H relative to modern rainfall by up to 3.16 ‰ in 18O. Thirdly the deep groundwater is notably depleted in heavy isotopes in comparison with shallow groundwater, suggestive of cooler (T ≈ 10 °C) and humid (P ≈ 1,500 mm/year) recharge conditions than at present day (Hamed et al. 2013a).
The stable isotope composition of these aquifers ranges from −5.74 to −8.11 ‰ for the δ18O and from −55.1 to −33.7 ‰ for δ2H (Table 2). This isotopically difference attests to the heterogeneity of recharge modes and probably longer residence time in the aquifer (Gonfiantini et al. 1974; Fontes et al. 1983; Zouari et al. 2003).
Radiocarbon activities “DIC” were measured on 20 selected samples to establish the groundwater residence time (Table 2) using the Pearson model (Fontes et al. 1983) in which the measured δ13C values have enabled corrections to be made, using a value of 100 % modern carbon together with a soil CO2 value of −26 ‰ and carbon of 0.8 ‰. The radiocarbon values, expressed as percent modern carbon, (pmC) exhibit a range from 5 to 65.2. The deep groundwater samples have values of <10 pmC, indicating a predominance of ancient recharge. The deep groundwater is old, approximately 40 kyear. In contrast, the radio-isotopes data of shallow groundwater show a clear evolution along the groundwater flow path. From the beginning of the groundwater flow path to ≈20 km the shallow groundwater have values more than 50 pmC with ages <3.5 kyear. The shallow groundwater is younger but generally older than 0.5 kyear, and likely represents a mixture of modern waters limited palao-recharge.
Tritium (3H-H2O: radioactive isotope of hydrogen) contents in groundwaters from the study area vary widely from 0.65 to 3.75 TU (Table 2). These values are interpreted with respect to 3H contents in precipitation from the Tunisian GNIP-stations of Sfax and Tunis–Carthage for the period 1967–2000 (Hamed et al. 2013b). These contents that show a substantial increase after the onset of atmospheric nuclear weapons testing, during the early 1960s, decreasing gradually during the 1970s and 1980s to stabilize between 4 and 7 TU during the 1990s (IAEA/WMO 1999). Groundwaters with 3H concentration less than the detection limit (2 TU) were either derived from pre-nuclear recharge or contain significant amounts of pre-1952 waters. They include the ancient groundwaters from the deep aquifer and the mixed groundwaters from the intermediate and shallow aquifers. Groundwaters with 3H between 2 and 4 TU were mainly recharged during the 1990s. However, the tritium concentrations show the contribution of recently recharged water coming from direct rain infiltration through sand dunes. Downstream, near the Gafsa sill, geothermal (≈30 °C), deep and old groundwaters coming from the northern outcrops mix with some recent water recharged through the local Cretaceous outcrops (fractured and karstified of carbonate rock) and/or from the alluvial aquifer, which is recharge by the drainage network (El Kebir, Sidi Aïch, Bayeich and El Maleh) of the North Gafsa basin and also by the recent and evaporate water Dam of Lalla region. This mixing is marked by measurable tritium concentration, by a lowering of carbon-14 activity and by a decrease of the groundwater TDS (dilution effect).
Conclusion
Saharan hot strong winds and sands, serious soil salinization and water insufficiency are the natural causes for the disappearance of some ancient oases. The geochemical evolution of groundwater facies from the recharge area towards the basin outlet is marked by a gradual increase of sodium, chloride and sulfate as a consequence of evaporitic rocks leaching, over-exploitation, mixing and of evaporation processes. These large variations in chemical compositions may imply discontinuities in the hydrogeology (change of lithology, faults and variations in the flow patterns). In addition to these natural processes, return flow of irrigation water, which is modified by the excessive addition of fertilizers and pesticides, plays also a significant role in the salinization of groundwater.
The stable isotopes data indicate that the El Guettar unconfined aquifer is mainly recharged by depleted rainfall water, particularly in the foot of the surrounding mountains, with a significant contribution of isotopically enriched agricultural return flow waters. Tritium and 14C data reveal modern groundwater, likely recharged during the last 50 years. The spatial distribution of these radiogenic tracers, which show a decrease in the direction of flow, suggests that the hydrodynamic system acts as piston flow model (Hamed et al. 2010b).
Abbreviations
- 1 Ky:
-
1,000 years
- CPG:
-
Phosphate Company of Gafsa
- PDB:
-
Pee Dee Belemnite
- TU:
-
Tritium unit
- VSMOW:
-
Vienna Standard Mean Oceanic Water
- CI:
-
Continental intercalaire
- CT:
-
Complex terminal
- TDS:
-
Total dissolved solid
- DIC:
-
Dissolved inorganic carbon
References
Abaurrea J, Asín J, Cebrián AC, Centelles A (2007) Modeling and forecasting extreme hot events in the central Ebro valley, a Continental-Mediterranean area. Glob Planet Change 57:43–58
Adams S, Tredoux G, Harris C, Titus R, Pietersen K (2001) Hydrochemical characteristics of aquifers near Sutherland in the Western Karoo, South Africa. J Hydrol 241:91–103
Ahmadi R, Ouali J, Mercier E, Mansy JL (2006) The geomorphologic responses to hinge migration in the fault-related folds in the southern Tunisian Atlas. J Struct Geol 28:721–728
Ali MS (2004) Use of chloride-mass balance and environmental isotopes for evaluation of groundwater recharge in the alluvial aquifer, Wadi Tharad, west Saudi Arabia. Environ Geol 46:741–749
Alpert P, Osetinsky I, Ziv B, Shafir H (2004) Semi-objective classification for daily synoptic systems: application to the eastern Mediterranean climate change. Int J Climatol 24:1001–1011
Alpert P, Krichak SO, Shafir H, Haim D, Osetinsky I (2008) Climatic trends to extremes employing regional modeling and statistical interpretation over the E Mediterranean. Glob Planet Change 63:163–170
Baali F, Rouabhia A, Kherici N, Djabri L, Bouchaou L, Hani A (2007) Underground water quality and contamination risk. The case of the basin of Chéria. N.E. Algeria. Estud Geol 63(1):127–133. ISSN: 0367–0449
Bakalowicz M (2004) The epikarst, the skin of karst. In: Jones WK, Culver DC, Herman JS (eds) The Epikarst Conference. Karst Water Institute Special Publication no. 9. The Karst Water Institute, Shepherdstown, WVA, pp 16–22
Ball JW, Nordstrom DK (1991) WATEQ4F—user’s manual with revised thermodynamic database and test cases for calculating speciation of major, trace and redox elements in natural waters. United States Geological Survey, Open-File Report, pp 90–129
Beniston M (2005) Warm winter spells in the Swiss Alps: strong heat waves in a cold season? A study focusing on climate observations at the Saentis high mountain site. Geophys Res Lett 32:L01812. doi:10.1029/2004GL021478
Beniston M, Jungo P (2002) Shifts in the distribution of pressure, temperature and moisture and changes in the typical weather patterns in the Alpine region in response to the behavior of the North Atlantic Oscillation. Theor Appl Climatol 71:29–42
Bertin RI (2008) Plant phenology and distribution in relation to recent climate change. J Torrey Bot Soc 135:126–146
Bethoux JP, Gentili B, Tailliez D (1998) Warming and freshwater budget change in the Mediterranean since the 1940s, their possible relation to the greenhouse effect. Geophys Res Lett 25:1023–1026
Böhm R, Auer I, Brunetti M, Maugeri M, Nanni T, Schöner W (2001) Regional temperature variability in the European Alps: 1760–1998 from homogenized instrumental time series. Int J Climatol 21(14):1779–1801
Bousnina L (1997) Le climat de Sfax, Altaïr-Éditions
Brohan P, Kennedy JJ, Harris I, Tett SFB, Jones PD (2006) Uncertainty estimates in regional and global observed temperature changes: a new data set from 1850. J Geophys Res 111:D12106. doi:10.1029/2005JD006548
Brunetti M, Buffoni L, Maugeri M, Nanni T (2000) Trends in minimum and maximum daily temperatures in Italy from 1865 to 1996. Theor Appl Climatol 66:9–60
Brunetti M, Buffoni L, Mangianti F, Maugeri M, Nanni T (2004) Temperature, precipitation and extreme events during the last century in Italy. Glob Planet Change 40:141–149
Camuffo D, Bertolin C, Barriendos M, Dominguez-Castro F, Cocheo C, Enzi S, Sghedoni M, Della Valle A, Garnier E, Alcoforado MJ, Xoplaki E, Luterbacher J, Diodato N, Maugeri M, Nunes MF, Rodriguez R (2010) 500-year temperature reconstruction in the Mediterranean Basin by means of documentary data and instrumental observations. Clim Change 10:1169–1199
Castany G (1982) Bassin sédimentaire du Sahara septentrional (Algérie–Tunisie)-Aquifères du Continental Intercalaire et du Complexe Terminal. Bulletin Bureau Recherches Géologiques Minières (BRGM), Série 2, vol 3, pp 127–147
Casty C, Wanner H, Luterbacher J, Esper J, Bohn R (2005) Temperature and precipitation variability in the European Alps since 1500. Int J Climatol 25:1855–1880
Celle H, Daniel M, Mudry J, Blavoux B (2000) Signal pluie et traçage par les isotopes stables en Méditerranée Occidentale. Exemple de la région avignonnaise (Sud-Est de la France). C R Acad Sci 333:647–650
Celle-Jeanton H, Travi Y, Blavoux B (2001a) Isotopic typology of the precipitation in the Western Mediterranean region at three different time scales. Geophys Res Lett 28:1215–1218
Celle-Jeanton H, Zouari K, Travi Y, Daoud A (2001b) Caractérisation isotopique des pluies en Tunisie. Essai de typologie dans la région de Sfax. C R Acad Sci 333:625–631
Coleman ML, Shepherd TJ, Durham JJ, Rouse JE, Moore GR (1982) Reduction of water with zinc for hydrogen isotope analysis. Anal Chem 54:993–995
Coplen TB (1996) New guidelines for reporting stable hydrogen, carbon, and oxygen isotope-ratio data. Geochim Cosmochim Acta 60:3359–3360
Coque R (1962) La Tunisie présaharienne: étude géomorphologique. Armond Colin, Paris
CPG-GCT (2010) Tunisian phosphate industry. http://www.gct.com.tn/francais/secteur.htm
DGRE (Direction Générale des Ressources en Eau) (1993) Annuaire de l’exploitation des nappes de la Tunisie. DGRE, Tunis, Tunisie
DGRE (Direction Générale des Ressources en Eau) (2010) Annuaire de l’exploitation des nappes de la Tunisie. DGRE, Tunis, Tunisie
DGRE (Direction Générale des Ressources en Eau) (2012) Annuaire de l’exploitation des nappes de la Tunisie. DGRE, Tunis, Tunisie
Dlala M, Hfaiedh M (1993) Le séisme du 7 Novembre à Métlaoui (Tunisie méridionale): une tectonique active en compression. C R Acad Sci Paris 317(II):1297–1307
Domroes M, El-Tantawi A (2005) Recent temporal and spatial temperature changes in Egypt. Int J Climatol 25:51–63
El Kenawy AM, López-Moreno JI, Vicente-Serrano SM, Mekld MS (2009) Temperature trends in Libya over the second half of the 20th century. Theor Appl Climatol 98:1–8
Elmejdoub N, Jedoui Y (2009) Pleistocene raised marine deposits of the Cap Bon peninsula (N–E Tunisia): records of sea-level highstands, climatic changes and coastal uplift. Geomorphology 112:179–189
Epstein S, Mayeda TK (1953) Variations of 18O content of waters from natural sources. Geochim Cosmochim Acta 4:213–224
Farhat H, Moumni L (1989) Etude hydrogéologique de la nappe de Gafsa Nord. DGRE, Tunis
Folland CK, Karl T, Salinger M (2002) Observed climate variability and change. Weather 57:269–278
Fontes JC, Coque R, Dever L, Filly A, Mamou A (1983) Paléohydrologie isotopique de l’wadi el Akarit (Sud Tunsien) au Pléistocène Supérieur et à l’Holocène [Isotopic Paleohydrology of Wadi el Akarit (Southern Tunsien) during the Late Pleistocene and Holocene]. Palaeolgeogr Palaeoclimatol Palaeoecol 43:41–61
Ford DC, Williams PW (2007) Karst hydrogeology and geomorphology. Wiley, London
Gabtni H, Jallouli C, Mickus KL, Zouari H, Turki MM (2009) Deep structure and crustal configuration of the Jeffara basin (Southern Tunisia) based on regional gravity, seismic reflection and borehole data: how to explain a gravity maximum within a large sedimentary basin? J Geodyn 47:142–152
Geyh MA (2000) An overview of 14C analysis in the study of groundwater. Radiocarbon 42(1):99–114
Gonfiantini R, Conrad G, Fontes JC, Sauzy G, Payne BR (1974) Etude isotopique de la nappe du Continental Intercalaire et ses relations avec les autres nappes du Sahara Septentrional [Isotopic study of the Continental intercalaire aquifer and its relations with the other aquifers of the Septentrional Sahara]. In: Isotope techniques in groundwater hydrology, vol I. IAEASM-182/25, IAEA, Vienna, pp 227–241
Gouasmia M (2008) Etude géophysique des potentialités hydrauliques au SW de la région de Gafsa. Ph.D. Thesis, University of Tunis II, Tunis, p 200
Guendouz A, Moulla AS, Remini B, Michelot JL (2003) Hydrochemical and isotopic behaviour of a Saharan phreatic aquifer suffering severe natural and anthropic constraints (case of Oued-Souf region, Algeria). J Hydrol 14:955–968
Hamed Y (2009) Caractérisation hydrogéologique, hydrochimique et isotopique du système aquifére de Moularés-Tamerza. Ph.D. thesis, University of Sfax, pp 280
Hamed Y (2011) The hydrogeochemical characterization of groundwater in Gafsa–Sidi Boubaker region (Southwestern Tunisia). Arab J Geosci. doi:10.1007/s12517-011-0393-5
Hamed Y, Dhahri F (2013) Hydro-geochemical and isotopic composition of groundwater and meteoric water, with emphasis on sources of salinity, in the aquifer system in Northwestern Tunisia. J Afr Earth Sci. doi:10.1016/j.jafrearsci.2013.02.004
Hamed Y, Dassi L, Ahmadi R, Ben Dhia H (2008) Geochemical and isotopic study of the multilayer aquifer system in the Moulares–Redayef basin, southern Tunisia. J Hydrol Sci 53(5):1241–1252
Hamed Y, Zairi M, Ali W, Ben Dhia H (2010a) Estimation of residence times and recharge area of groundwater in the Moulares mining basin by using carbon and oxygen isotopes (South Western Tunisia). J Environ Prot 1:466–474. doi:10.4236/jep.2010.14054
Hamed Y, Dassi L, Ahmadi R, Gouasmia M (2010b) La guerre de l’ « or bleu » dans les régions Méditerranéennes (Afrique du Nord)- la Tunisie exemple typique. In: 5th international conference on water resources in Mediterranean basin “Lille-France 26–28 May
Hamed Y, Ahmadi R, Hadji R, Mokadem N, Ben Dhia H, Ali W (2013a) Groundwater evolution of the Continental intercalaire aquifer of Southern Tunisia and a part of Southern Algeria: use of geochemical and isotopic indicators. Desalin Water Treat J. doi:10.1080/19443994.2013.806221
Hamed Y, Awad S, Ben Sâad A (2013b) Nitrate contamination in groundwater in the Sidi Aïch–Gafsa oases region Southern Tunisia. Environ Earth Sci. doi:10.1007/s12665-013-2445-5
Hamed Y, Hadj R, Mokadem N (2013c) The Continental intercalaire groundwater salinization in Southern Tunisia. International conference, 18–19 November Tebessa, Algeria
Hansen J, Sato M, Ruedy R, Lo K, Lea DW, Medina-Elizade M (2006) Global temperature change. PNAS 103:14288–14293
Hassanean HM, Abdel Basset H (2006) Variability of summer temperature over Egypt. Int J Climatol 26:1619–1634
IAEA/WMO (1999) Global network of isotopes in precipitation. The GNIP database, Station 7622500. http://isohis.iaea.org
IIR (2012) The existence of an area contaminated by waste nuclear in Gabes basin, Tunisia
Jacobeit J (2000) Rezente Klimaentwicklung im Mittelmeerraum. Petermanns Geogr Mitt 144:22–33
Jallouli C, Inoubli MH, Albouy YY (1996) Le corps igne de Nefza (Tunisie septentrionale): caractéristiques géophysiques et discussion du mécanisme de sa mise en place. Notes Serv Geol Tunis 62:109–123
José M, García-Ruiz J, Ignacio LM, Sergio M, Vicente S, Teodoro LM, Santiago B (2011) Mediterranean water resources in a global change scenario. Earth Sci Rev 105:121–139
Kamel S, Hamed Y, Chkir N, Zouari K (2008) The hydrogeochemical characterization of ground waters in Tunisian Chott’s region. Environ Geol 54:843–854
Kostopoulou E, Jones PD (2005) Assessment of climate extremes in the Eastern Mediterranean. Meteorol Atmos Phys 89:69–85
Kpomblekou AK, Killorn R (1996) Nitrification of ammonium nitrogen in soils treated with XDE-474. Soil Sci Soc Am J 60:1482–1489
Kraft GJ, Sities W, Mechenich DJ (1999) Impacts of irrigated vegetable agriculture on a humid north-central US sand plain aquifer. Ground Water 37:572–580
Mamou A (1989) Caractéristiques, évaluation et gestion des ressources en eau du sud tunisien. Thèse 3ème cycle, Univ. de Paris-Sud, Orsay, France
Mamou A (1990) Caractéristiques et évaluation des ressources en eau du sud tunisien. Thèse Doctorat Univ Paris-Sud, Orsay, p 320
Mamou A, Kassah A (2002) Eau et développement dans le sud tunisien. Cahier du CERES, Série Géographique no. 23, Tunis 268 p
Mangin A (1994) Karst hydrogeology. In: Stanford J, Gibert J, Danielopol D (eds) Groundwater ecology. Academic Press, New York, pp 43–67
Mokadem N (2012) Hydrodynamic and mineralization of deep groundwater in El Guettar–Gafsa basin “SW Tunisia”. Master-Faculty of Sciences of Bizerte-Tunisia, 145 p
Mokadem N, Hamed Y, Ben Sâad A, Gargouri I (2012) Atmospheric pollution in North Africa (ecosystems–atmosphere interactions): a case study in the mining basin of El Guettar–M’Dilla (Southwestern Tunisia). Arab J Geosci. doi:10.1007/s12517-013-0852-2
Mokadem N, Inoubli A, Redhaounia B, Hamed Y, Bouri S (2013) Geochemical approach to the functioning of the Gafsa north deep aquifers (central Tunisia). In: International Conference, 18–19 November, Tebessa, Algeria
ONM (Office National de la Météorologie) (2010) Revue de l’Office National de la Météorologie 98(5):3–9
Oostindie K, Bronswijk JJB (1995) Consequences of preferential flow in cracking clay soils for contamination-risk of shallow aquifers. J Environ Manag 43(4):359–373
Palutikof JP, Holt T (2004) Climate change and the occurrence of extremes: some implications for the Mediterranean Basin. In: Marquina A (ed) Environmental challenges in the Mediterranean 2000–2050, Chapter 4. Kluwer Academic Publishers, Dordrecht, pp 61–73
Pearson FJJ, Rightmire CT (1980) Sulphur and oxygen isotopes in aqueous sulphur compounds. In: Fritz P, Fontes JCh (eds) Handbook of environmental isotope geochemistry, vol I. Elsevier, Amsterdam, pp 227–258
Piervitali E, Colacino M, Conte M (1998) Rainfall over the central-western mediterranean basin in the period 1951–1995. Part I: precipitation trends. Nuovo Cimento Soc Ital Fis C 21:331–344
Piper AM (1944) A graphic procedure in the geochemical interpretation of water-analyses. Trans Am Geophys Union 25:914–923
Postma D, Boesen C, Kristiansen H, Larsen F (1991) Nitrate reduction in an unconfined sandy aquifer: water chemistry, reduction processes, and geochemical modeling. Water Resour Res 27:2027–2045
Repapis CC, Philastras CM (2004) A note on the air temperature trends of the last 100 years as evidenced in the eastern Mediterranean time series. Theor Appl Climatol 39:93–97
Rightmire CT (1978) Seasonal variation in pCO2 and 13C content of soil atmosphere. Water Resour Res 14:691
Rindsberger M, Jaffe S, Rahamim S, Gat JR (1990) Patterns of the isotopic composition of precipitation in time and space: data from the Israeli storm water collection program. Tellus 42:263–271
Saaroni H, Ziv B, Edelson J, Alpert P (2003) Long-term variations in summer temperatures over the Eastern Mediterranean. Geophys Res Lett 30. doi:10.1029/2003GL017742
Santos J, Corte-Real J (2006) Temperature extremes in Europe and winter time large-scale atmospheric circulation: HadCM3 future scenarios. Clim Res 31:3–18
Sanz-Elorza M, Dana ED, González A, Sobrino E (2003) Changes in the high-mountain vegetation of the central Iberian Peninsula as a probable sign of global warming. Ann Bot 92:273–280
SASS (2002) Le système aquifère du Sahara septentrional: une conscience de bassin. OSS, Vol et Annexes. OSS/SASS, Tunis
Strong DT, Fillery IRP (2002) Denitrification response to nitrate concentrations in sandy soils. Soil Biol Biochem 34(7):945–954
Stuiver M, Polach H (1977) Reporting of 14C data. Radiocarbon 19:355–363
Swezey CS (1996) Structural controls on Quaternary depocentres within the Chotts Trough region of southern Tunisia. J Afr Earth Sci 22(3):335–347
Thatcher LL, Janzer VJ, Edward RW (1977) Methods for determination radioactive substances in water and fluvial sediments. In: Techniques of water resources investigations. US Geological Survey Washington, pp 79–81
Thornthwaite CW (1948) An approach toward a rational classification of climate. Geogr Rev 38:55–94
UNESCO (1972) Etude des ressources en eau du Sahara Septentrional. Projet ERESS. Nappe du Complexe Terminal Tech Rep 6:44
Van der Weijden CH, Pacheco FAL (2003) Hydrochemistry, weathering and weathering rates on Madeira Island
Vargas-Yáñez M, Jesús García M, Salat J, García-Martínez MC, Pascual J, Moya F (2008) Warming trends and decadal variability in the Western Mediterranean shelf. Glob Planet Change 63:177–184
WHO (2006) World Health Organization. Guidelines for drinking water quality, 3rd edn, incorporating first addendum (available IAEA/WMO (1999) Global network for isotopes in precipitation, the GNIP database, Release 3. http://www.iaea.org/programs/ri/gnip/gnipmain.htm)
Yermani M (2000) Contribution à l’étude du fonctionnement hydrodynamique du système aquifère de Gafsa Nord (Tunisie centrale). Thesis, Tunis II Univ., Tunisia
Zargouni F (1985) Tectonique de l’Atlas Méridional de Tunisie : évolution géométrique et cinématique des structures en zone de cisaillement. Rev Sci Terre 3:304
Ziv B, Saaroni H, Baharad A, Yekutieli D, Alpert P (2005) Indications for aggravation in summer heat conditions over the Mediterranean Basin. Geophys Res Lett 32:L12706. doi:10.1029/2005GL022796
Zouari K, Chkir N, Ouda B (2003) Palaeoclimatic variation in Meknassi basin (central Tunisia) during Holocene period using multidisciplinary approaches. IAEA, Vienna, pp 80–28
Acknowledgments
The authors are thankful to the Editor and the anonymous reviewers for their valuable suggestions and would like to thank the International Agency of Atomic Energy (IAEA) for financial support to carry out this work, to the Radio-Analysis Laboratory of the National School of Engineers of Sfax (LRAE) and to the Water, Energy and Environmental Laboratory (L3E)-ENIS-Sfax-Tunisia. Special thanks are directed to the staff members of Gafsa Water Resources Division/Agriculture Ministry and the staff members of the CPG.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mokadem, N., Hamed, Y., Hfaid, M. et al. Hydrogeochemical and isotope evidence of groundwater evolution in El Guettar Oasis area, Southwest Tunisia. Carbonates Evaporites 30, 417–437 (2015). https://doi.org/10.1007/s13146-015-0235-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13146-015-0235-8