Abstract
Solid pseudopapillary tumor (SPT) of the pancreas is a neoplasm with low malignant potential. It is often challenging to diagnose SPT due to its nonspecific clinical and radiological features, and [18F]FDOPA is effective in diagnosing SPT, particularly in differentiating SPT from benign conditions such as splenosis. A 55-year-old woman underwent distal pancreatectomy and splenectomy for histologically confirmed SPT. She was also initially diagnosed with splenosis. During follow-up, sizes of multiple nodular lesions were increased, raising the possibility of peritoneal seeding of SPT. For diagnosis, a spleen scan and SPECT/CT were performed using 99mTc-labeled damaged red blood cells, which showed no uptake in the peritoneal nodules. Subsequent [18F]FDOPA PET/CT revealed [18F]FDOPA-avidity of the nodules. The patient underwent tumor resection surgery, and the nodules were pathologically confirmed as SPT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Solid pseudopapillary tumor (SPT) of the pancreas is an uncommon, low-grade malignant neoplasm that predominantly affects young women. It has a low incidence of recurrence or distant metastasis [1,2,3,4]. SPT often remains asymptomatic, though some cases may manifest symptoms like abdominal pain or distension [1, 3, 4]. Diagnostic approaches encompass diverse methods including barium meal examinations, ultrasonography, computed tomography (CT), magnetic resonance imaging (MRI), angiography, endoscopic retrograde cholangiopancreatography (ERCP), and fine-needle aspiration cytology. However, there is no imaging modality of choice, so it should be determined according to the clinical situation [1, 4].
In general, complete surgical excision is regarded as the treatment of choice [4, 5]. Surgical procedures depend on the location of the tumor in the pancreas. Distal pancreatectomy with splenectomy is a feasible option for tumors located in the body or tail of the pancreas. It is important to be aware of the potential occurrence of splenic tissue implantation following the surgery [6]. Various imaging exams could be performed in this clinical context, although none is specific [7].
We report the usefulness of [18F]FDOPA PET/CT in a case of SPT with peritoneal seeding that mimicked splenosis.
Case Report
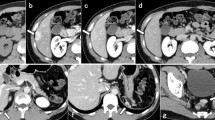
A 55-year-old woman underwent laparoscopic distal pancreatectomy with splenectomy for incidentally detected SPT. The patient was followed up by an annual CT scan, and 34 months later, six enhancing nodular lesions were identified near the splenectomy site. Initially, the lesions were reported as splenosis. On follow-up CT scans over 2 years, the nodules progressively enlarged and the long diameter of the largest lesion had expanded from 2.2 to 7.7 cm (Fig. 1).
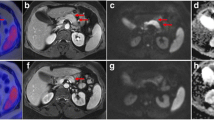
The nodules were suspected to be peritoneal seeding and a spleen scan with single photon emission computed tomography (SPECT)/CT was performed using 99mTc-labeled damaged RBC. This scan showed no uptake in the peritoneal nodules (Fig. 2). On [18F]FDOPA PET/CT, the peritoneal nodules showed high avidity (Fig. 3). The maximum standardized uptake value (SUV) of the lesion was 6.90, whereas the mean liver SUV was only 1.49. The multiple peritoneal nodules with high [18F]FDOPA-avidity were suggested as recurred tumors. The peritoneal nodules were surgically removed and pathologically confirmed as SPT (Fig. 4).
Discussion
[18F]FDOPA PET/CT has been used in various diseases, especially for the diagnosis of neuroendocrine tumors [8,9,10]. [18F]FDOPA, a radiolabeled analog of amino acid, is taken up into cells via the L-type amino acid transporter (LAT), decarboxylated by amino acid decarboxylase (AADC), and transported into secretory vesicles [11, 12]. Therefore, cells with neuroendocrine features or only high expression of LAT show a high uptake of [18F]FDOPA. Its uptake is also shown in other organs or tumors without a neuroendocrine feature. Considering its normal distribution, the basal ganglia, pancreas, adrenal glands, hepatobiliary, and urinary system show mild-to-moderate radioactivity, but the spleen shows faint or no radioactivity [13, 14].
Some cases of SPT have shown uptake of [18F]FDOPA in PET/CT scans, but the mechanisms behind this remain uncertain [15,16,17,18,19]. SPT originates from the exocrine tissue of the pancreas and, unlike neuroendocrine tumors (NET), it does not consistently display neuroendocrine features and has a different amino acid profile [18, 20]. This suggests that the mechanism of [18F]FDOPA uptake in SPT may differ from that in NET. One hypothesis for [18F]FDOPA uptake in SPT is elevated LAT-1 expression. Elevated LAT-1 expression is a common feature in multiple solid tumors and serves as a potential prognostic indicator [21]. It is associated with tumor growth and proliferation [22]. In the context of SPT, increased LAT-1 expression could explain the observed [18F]FDOPA uptake. However, research focusing on LAT-1 expression in SPT is limited. Further studies are needed to clarify the role of LAT-1 in SPT.
Splenosis is a benign condition in which splenic tissues appear at heterotopic sites, mainly in the abdominal cavity or organs. It is incidentally detected after trauma or splenic surgery [23]. Sometimes it is necessary to differentiate with tumors [24]. For differentiating, we use many imaging modalities, such as CT, MRI, or USG. But none of these imaging modalities is the gold standard [7, 25, 26]. Pathologic examination is the gold standard, but surgery or biopsy is invasive, so it is not preferred. Spleen scan using 99mTc-labeled damaged RBC was a noninvasive, highly sensitive, and specific exam. If the results are negative, it largely rules out the possibility of splenic tissue implantation, but additional diagnostic work-up is still required for differential diagnosis.
In situations where differentiation between splenic tissue and tumor is required during SPT evaluation, [18F]FDG PET/CT is not helpful because both conditions can be mildly [18F]FDG-avid. Also in our case, the baseline [18F]FDG PET/CT taken before surgery showed low FDG-avidity of the SPT. If a spleen scan was done, it would find splenic tissue but not other tumor lesions. Therefore, if progression is clinically suspected, further testing is needed. However, [18F]FDOPA PET/CT shows a low spleen uptake and a high SPT uptake. Therefore, it may be more efficient to perform a [18F]FDOPA PET/CT in this kind of case. Given these points, [18F]FDOPA PET/CT in SPT evaluation has potential, although further studies are needed. However, because low-grade NET also shows [18F]FDOPA-avidity, in some situations, it may not be efficient to differentiate SPT from NET. In conclusion, the use of [18F]FDOPA PET/CT can be a valuable diagnostic tool in cases of uncertain diagnosis or suspected metastatic disease, as seen in our case of SPT with peritoneal seeding.
To the best of our knowledge, this is the first case report where [18F]FDOPA PET/CT was employed to diagnose recurred SPT, and the utility of [18F]FDOPA PET/CT for distinguishing between splenosis and tumor recurrence was shown. Further studies are warranted to validate the utility of [18F]FDOPA PET/CT in SPT.
Data Availability
Please contact the author for data requests.
References
Guo N, Zhou QB, Chen RF, Zou SQ, Li ZH, Lin Q, et al. Diagnosis and surgical treatment of solid pseudopapillary neoplasm of the pancreas: analysis of 24 cases. Can J Surg. 2011;54:368–74.
Lüttges J. Was ist neu? Pathol. 2011;32:332.
Lam KY, Lo CY, Fan ST. Pancreatic solid-cystic-papillary tumor: clinicopathologic features in eight patients from Hong Kong and review of the literature. World J Surg. 1999;23:1045–50.
Mazzarella G, Muttillo EM, Coletta D, Picardi B, Rossi S, Rossi Del Monte S, et al. Solid pseudopapillary tumor of the pancreas: a systematic review of clinical, surgical and oncological characteristics of 1384 patients underwent pancreatic surgery. Hepatobiliary Pancreat Dis Int. 2023. https://doi.org/10.1016/j.hbpd.2023.05.004
Coelho JCU, Costa MAR da, Ramos EJB, Torres AR, Savio MC, Claus CMP. Surgical management of solid pseudopapillary tumor of the pancreas. JSLS. 2018;22(4):e2018.00032. https://doi.org/10.4293/JSLS.2018.00032.
Kiroff GK. Splenosis following splenectomy. Arch Surg. 1984;119:351.
Vernuccio F, Dimarco M, Porrello G, Cannella R, Cusmà S, Midiri M, et al. Abdominal splenosis and its differential diagnoses: what the radiologist needs to know. Curr Probl Diagn Radiol. 2021;50:229–35.
Santhanam P, Taïeb D. Role of 18F-FDOPA PET/CT imaging in endocrinology. Clin Endocrinol (Oxf). 2014;81:789–98.
Chen W, Silverman DHS, Delaloye S, Czernin J, Kamdar N, Pope W, et al. 18F-FDOPA PET imaging of brain tumors: comparison study with 18F-FDG PET and evaluation of diagnostic accuracy. J Nucl Med. 2006;47:904–11.
Darcourt J, Schiazza A, Sapin N, Dufour M, Ouvrier MJ, Benisvy D, et al. 18F-FDOPA PET for the diagnosis of parkinsonian syndromes. Q J Nucl Med Mol Imaging. 2014;58:355–65.
Koopmans KP, Neels ON, Kema IP, Elsinga PH, Links TP, de Vries EGE, et al. Molecular imaging in neuroendocrine tumors: molecular uptake mechanisms and clinical results. Crit Rev Oncol Hematol. 2009;71:199–213.
Minn H, Kauhanen S, Seppänen M, Nuutila P. 18F-FDOPA: a multiple-target molecule. J Nucl Med. 2009;50:1915–8.
Chondrogiannis S, Cristina Marzola M, Al-Nahhas A, Venkatanarayana TD, Mazza A, Opocher G, et al. Normal biodistribution pattern and physiologic variants of 18F-DOPA PET imaging. Nucl Med Commun. 2013;34:1141–9.
Chondrogiannis S, Grassetto G, Marzola MC, Rampin L, Massaro A, Bellan E, et al. 18F-DOPA PET/CT biodistribution consideration in 107 consecutive patients with neuroendocrine tumours. Nucl Med Commun. 2012;33:179.
Imperiale A, Addeo P, Averous G, Namer IJ, Bachellier P. Solid pseudopapillary pancreatic tumor mimicking a neuroendocrine neoplasm on 18F-FDOPA PET/CT. J Clin Endocrinol Metab. 2013;98:2643–4.
Somme F, Montaz-Rosset M-S, Averous G, Deur J, Goichot B, Bachellier P, et al. Solid pseudopapillary tumour should be part of differential diagnosis of focal pancreatic lesions with increased 18F-FDOPA uptake. Clin Endocrinol (Oxf). 2020;93:78–81.
Youland RS, Kitange GJ, Peterson TE, Pafundi DH, Ramiscal JA, Pokorny JL, et al. The role of LAT1 in (18)F-DOPA uptake in malignant gliomas. J Neurooncol. 2013;111:11–8.
Notohara K, Hamazaki S, Tsukayama C, Nakamoto S, Kawabata K, Mizobuchi K, et al. Solid-pseudopapillary tumor of the pancreas: immunohistochemical localization of neuroendocrine markers and CD10. Am J Surg Pathol. 2000;24:1361–71.
Berry MD, Juorio AV, Li XM, Boulton AA. Aromatic L-amino acid decarboxylase: a neglected and misunderstood enzyme. Neurochem Res. 1996;21:1075–87.
Hiraoka N, Toue S, Okamoto C, Kikuchi S, Ino Y, Yamazaki-Itoh R, et al. Tissue amino acid profiles are characteristic of tumor type, malignant phenotype, and tumor progression in pancreatic tumors. Sci Rep. 2019;9:9816.
Lu J, Li P, Yang Y, Wang L, Zhang Y, Zhu J, et al. Prognostic value of LAT-1 status in solid cancer: a systematic review and meta-analysis. PLoS One. 2020;15: e0233629.
McGivan JD, Pastor-Anglada M. Regulatory and molecular aspects of mammalian amino acid transport. Biochem J. 1994;299:321–34.
Fremont RD, Rice TW. Splenosis: a review. South Med J. 2007;100(6):589–93.
Tandon YK, Coppa CP, Purysko AS. Splenosis: a great mimicker of neoplastic disease. Abdom Radiol. 2018;43:3054–9.
Lin W-C, Lee R-C, Chiang J-H, Wei C-J, Chu L-S, Liu R-S, et al. MR features of abdominal splenosis. Am J Roentgenol. 2003;180:493–6.
Lake ST, Johnson PT, Kawamoto S, Hruban RH, Fishman EK. CT of splenosis: patterns and pitfalls. Am J Roentgenol. 2012;199:W686-93.
Acknowledgements
We thank our colleagues from Seoul National University Hospital, who provided insight and expertise that greatly assisted the research.
Funding
None.
Author information
Authors and Affiliations
Contributions
The study was designed by Joonhyung Gil and Minseok Suh. The first draft of the manuscript was written by Joonhyung Gil and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Joonhyung Gil, Minseok Suh, Hongyoon Choi, Jin Chul Paeng, Gi Jeong Cheon, and Keon Wook Kang declare no conflict of interest.
Ethics Approval and Consent to Participate
The study was approved by the Institutional Review Board of Seoul National University Hospital (2309-009-1462). All procedures followed were performed in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2013. The institutional review board waived the need to obtain informed consent.
Consent for Publication
The institutional review board waived the need to obtain informed consent because of the anonymity and the retrospective nature of the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gil, J., Suh, M., Choi, H. et al. [18F]FDOPA PET/CT in Solid Pseudopapillary Tumor of the Pancreas: a Recurred Tumor Mimicking Splenosis. Nucl Med Mol Imaging 58, 81–85 (2024). https://doi.org/10.1007/s13139-023-00826-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13139-023-00826-1