Abstract
Objectives
This study aimed to determine the value of clinical prognostic factors and semiquantitative metabolic parameters from initial staging fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography (F-18 FDG PET/CT) in non-Hodgkin lymphoma (NHL) patients treated with stem cell transplantation (SCT).
Methods
A total of 39 malignant lymphoma patients who underwent initial staging F-18 FDG PET/CT were enrolled in this study. SUVmax, MTV_wb, and TLG_wb were measured during the initial staging PET/CT. Receiver operating characteristic curve (ROC) analysis was adopted to dichotomize continuous variables. Log-rank test and Cox proportional hazard regression analysis were used to evaluate disease-free survival (DFS) rate.
Results
Among the 39 patients with malignant lymphoma, 17 (43.6%) had a relapse. For several clinical factors such as age, ECOG performance score, AMC/ALC score, stages, and revised International Prognostic Index score, differences between the two dichotomized groups were statistically insignificant. In univariate analysis, DFS estimates were 71.0 ± 7.8 months and 18.0 ± 5.9 months in high-SUVmax and low-SUVmax group, respectively (P < 0.01). For MTV_wb, DFS estimates were 46.6 ± 12.4 months and 69.1 ± 8.5 months in high-MTV_wb and low-MTV_wb group, respectively (P = 0.12). For TLG_wb, DFS estimates were 65.3 ± 7.5 months and 13.7 ± 8.6 months in high-TLG_wb and low-TLG_wb group, respectively (P = 0.02). In Cox proportional hazard regression analysis, only MTV_wb showed statistical significance (HR 3.01, 95% CI 1.04–8.74, P = 0.04).
Conclusion
In NHL patients treated with SCT, the MTV_wb of initial staging F-18 FDG PET/CT was an independent prognostic factor.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since the establishment of combined rituximab chemotherapy complex, the survival rate and prognosis of malignant non-Hodgkin lymphoma (NHL) patients improved [1, 2]. However, some patients show poor treatment response or recurrence after the initial response. High-dose chemotherapy with hematopoietic stem cell transplantation (SCT) has been used as the standard treatment for recurrent malignant lymphoma, refractory malignant lymphoma, or some types of aggressive NHL [3,4,5,6,7,8]. Although allogeneic and autologous SCT are a promising treatment modality for relapsed or recurrent malignant lymphoma, these options are costly and have morbidity and mortality cases [9]. Therefore, predicting patient’s treatment response can contribute to improved prognosis by enabling early changes to appropriate therapy. Fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography (F-18 FDG PET/CT) is a diagnostic imaging tool capable of qualitative or semi-quantitative analysis using imaging glucose metabolism in lesions and widely used in the diagnosing, staging, and treatment response evaluation of malignant tumors. Several studies exist on the effects of initial staging F-18 FDG PET/CT on the prognosis of patients with lymphoma [10,11,12,13,14]. In these studies, SUV representing glucose metabolism of malignant lymphoma was statistically and significantly correlated with patients’ prognosis. Regarding the study for F-18 FDG PET/CT in predicting the prognosis of patients with malignant lymphoma who underwent SCT, most studies are designed to evaluate the role of initial staging F-18 FDG PET/CT performed after the salvage chemotherapy as prognostic factors for the outcome after SCT [15,16,17,18,19,20,21,22,23]. To the best of our knowledge, there was no study that investigated the correlation between metabolic parameters of initial staging F-18 FDG PET/CT and prognostic outcomes after a hematopoietic SCT. This study evaluated the prognostic value of initial staging F-18 FDG PET/CT assessed using semiquantitative metabolic parameters (SUVmax, whole body metabolic tumor volume (MTV_wb), and whole body total lesion glycolysis (TLG_wb) in patients with NHL undergoing hematopoietic SCT.
Materials and Methods
Patients
In this retrospective study, 39 malignant NHL patients who underwent autologous or allogenic SCT with frontline or second-line strategy at our institution were evaluated, from January 2007 to August 2014. Patients who underwent initial staging F-18 FDG PET/CT were enrolled, but patients who underwent resection or any kind of anticancer treatment for malignant lymphoma lesion before the initial staging F-18 FDG PET/CT were excluded. This retrospective study was approved by the institutional review board (IRB No. 16–0029). To evaluate the predictive ability of clinical and metabolic parameters, disease-free survival (DFS) was used as endpoint. DFS was defined as the number of months from the date of SCT to the date of recurrence, or the date of last follow-up in the patients without recurrence.
Image Acquisition
All patients fasted for >6 h before undergoing PET/CT, and the blood glucose level was <180 mg/dl (74–143 mg/dl). A whole-body scan from head to thigh was acquired 60 min following intravenous injection of 0.1–0.14 MBq/kg of F-18 FDG. PET/CT examinations were performed using a PET/CT scanner (Discovery STE; GE Healthcare, Milwaukee, WI, USA). The CT images were acquired using multidetector CT equipment with the standard protocol that consists of 140 kV, 60–80 mA, a tube rotation time of 0.4 s per rotation, a pitch of 0.984, and a section thickness 3.75 mm. Emission PET data were acquired for 2.5 or 3 min per bed. PET images were reconstructed using an ordered-subset expectation maximization iterative reconstruction algorithm with three iterations, 18 subsets, matrix size of 256 × 256, 50 cm transaxial field-of-view (FOV). PET images were then fused with CT images.
Image Analysis
Two nuclear medicine specialists independently reviewed the images, and discussed for equivocal findings to select true lesions. For semiquantitative analysis, all images were analyzed using PET VCAR on Advantage Workstation 4.6 (GE Medical System, Milwaukee, WI) by a nuclear physician. Physiologic uptake in kidney, urinary bladder, etc. was carefully excluded. SUVmax was measured within a designated region of interest (ROI) and defined as the highest SUV of pixel. MTV was determined by measuring the volume of the lesion above the 2.5 SUV value and the TLG was determined by the MTV × SUVmean [24]. MTV_wb and TLG_wb were calculated as the summation of individual MTV and TLG within the field of image (Fig. 1).
Clinical Prognostic Factors
Clinical staging of malignant lymphoma followed the Ann Arbor staging [25]. Imaging studies including the initial staging CT and F-18 FDG PET/CT and pathologic findings from the bone marrow biopsy of the hip were used to establish the clinical stage. If typical findings on the F-18 FDG PET/CT or positive results on the bone marrow biopsy were observed, patients were regarded as having bone marrow involvement. The revised International Prognostic Index (R-IPI) score was calculated using the patient’s age, Lactate dehydrogenase (LDH) level, stage, extra-nodal lesion status, and performance status at the time of diagnosis. The absolute monocyte/lymphocyte count prognostic score (AMC/ALC score) was determined based on the number of neutrophils and lymphocytes in the blood at the time of diagnosis [26].
Recurrence Assessment
The follow-up duration was at least 2 years after hematopoietic SCT (median follow-up duration: 34 months). All patients were regularly followed every 3 or 6 months. During the follow-up period, physical examination, blood chemistries, and contrast-enhanced CT were used for surveillance. The recurrence of NHL was mostly confirmed through histologic examination. If suspected recurrent lesions were observed in the area where assessment for biopsy is difficult, recurrence was determined only based on the finding of F-18 FDG PET/CT or interval image finding.
Statistical Analysis
Medcalc statistical software version 14.12.0 (Medcalc software bvba, Ostend, Belgium: 2014) was used for statistical analysis. For the univariate analysis, the semi-quantitative metabolic parameters such as SUVmax, MTV_wb, and TLG_wb and clinical prognostic factors such as clinical staging, R-IPI, and AMC/ALC score were grouped into dichotomized variables by optimal cut-off value using the receiver operating characteristic (ROC) curve analysis. Kaplan–Meier survival analysis and log-rank test were used to compare the recurrence rates between groups, and P-value <0.2 was considered significant. Analysis for multicollinearity between independent variables was performed using the statistical software package SPSS 18.0 (SPSS Inc.,Chicago, IL).
Cox proportional hazard regression analysis was used for multivariate analysis and P-value <0.05 was considered statistically significant.
Results
Patients’ Characteristics
A total of 39 patients were included in this study and the mean age was 50.3 years, consisting of 20 men and 19 women (Table 1). The mean follow-up duration was 39.5 ± 10.4 months, and 17 patients (43.6%) had relapsed. Among the NHL patients, diffuse large B-cell lymphoma (DLBCL) was commonly found in 23 patients and followed by peripheral T-cell lymphoma (n = 5), extranodal NK−/T-cell lymphoma (n = 3), follicular lymphoma (n = 3), anaplastic large cell lymphoma (n = 2), marginal zone B-cell lymphoma (n = 1), mantle cell lymphoma (n = 1), and angioimmunoblastic T-cell lymphoma (n = 1) (Table 2). In cases of DLBCL, the first-line of chemotherapy consists of cyclophosphamide, doxorubicin (Adriamycin), vincristine, and prednisone (CHOP) with or without rituximab, an anti-CD-20 antibody (RCHOP). The second-lines are dexamethasone/cisplatin/cytararbine (DHAP), etoposide/methylprednisolone/cytarabine/cisplatin (ESHAP), gemcitabine/dexamethasone/cisplatin (GDP), gemcitabine/oxlaliplatin (GemOx), and ifosfamide/carboplatin/etoposide (ICE). For the other types of NHL, chemotherapeutic regimen prior to SCT followed the National Comprehensive Cancer Network (NCCN) guideline for treatment.
Prognostic Evaluation of Clinical Parameters
The age at diagnosis was divided into two groups: elderly group (≧60 years) and young group (<60 years). DFS was 48.0 ± 13.5 months (95% CI, 21.5–74.5) in the elderly group and 62.6 ± 8.1 months (95% CI, 46.7–78.6) in the young group, and no statistically significant difference was observed between the two groups (P = 0.67). The ECOG performance score was divided into two groups: high group (≧1) and low group (=0), with DFS of 55.5 ± 11.8 months (95% CI, 32.3–78.7) in high group and 61.0 ± 8.7 months (95% CI, 44.0–78.0) in low group, and no statistically significant difference was observed between the two groups (P = 0.99). The AMC/ALC score was divided into two groups: high-risk group (AMC ≧0.63 × 109/L and ALC ≦1.0 × 109/L) and low-risk group, with DFS of 62.3 ± 94 months (95% CI, 43.9–80.6) and 48.7 ± 9.2 months (95% CI, 30.7–66.7) in high- and low-risk groups, respectively, and no statistically significant difference was observed between the two groups (P = 0.99). The stage was divided into two groups: high (III–IV) and low (I–II), with DFS of 63.2 ± 8.0 months (95% CI,47.4–79.0) and 53.9 ± 16.3 months (95% CI, 21.9–85.9) in high- and low-stage groups, respectively, and no statistically significant difference was observed between the two groups (P = 0.53). The revised IPI score was divided into two groups: high- (poor) and low-risk groups (very good and good), with DFS of 66.9 ± 10.2 months (95% CI, 46.8–86.9) and 53.2 ± 9.7 months (95% CI, 34.2–72.2) in high- and low-risk groups, respectively, and no statistically significant difference was observed between the two groups (P = 0.44) (Fig. 2).
Prognostic Evaluation of Metabolic Parameters on F-18 FDG PET/CT
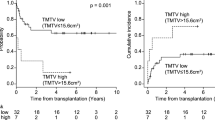
The optimal cutoff of SUVmax, MTV_wb, and TLG_wb obtained using the ROC curve analysis was 8.2, 673 cm3, and 81, respectively (Table 3). Using these cutoff values, PET/CT parameters were dichotomized. DFS was 71.0 ± 7.8 months (95% CI, 55.7–86.4) in patients with high SUVmax (>8.2) and 18.0 ± 5.9 months (95% CI, 6.5–29.5) in patients with low SUVmax, and a statistically significant difference was observed between the two groups (P < 0.01). The optimal cutoff of MTV_wb obtained using the ROC curve analysis was 673 cm3. The MTV_wb at initial staging FDG-PET/CT was divided into two groups: high (>673 cm3) and low MTV_wb groups. DFS was 46.6 ± 12.4 months (95% CI, 22.3–71.0) in patients with high MTV_wb (>673 cm3) and 69.1 ± 8.5 months (95% CI, 52.4–85.8) in patients with low MTV_wb. A statistically significant difference was observed between the two groups (P = 0.12). Regarding TLG_wb, DFS was 65.3 ± 7.5 months (95% CI, 50.7–80.0) in patients with high TLG_wb (>81) and 13.7 ± 8.6 months (95% CI, 0–30.5) in patients with low TLG_wb group, and a statistically significant difference was observed between the two groups (P = 0.02) (Fig. 3).
Cox proportional hazards regression analysis was performed for multivariate analysis using the SUVmax, MTV_wb, and TLG of initial staging F-18 FDG PET/CT, which showed significant differences between the groups in log-rank test. The analysis showed that SUVmax (HR, 0.38; 95% CI, 0.08–1.94 P = 0.25), MTV_wb (HR, 3.01; 95% CI, 1.04–8.74; P = 0.04), TLG (HR, 0.32; 95% CI, 0.09–1.05; P = 0.32). MTV_wb was statistically significant different in Cox proportional hazards regression analysis (Table 4).
Discussion
Hematopoietic SCT is a promising therapeutic tool for recurrent or relapsed malignant lymphoma or some types of aggressive NHL [3, 4, 6, 7]. Almost all studies for the prognostic role of F-18 FDG PET/CT in SCT were approximately recurrent or relapsed malignant lymphoma, and these studies used the qualitative analysis or metabolic parameters using the semiquantitative analysis on the pre-SCT and post-SCT F-18 FDG PET/CT [18,19,20,21,22]. In their studies, researchers concluded that FDG uptake of lesions were significantly correlated with the clinical outcomes of patients with malignant lymphoma who underwent SCT. This study aimed to evaluate the prognostic role of metabolic parameters for the initial staging F-18 FDG PET/CT in NHL patients who underwent SCT as the frontline or second-line strategy.
This study showed that only MTV was a statistically significant prognostic factor among metabolic parameters (SUVmax_rep, MTV, and TLG) of F-18 FDG PET/CT. This result is different from other studies in that SUVmax on initial staging F-18 FDG PET/CT was a significant prognostic factor [27,28,29]. However, the difference of enrolled patients’ characteristics and treatment modality was incomparable. These patients were clinically high-risk groups or recurrent or relapsed patients with candidates for SCT as the frontline or second-line strategy.
Regarding the correlation between lesional SUVmax and clinical outcome of patients with aggressive malignant lymphoma, a few studies showed different results. Hwang et al. revealed that no significant correlation was observed between lesional SUVmax and patients’ survival outcome in patients with aggressive NHL patients among their study population [30]. Yi et al.’s study also showed that although patients with higher SUVmax showed inferior overall survival than those with lower SUVmax, the difference was not statistically significant [31]. Contrary to Hwang et al. and Yi et al.’s results, other researchers showed that lesional SUVmax was a significant prognostic factor in patients with T-cell lymphoma, a subtype of aggressive NHL [32, 33]. Unlike those studies, this study enrolled patients with various subtypes of NHL and different treatment modality. This difference might be the reason why the prognostic role of lesional SUVmax is different depending on each study. In addition, measurement of SUV from a single ROI, which does not represent the overall tumor profile, can be the other reason for the different results especially in aggressive NHL.
Similar explanations might be applicable to the IPI result in this study, which was not correlated with the patients’ outcome. IPI has been an important tool in predicting outcomes of patients with aggressive NHL based on the number of negative prognostic factors present at the time of diagnosis [34]. However, an effective new treatment modality can affect the clinical significance of these prognostic markers. In fact, according to analysis of PARMA trial, IPI was highly correlated with OS in the conventional chemotherapy arm, but was not a significant prognostic factor in the transplant arm [35].
TLG_wb as a prognostic factor was insignificantly correlated with patients’ outcome and is calculated as the summation of each lesional MTV multiplied by its SUVmean. Although this parameter reflects metabolically active tumor volume, glucose metabolism of each tumor lesion can also affect the TLG value. This result might be due to the relationship between TLG and SUV of the tumor.
In this study, only MTV was a prognostic factor. Many studies revealed that tumor bulk of malignant lymphoma is one of the prognostic factors [36,37,38,39]. Therefore, we believe that measured MTV based on the cutoff value of SUV 2.5 can reflect the tumor size as a prognostic factor on the initial staging F-18 FDG PET/CT for NHL patients who are scheduled for hematopoietic SCT. Although the MTV was not measured by various cutoff values, the tumor from physiologic activity could be delineated at this cutoff value. Application of measured MTV using various cutoff values for larger patient cohorts might be warranted. Deauville criteria can be used for the evaluation of therapeutic response and prognosis. Although Deauville criteria might be reproducible, this criteria does not represent the volumetric aspect of tumor.
However, this study has several limitations. This is not a prospective study; thus, it is subject to the inherent limitations of retrospective data. In addition, the subtypes of NHL and treatment strategy, i.e., heterogeneous frontline and second-line strategies, in enrolled patients were heterogeneous. This situation might affect the results of the outcome. Therefore, further controlled prospective studies with larger population are necessary.
Conclusion
The results of this study show that MTV_wb of initial staging F-18 FDG PET/CT has a prognostic role in the aggressive and high-risk NHL patients who are scheduled for SCT as the frontline or second-line strategy. For SCT as the frontline or second-line strategy, patients with high MTV_wb might be classified as a group with poor outcomes. Therefore, appropriate therapeutic plans are necessary.
References
Pfreundschuh M, Kuhnt E, Trumper L, Osterborg A, Trneny M, Shepherd L, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the Mab Thera international trial (MInT) group. Lancet Oncol. 2011;12:1013–22.
Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–42.
Greb A, Bohlius J, Trelle S, Schiefer D, De Souza CA, Gisselbrecht C, et al. High-dose chemotherapy with autologous stem cell support in first-line treatment of aggressive non-hodgkin lymphoma — results of a comprehensive meta-analysis. Cancer Treat Rev. 2007;33:338–46.
Linch DC, Winfield D, Goldstone AH, Moir D, Hancock B, McMillan A, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant hodgkin's disease: results of a BNLI randomised trial. Lancet. 1993;341:1051–4.
Philip T, Guglielmi C, Hagenbeek A, Somers R, Van der Lelie H, Bron D, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-hodgkin's lymphoma. N Engl J Med. 1995;333:1540–5.
Schmitz N, Pfistner B, Sextro M, Sieber M, Carella AM, Haenel M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive hodgkin's disease: a randomised trial. Lancet. 2002;359:2065–71.
Villa D, Crump M, Panzarella T, Savage KJ, Toze CL, Stewart DA, et al. Autologous and allogeneic stem-cell transplantation for transformed follicular lymphoma: a report of the canadian blood and marrow transplant group. J Clin Oncol. 2013;31:1164–71.
Moskowitz AJ, Moskowitz CH. Controversies in the treatment of lymphoma with autologous transplantation. Oncologist. 2009;14:921–9.
Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–26.
Cazaentre T, Morschhauser F, Vermandel M, Betrouni N, Prangere T, Steinling M, et al. Pre-therapy 18F-FDG PET quantitative parameters help in predicting the response to radioimmunotherapy in non-hodgkin lymphoma. Eur J Nucl Med Mol Imaging. 2010;37:494–504.
Byun BH, Na II, Cheon GJ, Kang HJ, Kim KM, Lee SS, et al. Clinical significance of 18F-FDG uptake by primary sites in patients with diffuse large B cell lymphoma in the head and neck: a pilot study. Ann Nucl Med. 2008;22:645–51.
Nguyen XC, Lee WW, Amin AM, Eo JS, Bang SM, Lee JS, et al. Tumor burden assessed by the maximum standardized uptake value and greatest diameter on FDG-PET predicts prognosis in untreated diffuse large B-cell lymphoma. Nucl Med Mol Imaging. 2010;44:39–44.
Karam M, Ata A, Irish K, Feustel PJ, Mottaghy FM, Stroobants SG, et al. FDG positron emission tomography/computed tomography scan may identify mantle cell lymphoma patients with unusually favorable outcome. Nucl Med Commun. 2009;30:770–8.
Kawai N, Zhen HN, Miyake K, Yamamaoto Y, Nishiyama Y, Tamiya T. Prognostic value of pretreatment 18F-FDG PET in patients with primary central nervous system lymphoma: SUV-based assessment. J Neuro-Oncol. 2010;100:225–32.
Adams HJ, Kwee TC. Prognostic value of pretransplant FDG-PET in refractory/relapsed hodgkin lymphoma treated with autologous stem cell transplantation: systematic review and meta-analysis. Ann Hematol. 2016;95:695–706.
Gentzler RD, Evens AM, Rademaker AW, Weitner BB, Mittal BB, Dillehay GL, et al. F-18 FDG-PET predicts outcomes for patients receiving total lymphoid irradiation and autologous blood stem-cell transplantation for relapsed and refractory hodgkin lymphoma. Br J Haematol. 2014;165:793–800.
Lim SH, Hyun SH, Kim HS, Lee JY, Yoo KH, Jung KS, et al. Prognostic relevance of pretransplant deauville score on PET-CT and presence of EBV DNA in patients who underwent autologous stem cell transplantation for ENKTL. Bone Marrow Transplant. 2016;51:807–12.
Qiao W, Zhao J, Xing Y, Wang C, Wang T. Predictive value of [(1)(8)F]fluoro-2-deoxy-D-glucose positron emission tomography for clinical outcome in patients with relapsed/refractory diffuse large B-cell lymphoma prior to and after autologous stem cell transplant. Leuk Lymphoma. 2014;55:276–82.
Reyal Y, Kayani I, Bloor AJ, Fox CP, Chakraverty R, Sjursen AM, et al. Impact of pretransplantation (18)F-fluorodeoxyglucose-positron emission tomography on survival outcomes after T cell-depleted allogeneic transplantation for hodgkin lymphoma. Biol Blood Marrow Transplant. 2016;22:1234–41.
Sauter CS, Matasar MJ, Meikle J, Schoder H, Ulaner GA, Migliacci JC, et al. Prognostic value of FDG-PET prior to autologous stem cell transplantation for relapsed and refractory diffuse large B-cell lymphoma. Blood. 2015;125:2579–81.
Spaepen K, Stroobants S, Dupont P, Vandenberghe P, Maertens J, Bormans G, et al. Prognostic value of pretransplantation positron emission tomography using fluorine 18-fluorodeoxyglucose in patients with aggressive lymphoma treated with high-dose chemotherapy and stem cell transplantation. Blood. 2003;102:53–9.
Ulaner GA, Goldman DA, Sauter CS, Migliacci J, Lilienstein J, Gonen M, et al. Prognostic value of FDG PET/CT before allogeneic and autologous stem cell transplantation for aggressive lymphoma. Radiology. 2015;277:518–26.
Wang C, Li P, Wu S, Lu J, Liu Q, Luo H, et al. The role of fluorine-18 fluorodeoxyglucose PET in prognosis evaluation for stem cell transplantation of lymphoma: a systematic review and meta-analysis. Nucl Med Commun. 2016;37:338–47.
Ryu IS, Kim JS, Roh JL, Lee JH, Cho KJ, Choi SH, et al. Prognostic value of preoperative metabolic tumor volume and total lesion glycolysis measured by 18F-FDG PET/CT in salivary gland carcinomas. J Nucl Med. 2013;54:1032–8.
Meignan M, Gallamini A, Meignan M, Gallamini A, Haioun C. Report on the first international workshop on interim-PET-scan in lymphoma. Leuk Lymphoma. 2009;50:1257–60.
Sehn LH, Berry B, Chhanabhai M, Fitzgerald C, Gill K, Hoskins P, et al. The revised international prognostic index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007;109:1857–61.
Okada J, Oonishi H, Yoshikawa K, Itami J, Uno K, Imaseki K, et al. FDG-PET for predicting the prognosis of malignant lymphoma. Ann Nucl Med. 1994;8:187–91.
Chihara D, Oki Y, Onoda H, Taji H, Yamamoto K, Tamaki T, et al. High maximum standard uptake value (SUVmax) on PET scan is associated with shorter survival in patients with diffuse large B cell lymphoma. Int J Hematol. 2011;93:502–8.
Park S, Moon SH, Park LC, Hwang DW, Ji JH, Maeng CH, et al. The impact of baseline and interim PET/CT parameters on clinical outcome in patients with diffuse large B cell lymphoma. Am J Hematol. 2012;87:937–40.
Hwang JP, Lim I, Byun BH, Kim BI, Choi CW, Lim SM. Prognostic value of SUVmax measured by pretreatment 18F-FDG PET/CT in patients with primary gastric lymphoma. Nucl Med Commun. 2016;37:1267–72.
Yi JH, Kim SJ, Choi JY, Ko YH, Kim BT, Kim WS. 18F-FDG uptake and its clinical relevance in primary gastric lymphoma. Hematol Oncol. 2010;28:57–61.
Kim CY, Hong CM, Kim DH, Son SH, Jeong SY, Lee SW, et al. Prognostic value of whole-body metabolic tumour volume and total lesion glycolysis measured on (1)(8)F-FDG PET/CT in patients with extranodal NK/T-cell lymphoma. Eur J Nucl Med Mol Imaging. 2013;40:1321–9.
Suh C, Kang YK, Roh JL, Kim MR, Kim JS, Huh J, et al. Prognostic value of tumor 18F-FDG uptake in patients with untreated extranodal natural killer/T-cell lymphomas of the head and neck. J Nucl Med. 2008;49:1783–9.
International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-hodgkin's lymphoma. N Engl J Med. 1993;329:987–94.
Blay J, Gomez F, Sebban C, Bachelot T, Biron P, Guglielmi C, et al. The international prognostic index correlates to survival in patients with aggressive lymphoma in relapse: analysis of the PARMA Trial. Parma group. Blood. 1998;92:3562–8.
Coiffier B, Gisselbrecht C, Vose JM, Tilly H, Herbrecht R, Bosly A, et al. Prognostic factors in aggressive malignant lymphomas: description and validation of a prognostic index that could identify patients requiring a more intensive therapy. The groupe d'etudes des lymphomes agressifs. J Clin Oncol. 1991;9:211–9.
Oguchi M, Ikeda H, Isobe K, Hirota S, Hasegawa M, Nakamura K, et al. Tumor bulk as a prognostic factor for the management of localized aggressive non-hodgkin's lymphoma: a survey of the japan lymphoma radiation therapy group. Int J RadiatOncolBiol Phys. 2000;48:161–8.
Shipp MA, Harrington DP, Klatt MM, Jochelson MS, Pinkus GS, Marshall JL, et al. Identification of major prognostic subgroups of patients with large-cell lymphoma treated with m-BACOD or M-BACOD. Ann Intern Med. 1986;104:757–65.
Velasquez WS, McLaughlin P, Tucker S, Hagemeister FB, Swan F, Rodriguez MA, et al. ESHAP—an effective chemotherapy regimen in refractory and relapsing lymphoma: a 4-year follow-up study. J Clin Oncol. 1994;12:1169–76.
Acknowledgements
The authors thank D Kang, staff of our department for her help during this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Yun Soo Park, Seok Mo Lee, Ji Sun Park, Sang Kyun Bae, Hye-Kyung Shim, Won-Sik Lee, Sang-Min Lee declare that they have no conflict of interest.
Ethical Statement
The study was approved by the Institutional Review Board of Inje University Busan Paik Hospital (IRB No. 16–0029) and has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed Consent
The institutional review board waived the need to obtain informed consent for this retrospective study.
Rights and permissions
About this article
Cite this article
Park, Y.S., Lee, S.M., Park, J.S. et al. Evaluating the Predictive Ability of Initial Staging F-18 FDG PET/CT for the Prognosis of Non-Hodgkin Malignant Lymphoma Patients Who Underwent Stem Cell Transplantation. Nucl Med Mol Imaging 52, 216–223 (2018). https://doi.org/10.1007/s13139-017-0503-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13139-017-0503-8