Abstract
Purpose
The purpose of this study was to investigate the value of 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT) parameters in the detection of regional lymph node (LN) metastasis in patients with cutaneous melanoma.
Methods
We evaluated patients with cutaneous melanoma who underwent FDG PET/CT for initial staging or recurrence evaluation. A total of 103 patients were enrolled, and 165 LNs were evaluated. LNs that were confirmed pathologically or by follow-up imaging were included in this study. PET parameters, including maximum standardized uptake value (SUVmax), total lesion glycolysis and tumour-to-liver ratio, were used to determine the presence of metastases, and the results were compared with CT-determined LN metastasis. Receiver operating characteristic (ROC) curve analysis was used to determine the optimal cut-off values of the FDG PET parameters.
Results
A total of 93 LNs were malignant, and 84 LNs were smaller than 10 mm. In all 165 LNs, an SUVmax of >2.51 showed a sensitivity of 73.1%, a specificity of 88.9%, and an accuracy of 80.0% in detecting metastatic LNs. CT showed a higher specificity (87.3%) and lower accuracy (65.5%). For non-enlarged regional LNs (<10 mm), an SUVmax cut-off value of 1.4 showed the highest negative predictive value (81.3%). For enlarged LNs (≥10 mm), an SUVmax cut-off value of 2.4 showed the highest sensitivity (90.7%) and accuracy (88.9%) in detecting metastatic LNs.
Conclusions
In patients with cutaneous melanoma, an SUVmax of >2.4 showed a high sensitivity (91%) and accuracy (89%) in detecting metastasis in LNs ≥1 cm, and LNs <1 cm with an SUVmax <1.4 were likely to be benign.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The incidence of cutaneous melanoma has shown a recent increase in Western countries, and in low-incidence areas, such as Asian countries. The World Health Organization (WHO) classification of cutaneous melanoma categorizes melanoma into four main subtypes, of which superficial spreading melanoma is the most common in Caucasians. In contrast, acral lentiginous melanoma [1] is the most common in Asians and is generally detected during later stages compared to other melanoma subtypes [2]. Also, due to the aggressive nature of melanoma, prognosis is dependent on precise diagnosis and treatment. Patients with localized disease have been reported to have a 5-year survival rate of 90%, whereas if the disease has already spread at the time of diagnosis, the 5-year survival rate is 10%. As one of the most common sites of metastasis has been reported to be the lymph nodes (LN; 73.6% of cases) [3, 4], an evaluation of the imaging modalities used for determining LN metastasis is needed.
Before surgical treatment and sentinel node biopsy, imaging (ultrasonography for locoregional LN metastasis) is recommended for tumours of higher stages (pT1b–pT3a), and computed tomography (CT) or 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT) is recommended for tumours of stages >pT3a [5]. Although FDG PET has shown limited sensitivity for initial regional staging, especially in patients with American Joint Committee on Cancer (AJCC) stage I and II disease [6], FDG PET/CT is included in the imaging protocols of the National Comprehensive Cancer Network guidelines for patients with stage III and IV disease and for patients with stage IB or II disease with specific signs or symptoms [7]. Due to the relatively high uptake of FDG in melanoma metastases, combined with the often unpredictable metastatic pattern, functional imaging with whole-body PET using FDG has been shown to be useful for detecting metastasis [8, 9].
Studies evaluating clinically significant FDG uptake have been performed for various malignancies, with one of the most commonly evaluated malignancies being solid lung nodules [10, 11] and mediastinal LNs in lung cancer patients [12]. In the evidence-based medicine era, criteria such as these are helpful in guiding nuclear medicine specialists and clinicians in determining malignancy. However, most studies evaluating the clinical usefulness of FDG PET/CT for detecting metastatic LNs in patients with melanoma have relied on visual analysis.
The purpose of this study was to determine the value of FDG PET/CT in detecting LN metastasis in patients with cutaneous melanoma.
Materials and Methods
Patient Selection and Clinical Characteristics
The medical records of patients with cutaneous melanoma who underwent FDG PET/CT from January 2005 to December 2015 for initial staging or recurrence evaluation were reviewed. Contrast-enhanced CT (CE-CT) had to have been performed within 2 weeks of FDG PET/CT for the patient to be included in the study. A total of 103 patients were enrolled in the study. In patients who underwent FDG PET/CT for melanoma staging, the nodal stage was evaluated according to the seventh edition of the AJCC staging manual, with LNs not detected on CE-CT defined as “clinically occult” nodal metastases. However, due to the low sensitivity of FDG PET/CT in detecting microscopic LN metastases and the partial volume effect caused by very small LNs [9], LNs smaller than 5 mm were excluded from the study. Also, only LNs confirmed pathologically or on follow-up imaging were included in the study. Positive findings on imaging follow-up were defined as LNs that showed an increase in size or newly developed necrotic components on follow up CE-CT or ultrasonography. Finally, a total of 165 LNs were evaluated. This study was approved by our institutional review board, and informed consent was waived due to the retrospective nature of the study.
PET/CT Imaging Protocol
PET/CT was performed on a Discovery STE scanner (GE Healthcare, Milwaukee, WI) or a Biograph TruePoint 40 scanner (Siemens Medical Systems, Knoxville, TN) equipped with 16-slice or 40-slice CT, respectively. Patients fasted for at least 6 h before imaging, and the glucose level in the peripheral blood was confirmed to be no more than 140 mg/dL prior to injection of FDG. An FDG dose of approximately 5.5 MBq/kg body weight was administered intravenously 1 h before image acquisition. After the initial low-dose CT study (Discovery STE 30 mA and 130 kVp; Biograph TruePoint 40 36 mA and 120 kVp), a standard PET protocol was used to scan from the neck to the proximal thighs, with an acquisition time of 3 min per bed position in three-dimensional mode. Images were reconstructed using ordered subsets expectation maximization (Discovery STE two iterations, 20 subsets; Biograph TruePoint 40 three iterations, eight subsets).
FDG PET/CT and Contrast-Enhanced CT Analysis
Two experienced nuclear medicine physicians reviewed all PET/CT images. All images were evaluated on a lesion-by-lesion basis. Metabolic parameters were measured using MIM software. A volume of interest was drawn on each LN based on the contour seen on CT, and the maximum standardized uptake value (SUVmax) was recorded. In cases where there was a clear misregistration between the CT and PET images, the centre of the volume of interest was moved to the centre of the SUV. The relative SUVmax threshold (40%) was used to define metabolic tumour volume (MTV). Total lesion glycolysis (TLG) was calculated as MTV × mean SUV (SUVmean). Also, the SUVmean of the liver was determined by drawing three regions of interest on the normal liver parenchyma and obtaining the average SUV of these regions. The tumour-to-liver ratio (TLR) was also calculated by dividing the SUVmax of the LN by the SUVmean of the liver. CE-CT images were reviewed, and standard criteria for LN metastasis were used to identify metastatic LNs (short-axis diameter ≥15 mm for neck LNs, short-axis diameter ≥10 mm for non-neck LNs, or LNs with necrotic components or perilesional infiltration regardless of size).
Statistical Analysis
Patient data were collected, and PET and CT data were recorded. The gold standard was LN metastasis determined either pathologically or on follow-up imaging. SUVmax, TLG, and TLR were evaluated as continuous variables, and Student’s t test was performed for categorical variables. CT-determined LNs were categorized as positive or negative, and chi-squared analysis was performed. The Pearson correlation coefficient was used to compare LN size with FDG uptake parameters. Receiver operating characteristic (ROC) curve analysis was used to determine the optimal cut-off value of the FDG PET parameters to determine the highest sensitivity for the detection of LN metastasis. This cut-off value was used to determine the sensitivity, specificity, and accuracy of PET/CT in detecting LN metastasis. Differences between AUC values from the ROC analysis were analysed using a Z test according to the method of Hanley and McNeil. McNemar’s test was used to compare FDG PET/CT and CE-CT for evaluating LN metastasis. Finally, LNs were categorized as larger or smaller than 10 mm, and McNemar’s test was used to compare the sensitivity, specificity, and accuracy of CE-CT and PET/CT in detecting metastatic LNs.
Results
Patient Characteristics
Of the 103 patients (57 men; mean age 59.3 ± 11.9 years) who underwent FDG PET/CT, 67 (65%) underwent FDG PET/CT for melanoma staging, and 36 (35%) underwent FDG PET/CT for recurrence evaluation. A total of 165 LNs were assessed either pathologically (139 LNs, 84.2%) or on imaging follow-up (26 LNs, 15.8%). Of these 165 LNs, 93 (56.4%) were determined to be metastatic, and 72 (43.6%) were determined to be benign. Regarding size, 84 LNs (50.9%) were 5–10 mm in diameter, and 81 LNs (49.1%) were larger than 10 mm. The average SUVmax of the LNs was 4.85 ± 6.43. The characteristics of the 103 enrolled patients are summarized in Table 1 and of the 165 LNs evaluated are summarized in Table 2.
Analysis of Lymph Node Images
All FDG uptake parameters were significantly higher in metastatic LNs than in benign LNs (SUVmax 7.3 ± 7.6 vs. 1.6 ± 1.8, p < 0.001; TLG 43.2 ± 169.7 vs. 2.0 ± 9.3, p = 0.022; TLR 3.0 ± 3.1 vs. 0.7 ± 0.8, p < 0.001, respectively). Pathological LNs were significantly larger than benign LNs (14.9 ± 8.5 mm vs. 8.8 ± 3.0 mm, respectively; p < 0.001). FDG uptake was also significantly higher in metastatic LNs smaller than 10 mm than in benign LNs of the same size (SUVmax 2.6 ± 2.1 vs. 1.4 ± 1.0, respectively; p < 0.004; Table 3). SUVmax was significantly higher in larger metastatic LNs (≥10 mm) than in smaller metastatic LNs (<10 mm; p < 0.001), but there was no significant difference in SUVmax between larger and smaller benign LNs (p = 0.190). TLG and TLR also showed similar trends (Table 3).
The correlation between LN size and FDG uptake was analysed using the Pearson correlation coefficient. There was a significant but weak correlation between LN size and FDG uptake (r = 0.517 for SUVmax, r = 0.576 for TLG, r = 0.516 for TLR; p < 0.001 for all comparisons). When LNs were categorizing as benign or malignant, there was still a significant but weak correlation between LN size and FDG uptake in malignant LNs (r = 0.416 for SUVmax, r = 0.587 for TLG, r = 0.414 for TLR; p < 0.001 for all comparisons), but there was a minimal correlation in benign LNs (r = 0.372 for SUVmax, r = 0.443 for TLG, r = 0.372 for TLR; p < 0.001 for all comparisons).
Detection of LN Metastasis
The accuracy of FDG PET/CT and CE-CT in detecting metastasis was evaluated for all LNs. First, ROC analysis was performed to determine the cut-off values with the highest sensitivity (Fig. 1). The cut-off values with the highest sensitivities were 2.51 for SUVmax (area under curve, AUC, 0.864, p < 0.0001), 0.91 for TLR (AUC 0.858, p < 0.0001) and 3.5 for TLG (AUC 0.831; p < 0.0001). Applying these cut-off values, SUVmax showed high sensitivity (73.1%), high specificity (88.9%), and high accuracy (80%) in detecting metastasis in all LNs. In contrast, CE-CT showed a similar sensitivity (76.3%), but lower specificity (66.7%) and lower accuracy (72.1%). The values for the other FDG parameters are shown in Table 4. The AUC values of PET parameters provided significantly better detection of metastatic LNs than CE-CT (SUVmax vs. CE-CT Z = 3.745, p < 0.001; TLR vs. CE-CT Z = 3.571, p < 0.001; TLG vs. CE-CT Z = 3.112, p = 0.002). All other AUC comparison values were not significant (p > 0.05). Finally, McNemar’s test revealed that there was a significant difference in the detection of metastatic LNs between SUVmax and CE-CT (p = 0.003) and between TLG and CE-CT (p < 0.001).
Receiver operating characteristic curve analysis of FDG PET/CT parameters for all LNs. The optimal cut-off values are 2.51 for SUVmax (AUC 0.864, p < 0.0001), 0.91 for TLR (AUC 0.858, p < 0.0001), and 3.5 for TLG (AUC 0.831, p < 0.0001). The CE-CT AUC is 0.715 (p < 0.0001). The AUCs for all PET parameters were significantly higher than the CE-CT AUC (p < 0.05; individual p values are provided in the main text)
For the 81 LNs ≥10 mm, the optimal cut-off values from the ROC analysis were 2.4 for SUVmax (AUC 0.881, p < 0.0001), 1.05 for TLR (AUC 0.892, p < 0.0001), and 3.46 for TLG (AUC 0.834, p < 0.0001). In this group of LNs, SUVmax showed high sensitivity (90.7%), high specificity (82.4%), and high accuracy (88.9%) in detecting LN metastasis. On CE-CT, all lesions were considered to be positive, resulting in 100% sensitivity and 0% specificity.
Finally, for the 84 LNs <10 mm, the optimal cut-off values were 1.4 for SUVmax (AUC 0.723, p = 0.0002), 0.68 for TLR (AUC 0.691, p = 0.004), and 1.55 for TLG (AUC 0.637, p = 0.049; Fig. 2). In this group of LNs, SUVmax showed a sensitivity of 69.0%, a specificity of 71.0%, and an accuracy of 70.3%. Although the SUVmax cut-off value of 1.4 had a very low positive predictive value (PPV) for detecting metastases (55.6%), its negative predictive value (NPV) was relatively high (81.3%), which suggests that minimal FDG uptake in non-enlarged regional LNs most likely signifies that the LNs are benign (Fig. 3). In contrast, CE-CT showed very low sensitivity (24.1%), slightly higher specificity (87.3%), and lower accuracy (65.5%) than the PET parameters. Other PET parameters are shown in Table 4. The SUVmax AUC was significantly higher than the CE-CT AUC (Z = 2.089, p = 0.036) and higher than the TLG AUC (Z = 2.142, p = 0.032). McNemar’s test showed that the rate of detection of metastatic LNs with CE-CT was significantly different from the rates of detection with SUVmax (p < 0.001) and TLR (p = 0.003), but not different from the rate with TLG (p = 0.263).
Receiver operating characteristic curve analysis of FDG PET/CT parameters for LNs <10 mm. The optimal cut-off values are 1.4 for SUVmax (AUC 0.723, p = 0.0002), 0.68 for TLR (AUC 0.691, p = 0.004), and 1.55 for TLG (AUC 0.637, p = 0.049). The CE-CT AUC is 0.557 (p = 0.3936). The SUVmax AUC is significantly higher than the CE-CT AUC (p = 0.036) and higher than the TLG AUC (p = 0.032)
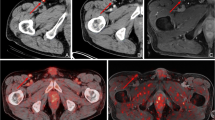
Representative images of benign and malignant LNs in patients with malignant melanoma. a–d A 57-year-old man who had cutaneous melanoma on the sole of the right foot: the coronal (a) and transaxial (b) PET images, and the PET/CT fusion image (c) show mild FDG uptake in the right inguinal area (SUVmax 2.04, TLR 0.76, TLG 2.04), which corresponds to a 9.7-mm LN seen on the CE-CT image (d). LN metastasis was confirmed by surgical excision. e–h A 62-year-old woman with cutaneous melanoma on the sole of the left foot: the coronal (e) and transaxial (f) PET images, and the PET/CT fusion image (g) show minimal FDG uptake in the left inguinal LN preoperatively (SUVmax 1.29, TLR 0.62, TLG 1.18, size 8.7 mm), which corresponds to the lesion seen on the CT image (h). Sentinel node biopsy showed no evidence of metastasis
Discussion
The present study evaluated various FDG parameters and CE-CT for detecting LN metastasis in patients with cutaneous melanoma. We showed that, the use of an SUVmax cut-off value of 2.5 was associated with an accuracy of 80.0%, which was superior to that of CE-CT (72.1%). For LNs smaller than 10 mm, SUVmax showed a much higher sensitivity than CE-CT in detecting LN metastasis. TLR showed similar results to those with SUVmax, but TLG showed lower sensitivity than the other PET parameters. Overall, FDG PET/CT showed a higher diagnostic value than conventional anatomical images in this cohort of patients with cutaneous melanoma. In a recent study, N and M stages were correctly determined by PET/CT in 243 of 250 patients (97.2%; 95% CI 95.2–99.4%) compared with 197 patients (78.8%; 95% CI 73.7–83.9%) by CT [13]. In this study, FDG PET/CT showed a higher value than CE-CT in detecting LN metastasis.
The clinical significance of this study is that it is one of the largest studies to use semiquantitative analysis to evaluate the ability of FDG PET/CT to detect LN metastasis. There have been many studies over the past decade that have evaluated the ability of FDG PET/CT to detect LN metastasis, although all but two studies used visual analysis to determine metastasis [1, 13,14,15,16,17,18,19]. Although visual analysis is generally used in the clinical setting, a more objective method may be more helpful when comparing the results of different studies. In one of the two studies that used SUVmax, a cut-off value of 2.5 was chosen, but this was arbitrarily determined [19]. More recently, Giesel et al. used SUVmax to detect LN metastasis in patients with cutaneous melanoma, as well as in patients with other malignancies, but they defined positive LNs as those with an SUVmax three times greater than the blood pool [15]. Similarly, we found that SUVmax adjusted to liver activity (TLR) showed similar diagnostic accuracy to SUVmax. In contrast, TLG showed significantly worse diagnostic accuracy than SUVmax, which is probably due to the contribution of the size component to the calculation of TLG. Overall, we showed that a few cut-off values could serve as guidelines in the detection of LN metastasis in patients with cutaneous melanoma, although further studies are needed to confirm our results.
We evaluated the sensitivity and specificity of FDG PET/CT in detecting metastatic LNs smaller than 10 mm. It is well-established that FDG PET/CT shows low sensitivity in detecting clinically occult (nonpalpable) LN metastasis [9, 14], and studies have suggested that FDG PET/CT is not suitable for determining the presence of LN micrometastasis [19]. However, PET/CT is often acquired to evaluate recurrence or for staging of melanoma in patients who might have in-transit metastasis without palpable LNs (N2c) or clinically occult regional metastatic LNs (N2a). Therefore, we included these smaller LNs (5–10 mm) to evaluate FDG PET/CT for assessing regional LNs with a high rate of metastasis. In regional or in-transit clinically occult LN, an SUVmax higher than 1.4 had a PPV of only 56% in detecting metastasis, but had a high NPV (81%) in detecting nonmalignant LNs. CE-CT showed a much lower PPV and NPV in detecting LN metastasis than both SUVmax and TLR.
The limitations of this study are that, due its retrospective nature, there was an inherent selection bias, which suggests the need for more studies using semiquantitative analysis to determine clinically significant cut-off values. Although we evaluated many regional LNs, a larger study is needed to confirm these results, as the relative number of metastatic LNs confirmed pathologically or on follow-up imaging is smaller for non-enlarged LNs.
Conclusions
In patients with cutaneous melanoma, an SUVmax higher than 2.4 showed high sensitivity (91%) and accuracy (89%) in detecting metastatic LNs ≥1 cm. LNs <1 cm with an SUVmax lower than 1.4 were likely to be benign.
References
Singh B, Ezziddin S, Palmedo H, Reinhardt M, Strunk H, Tuting T, et al. Preoperative 18F-FDG-PET/CT imaging and sentinel node biopsy in the detection of regional lymph node metastases in malignant melanoma. Melanoma Res. 2008;18:346–52.
Kim SY, Yun SJ. Cutaneous melanoma in Asians. Chonnam Med J. 2016;52:185–93.
Patel JK, Didolkar MS, Pickren JW, Moore RH. Metastatic pattern of malignant melanoma. A study of 216 autopsy cases. Am J Surg. 1978;135:807–10.
Leiter U, Meier F, Schittek B, Garbe C. The natural course of cutaneous melanoma. J Surg Oncol. 2004;86:172–8.
Dummer R, Hauschild A, Lindenblatt N, Pentheroudakis G, Keilholz U; ESMO Guidelines Committee. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v126–32.
Belhocine T, Pierard G, De Labrassinne M, Lahaye T, Rigo P. Staging of regional nodes in AJCC stage I and II melanoma: 18FDG PET imaging versus sentinel node detection. Oncologist. 2002;7:271–8.
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Melanoma. Fort Washington, PA: National Comprehensive Cancer Network; 2016.
Xing Y, Bronstein Y, Ross MI, Askew RL, Lee JE, Gershenwald JE, et al. Contemporary diagnostic imaging modalities for the staging and surveillance of melanoma patients: a meta-analysis. J Natl Cancer Inst. 2011;103:129–42.
Jimenez-Requena F, Delgado-Bolton RC, Fernandez-Perez C, Gambhir SS, Schwimmer J, Perez-Vazquez JM, et al. Meta-analysis of the performance of (18)F-FDG PET in cutaneous melanoma. Eur J Nucl Med Mol Imaging. 2010;37:284–300.
Lowe VJ, Hoffman JM, DeLong DM, Patz EF, Coleman RE. Semiquantitative and visual analysis of FDG-PET images in pulmonary abnormalities. J Nucl Med. 1994;35:1771–6.
Truong MT, Ko JP, Rossi SE, Rossi I, Viswanathan C, Bruzzi JF, et al. Update in the evaluation of the solitary pulmonary nodule. Radiographics. 2014;34:1658–79.
Hellwig D, Graeter TP, Ukena D, Groeschel A, Sybrecht GW, Schaefers HJ, et al. 18F-FDG PET for mediastinal staging of lung cancer: which SUV threshold makes sense? J Nucl Med. 2007;48:1761–6.
Reinhardt MJ, Joe AY, Jaeger U, Huber A, Matthies A, Bucerius J, et al. Diagnostic performance of whole body dual modality 18F-FDG PET/CT imaging for N- and M-staging of malignant melanoma: experience with 250 consecutive patients. J Clin Oncol. 2006;24:1178–87.
Crippa F, Leutner M, Belli F, Gallino F, Greco M, Pilotti S, et al. Which kinds of lymph node metastases can FDG PET detect? A clinical study in melanoma. J Nucl Med. 2000;41:1491–4.
Giesel FL, Schneider F, Kratochwil C, Rath D, Moltz J, Holland-Letz T, et al. Correlation between SUVmax and CT radiomic analysis using lymph node density in PET/CT-based lymph node staging. J Nucl Med. 2017;58:282–7.
Perng P, Marcus C, Subramaniam RM. (18)F-FDG PET/CT and melanoma: staging, immune modulation and mutation-targeted therapy assessment, and prognosis. AJR Am J Roentgenol. 2015;205:259–70.
Rodriguez Rivera AM, Alabbas H, Ramjaun A, Meguerditchian AN. Value of positron emission tomography scan in stage III cutaneous melanoma: a systematic review and meta-analysis. Surg Oncol. 2014;23:11–6.
Strauss LG. Sensitivity and specificity of positron emission tomography (PET) for the diagnosis of lymph node metastases. Recent Results Cancer Res. 2000;157:12–9.
Wagner JD, Schauwecker D, Davidson D, Logan T, Coleman JJ 3rd, Hutchins G, et al. Inefficacy of F-18 fluorodeoxy-D-glucose-positron emission tomography scans for initial evaluation in early-stage cutaneous melanoma. Cancer. 2005;104:570–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Jongtae Cha, Soyoung Kim, Jiyoung Wang, Mijin Yun, and Arthur Cho all declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Informed Consent
The institutional review board approved this retrospective study and the need for informed consent was waived.
Rights and permissions
About this article
Cite this article
Cha, J., Kim, S., Wang, J. et al. Evaluation of 18F-FDG PET/CT Parameters for Detection of Lymph Node Metastasis in Cutaneous Melanoma. Nucl Med Mol Imaging 52, 39–45 (2018). https://doi.org/10.1007/s13139-017-0495-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13139-017-0495-4