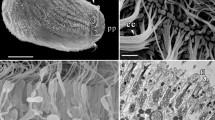
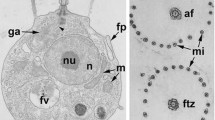
Abstract
The kinetid (flagellar/ciliary apparatus) of eukaryotic cells is an important source of phylogenetic information. It was found to be a prospective morphological phylogenetic marker in sponges, since its arrangement in choanocytes is congruent with the topology of the phylogenetic trees. However, investigation of the kinetid of sponge larval cells remains fragmentary. Here, we report the results of an ultrastructural study on the larval kinetids of the freshwater sponges Eunapius fragilis and Lubomirskia baikalensis (Demospongiae: Spongillida). Their kinetids were found to comprise a kinetosome associated with an accessory centriole and linked to the nucleus by a simple fibrillar root. The kinetosome bears a transverse cytoskeleton: filamentous train and microtubules which radiate from a microtubule organising centre (MTOC) shaped as a large hollow foot. In the short transition zone, between the central axonemal microtubules and kinetosome, a transverse plate with an axosome (central thickening) occurs. We have reviewed the kinetids of different sponge larvae to reconstruct the ancestral state of these traits. Thus, we suggest that spongillids retain the plesiomorphic characteristics of roots and an accessory centriole. But they possess the peculiarities of the transition zone, transverse cytoskeleton and MTOC structure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sponges (the phylum Porifera) are among the most ancient invertebrates on our planet (Simion et al. 2017; Nielsen 2019). They are crucial to interpreting the nature of the earliest animals. Researchers look into their simply organised bodies seeking features inherited from the dawn of metazoan history.
Sponges have only a few morphotraits available for comparison and classification. The main trait, skeleton structure, was evaluated somewhat differently and led to different conclusions (compare Hartman 1958; Berquist 1978; Hooper and van Soest 2002). When genetic analysis provided topological stability at the level of classes (Cárdenas et al. 2012; Voigt et al. 2012; Morrow and Cárdenas 2015), the opportunity arose to trace informative morphological features.
Kinetid (flagellar/ciliary apparatus) structure is a suitable feature for phylogenetic analysis. The kinetid consists of three main parts: (1) a free part (the flagellum/cilium); (2) a basal intracellular part, normally comprising two kinetosomes (the non-flagellated one is often called an accessory centriole) with attached microtubular or fibrillary rootlets; and (3) a transition zone connecting the free and basal parts of the flagellum (Lynn and Small 1981; Moestrup 1982 and others).
Kinetid structure is diverse in choanocytes. Previous studies showed that choanocyte kinetid structures of sponges are in agreement with the phylogenetic tree of Porifera, allowing a proposition of the ancestral choanocyte’s kinetid type (Pozdnyakov et al. 2017, 2018).
In addition to choanocytes, sponges have other essential flagellated cells—the covering cells of larvae. Sponge larvae are motile and relatively sophisticated organisms. Their morphological and behavioural complexity, which brings them closer to Eumetazoa, has long fascinated researchers. The larval flagellum serves for moving a larva through the water column, while the choanocyte flagellum creates a water flow through the sponge body. During metamorphosis, larval flagellated cells in calcareans and demosponges lose the flagellum and de-differentiate (Ivanova 1997b; Amano and Hori 2001; Ereskovsky and Willenz 2008; Gonobobleva and Ereskovsky 2004; Leys and Degnan 2002; Usher and Ereskovsky 2005; Sogabe et al. 2016). Kinetids in larval and adult cells of sponges differ in varying degrees (Amano and Hori 1996, 2001; Gonobobleva and Maldonado 2009; Maldonado 2009; Sokolova et al. 2019). These facts raise the questions of whether larval kinetids could have evolved independently and what the ancestral larval kinetid might have looked like?
It is not easy to obtain a comprehensive collection of sponge larvae, as many species have a short breeding periods, often with unknown time frames, or produce few larvae (Maldonado 2006; Ereskovsky et al. 2007). But data on the accessible species have been accumulated, and this study will complement them. The present work explores the larvae of Spongillida, the only freshwater order of Porifera. This taxon was traditionally classified within the marine order Haplosclerida. In Systema Porifera, it was assigned to Haplosclerida as the suborder Spongillina (Manconi and Pronzato 2002). Later, genetic data supported changing its status to a separate order, Spongillida, within the subclass Heteroscleromorpha (Morrow and Cárdenas 2015). Haplosclerida was recently shown to stand apart from the rest of Heteroscleromorpha (Morrow and Cárdenas 2015). Accordingly, the majority of data place Spongillida and Haplosclerida distant from one another (Morrow et al. 2012, 2013; Morrow and Cárdenas 2015; Simion et al. 2017). But data in some studies imply a joining of the Spongillida and Haplosclerida branches (Sperling et al. 2009; Hill et al. 2013). However, these studies have not been widely recognised due to the small sample size (Morrow and Cárdenas 2015), but the same outcome has continued to emerge in recent years (Schuster et al. 2018). Thus, the relationship between Haplosclerida and Spongillida remains uncertain. Interestingly, the choanocyte kinetids of haplosclerids and spongillids are similar to each other and different from the rest of the sponges (Pozdnyakov et al. 2018). Studying the kinetids of larval cells might provide additional information on their relationship.
We have studied the ultrastructure of kinetids in larval cells of the freshwater sponges Eunapius fragilis (Leidy, 1851) (family Spongillidae) and Lubomirskia baikalensis (Pallas, 1776) (family Lubomirskiidae). In addition to describing their kinetids, we intend to (1) suggest which characteristics of the kinetid resemble the ancestral kinetid, (2) estimate similarities between kinetid structures in the proposed ancestral larva and the adult, and (3) compare kinetids of spongillids and haplosclerids.
Materials and methods
Sample preparation
Eunapius fragilis specimens with mature larvae were collected in the Moscow Channel (Moscow region, Russia) in the middle of June 2017. Larvae were sampled by means of a glass Pasteur pipette, after putting a sponge in a tank with stagnant water. Larvae were fixed and prepared for electron microscopy according to the following protocol. For prefixation, 1 ml of 1% osmium tetroxide in cacodylate buffer (0.1 mol l−1, pH 7.4) was added to 3 ml of water containing the larvae. Next, 4 ml of 4% glutaraldehyde in the same buffer was added and the larvae were kept in this mixture for 15 min on ice in darkness. Then, the fixative mixture was replaced by 2% glutaraldehyde for 1 h on ice. Afterwards, the larvae were rinsed in buffer twice and postfixed in 1% osmium tetroxide for 1 h at room temperature.
The samples were then washed twice for 10 min in the same buffer, dehydrated in a graded ethanol series and embedded in Spurr resin. After polymerization, the resin blocks containing larvae were trimmed and treated with 10% hydrofluoric acid for 5 min to remove siliceous skeletal elements. Ultrathin sections (60 nm) were cut with a Leica EM UC6 ultramicrotome using a glass knife. The sections were mounted on Formvar-coated oval hole grids, double stained in 2% aqueous uranyl acetate (15 min) and afterwards in 2% lead citrate (3 min) and viewed with a JEM 1400 electron microscope equipped with an Olympus Veleta digital camera. Serial consecutive sections were studied.
Branch portions of Lubomirskia baikalensis were collected in Lake Baikal near Cape Listvenichny at a depth of 10–15 m using SCUBA in March 2017. The samples were immediately placed in containers with Baikal water where larvae were sampled by means of a glass Pasteur pipette. Fixation for semi-thin sections and TEM was performed according to the following protocol: pre-fixation in 1% OsO4, 10 min; washing in cacodylate buffer (0.1 mol l−1, pH 7.4), 10 min; fixation in 1.5% glutaraldehyde solution in cacodylate buffer, 1 h; washing in cacodylate buffer, 30 min; post-fixation in 1% OsO4 solution in cacodylate buffer, 2 h; washing in distillated water three times for 15 min at room temperature; dehydration in increasing concentrations of ethanol (30, 50, 70, 90 and 100%, 20 min in each) at room temperature; and finally embedding in Araldite resin. Blocks were then processed as previously described. Semi-thin sections (60–80 nm) were cut with an Ultramicrotome PowerTome XL and stained with 4% aqueous uranyl acetate. Digital photographs were taken with a Leica DMLB microscope.
Descriptions follow the terminology used by Andersen et al. (1991) and Woollacott and Pinto (1995).
Ancestral character state reconstruction
The phylogeny of taxa represented in this study was reconstructed using the maximum likelihood method implemented in RaxML (Stamatakis 2014) and Bayesian inference implemented in MrBayes (Ronquist and Huelsenbeck 2003) on 18S data from the GenBank database aligned using MUSCLE multiple alignment algorithm (Edgar 2004) (Suppl. Figs. 1, 2). A generalised phylogram based on recovered phylogenies was built for further manipulation. We used Mesquite (Maddison and Maddison 2019) to trace eight standard categorical characters (Suppl. Table 1) under the most parsimonious model for polymorphic terminal taxa data.
We took into account available data on most species (Suppl. Table 1). Crambe crambe (Schmidt, 1862) (Uriz et al. 2001) and Cacospongia mollior Schmidt, 1862 (Uriz et al. 2008) were excluded from the analysis as the position of these genera on the tree is not known. Haliclona indistincta (Bowerbank, 1866) (Stephens et al. 2013) was excluded because it is plausible that it was misassigned to Haplosclerida (for details, see Sokolova et al. 2019). Scopalina lophyropoda Schmidt, 1862 (Uriz et al. 2008), Chondrosia reniformes Nardo, 1847 (Lévi and Lévi 1976) and Halichondria moorei Bergquist, 1961 (Evans 1977) were not clearly illustrated for our purposes. Since information was not complete in some cases, we had to extrapolate the species data to the entire clade (Suppl. Table 1). A total of 38 sponge species from 15 monophyletic clades were included in the analysis. The names of clades are given in accordance with current classification (Van Soest et al. 2020), which does not, however, fully correspond with the molecular phylogeny at family level.
Results
The larvae of spongillid sponges are a peculiar parenchymella, often with a significant internal cavity in the anterior part (Fig. 1a). They are covered by relatively short, slightly elongated cells.
Kinetid structure of larval cells in Eunapius fragilis
Cells of the external layer in Eunapius fragilis larvae have an irregular shape but tightly contact each other in the distal zone (Fig. 1b). They are more or less wide, generally elongated in an apical-basal direction (Fig. 1b). The flagellum emerges from a deep apical pit and bears one to four flagellar vanes (Fig. 1b). The axoneme is conventional with a 9 × 2 arrangement of microtubules.
The transition zone of the flagellum is short. The central microtubules reach level of the cell surface and connect there to an axosome, a thickening in the middle of the transverse plate located on the distal end of kinetosome (Fig. 2c, f, h, i, k, n, o).
Ultrastructure of the kinetid in larval cells of Eunapius fragilis, longitudinal plane. Consecutive sections of three cells (a–d, e–g, i–j) and separate sections of six cells (h, k–o). Scale bars a–l 200 nm, m–o 250 nm. Abbreviations: afb, apical filamentous bundle; axs, axosome; bfb, basal filamentous bundle; bf, basal foot; fb, fibrillar bridge between the kinetosome and the centriole; c, centriole; cm, central microtubules; dz., dark zone; fr, fibrillar root; ftr, filamentous train; k, kinetosome; lmt, lateral microtubules; n, nucleus; pc, points of connection of transition fibres to the plasma membrane; tc, transition cylinder; tf, transition fibre; tp, transverse plate; Y-l, Y-linkers
A so-called dark zone of the flagellum, which seems to be characteristic for all cilia and flagella of sponges, locates at about 100 nm distal to the transverse plate (Fig. 2m–o). It is filled with electron-dense material for a length of approximately 150 nm. It includes a transitional cylinder distinguishable on the inside of peripheral microtubules (Fig. 2m, n). Outside of the axoneme, we observed Y-linkers: electron-dense fibres radiating from the microtubules to the flagellar membrane (Fig. 2m–o).
Nine transition fibres (=alar sheets) start from the apical edge of the kinetosome (Fig. 2b; Fig. 3a, e, f, j). A single microtubule organising centre (MTOC) on the kinetosome surface is a basal foot of an unusual shape. It looks like a fairly long (200 nm length) expanding tube with electron-dense walls (Fig. 2b, e, f, h, i, k, m; Fig. 3a, b, f, g, h, i). This tube is hollow, closed at the distal end and has no distinct head. The lateral microtubules start from the basal foot and radiate in different directions (Fig. 2b, g, k, m; Fig. 3b, c, f).
Ultrastructure of the kinetid in larval cells of Eunapius fragilis, transverse plane. Consecutive sections of three cells (a–d, e–g, i–k) and a section of another cell (h). Scale bar 200 nm. Abbreviations: bf, basal foot; c, centriole; ftr, filamentous train; lmt, lateral microtubules; k, kinetosome; tf, transition fibre
Opposite to the foot, several bundles of the filaments (Fig. 2c–e, h–k) arise from the kinetosome. Two of the bundles are prominent in longitudinal sections: apical and basal bundles (Fig. 2c–e, j). Close to the kinetosome, where the bundles arise, the filamentous material is so dense that sometimes it looks like a small dense body (Fig. 2c). The bundles expand and extend parallel to the plasma membrane for quite a long distance (up to 1.1 μm) and form a common filamentous train (Fig. 2j; Fig. 3a–d, f, g).
A broad fibrillar root connects the proximal end of the kinetosome to the nucleus (Fig. 2d, h, j, k, m, n). The nucleus occupies an apical, though in some cases a rather middle position in the cell, and has a beak connected to the fibrillar root (Fig. 2l).
The accessory centriole is located mostly at the side of the foot, slightly basal to the kinetosome and at a sharp angle to it (30–60° depending on the position of the nucleus and the shape of the cell) (Fig. 2b, c, e–h, i, k).
The centriole is connected to the kinetosome by a bridge of fibrillar material (Fig. 2h, i). The angle between the centriole and kinetosome axis varies from 30° to 60° depending on the nuclear position and the shape of the cell.
Kinetid structure of larval cells in Lubomirskia baikalensis
The kinetid of flagellated cells in Lubomirskia baikalensis larvae is similar to that in Eunapius fragilis (Fig. 4). However, some quantitative differences are noteworthy. In L. baikalensis, more filamentous bundles are attached to the kinetosome; that is why the filamentous train coming from the kinetosome is larger and denser than in E. fragilis (Fig. 4a–d, g). The fibrillar roots of L. baikalensis are generally longer than those in E. fragilis (Fig. 4b, c, e vs. Fig 2d, h–j). The centriole orientation relative to the kinetosome in L. baikalensis varies from nearly orthogonal to almost parallel in this species (Fig. 4e, g). Its position is more variable than in E. fragilis and less often associated with the position of the basal foot, but the top of the centriole is also mostly directed towards the basal foot. As in E. fragilis, the arrangement of the centriole apparently depends on the position of the nucleus in the cell.
Ultrastructure of the kinetid in larval cells of Lubomirskia baikalensis, longitudinal plane. a–c Series of consecutive sections through a cell. d–g Sections through the kinetid of different cells. Scale bars a–d, f, g 200 nm; e 250 nm. Abbreviations: afb, upper filamentous bundle; axs, axosome; bf, basal foot; bfb, basal filamentous bundle; c, centriole; cm, central microtubules; fb, fibrillar bridge; fr, fibrillar root; ftr, filamentous train; k, kinetosome, pc, points of connection of transition fibres to the plasma membrane
The principal scheme of kinetid structure in L. baikalensis and E. fragilis larval cells is summarized at Fig. 5.
Schematic reconstruction of the kinetid of a spongillid larva from lateral view. Abbreviations: afb, apical filamentous bundle; bfb, basal filamentous bundle; bf, basal foot; c, centriole; cm, central microtubules; dz, dark zone; fl, flagellum; fb, fibrillar bridge between the kinetosome and centriole; fr, fibrillar root; ftr, filamentous train; Ga, Golgi apparatus; k, kinetosome; lmt, lateral microtubules; n, nucleus; pr, cell protrusion; tc, transition cylinder; tf, transition fibre; tp+axs, transverse plate with axosome
Ancestral state reconstruction
Character state reconstructions illustrated following ancestral features in sponges: the accessory centriole presence, a MTOC shaped as a simple basal foot, the absence of additional cytoskeletal structures such as lateral bundles or lateral arms and a striated root (Fig. 6; Supl. Fig. 3).
The scheme of possible evolution of kinetid elements in sponge larval cells. Lateral bundle: a, filamentous; b, microtubular; c, absent. Roots: a, striated; b, simple unstriated; c, tubular; d, laminar. MTOC: a, simple basal foot; b, complex; c, large hollow foot. Accessory centriole: a, present; b, absent. Kinetosome-nucleus-link: a, absent; b, present. Lateral arm (imaged as a transverse section through the kinetosome): a, double; b, single; c, absent
The accessory centriole disappeared twice: in the Mycalidae group and the Haplosclerida order. MTOC transformations occurred on three occasions: in the order Spongillida, it took the shape of a large hollow tube, while in mycalids and some haplosclerids, it became a multi-component structure. The additional lateral bundle is found in the Irciniidae family, the Spongillida order and in the Calcarea+Homoscleromorpha lineage. This bundle is of microtubular nature in Homoscleromorpha, filamentous in Spongillida and unclear, possibly mixed, in Irciniidae and Calcarea. Lateral arms appeared twice: single in some Haplosclerida and double in the only investigated Dendroceratida (for description, see Woollacott and Pinto 1995; Sokolova et al. 2019).
Ancestral larval roots are shown to be striated, but larvae of the common ancestor of the Demospongiae had simple unstriated roots. In the order Poecilosclerida and subclass Verongimorpha complicated laminar roots appeared independently (for description, see Woollacott and Pinto 1995). In the order Haplosclerida the roots are also complicated, shaped as hollow large tubes (for description see Sokolova et al. 2019).
The kinetid linked to the beak of the pear-shaped nucleus may be ether derived or ancestral. It was found in the subclass Verongimorpha, orders Spongillida and Suberitida, class Calcarea and in at least some cells of the class Homoscleromorpha. The roundish nucleus separated from the kinetid was found in the orders Poecilosclerida and Haplosclerida, subclass Keratosa and class Homoscleromorpha.
The outgroup (choanoflagellates) shares with sponges striated roots and the accessory centriole, but shows differences in MTOC structure and the pattern of microtubules divergence, and the lack the dark zone (Suppl. Table 1, Suppl. Fig. 3).
Discussion
The kinetid in the observed spongillid larvae has these main elements: a fibrillar root connecting the kinetosome and nucleus, an accessory centriole located at a sharp to blunt angle to the kinetosome, a long hollow basal foot (MTOC) with radiating lateral microtubules, a filamentous train deriving from the kinetosome, transitional fibres, and a short transition zone with the transverse plate and axosome. Comparing kinetid composition in different branches of the sponge evolutionary tree allows us to trace the fate of kinetid elements. Then, we can seek to identify the apomorphic (derived) and plesiomorphic (ancestral) states of the kinetid of spongillid larvae. To meet this goal, we performed an ancestral state reconstruction analysis. Here, we will discuss the kinetid elements according to their position in the cell, proceeding in a basal to apical direction.
Our results imply that the ancestral root of sponge larval cells could be striated. This is plausible, because striated roots are present in the larvae of Calcarea and Homoscleromorpha (Boury-Esnault et al. 2003; Ereskovsky and Ereskovsky and Willenz 2008, Pozdnyakov et al. 2020 and others) and in the embryos of Demospongiae: the chondrillid Halisarca dujardini Johnston, 1842 (Gonobobleva 2007) and the poecilosclerid Lycopodina occidentalis (Lambe, 1893) (Riesgo et al. 2007). In H. dujardini (Gonobobleva 2007), the striation is shown to be ‘fluid’: it appears in embryos, disappears in larvae and reappears in adults. Striated roots are also common in Eumetazoa and diverse protists (e.g. Dingle and Larson 1981; Flammang et al. 1994; Tamm and Tamm 2002; Karpov 2000). Further studies will enable us to estimate the actual distribution of root structures among Porifera. Our results show that the simple fibrillar root replaced a striated one in Demospongiae. Spongillid roots, therefore, must be an ancient structure too. This structure is simple and looks unspecific, unlike the long, complicated roots of Poecilosclerida (Woollacott and Pinto 1995) or Haplosclerida (Sokolova et al. 2019).
The accessory centriole is a common structure in sponge larvae, and its presence in the reconstructed ancestral state seems not surprising. Its position may vary in different cells within the same larva of spongillids and other sponges but is fixed under the kinetosome in Homoscleromorpha larvae (Pozdnyakov et al. 2020).
The kinetosome of spongillids is anchored in the cells not only by the root but also by a transverse cytoskeleton of distinctive appearance. As in other sponges, it consists of lateral microtubules radiating from the MTOC, which has an unusual shape. A large, homogeneous hollow foot replaces the typical simple basal foot, which consists of a distal head attached to the kinetosome by stalks. Moreover, the additional filamentous train occurs on the other side of the kinetosome. This train was previously observed in larvae of another freshwater species, Baikalospongia bacillifera Dybowsky, 1880 and was thought to be a microfilament bundle (Efremova et al. 1988). Structures resembling this train are seen in sections of calcarean larvae (Borojevic 1969; Amano and Hori 2001; Ereskovsky and Ereskovsky and Willenz 2008; Lanna and Klautau 2012). They are considered by authors to be a microtubule bundle. In some cases, they resemble microtubules due to their diameter and arrangement (see Fig. 17 in Borojevic 1969), but other images do not exclude their filamentous nature (Amano and Hori 2001; Ereskovsky and Ereskovsky and Willenz 2008; Lanna and Klautau 2012), as was also supposed earlier (Efremova et al. 1988). More detailed studies are needed to confirm or deny the similarity of the transverse skeleton in Spongillida and Calcarea. Here, we consider the lateral bundle of Calcarea to be of unclear, probably mixed nature, as well as in the dictyoceratid Ircinia oros (Schmidt, 1864) (Ereskovsky and Tokina 2004); in Homoscleromorpha, it was shown to be microtubular (Pozdnyakov et al. 2020). Thus, the filamentous train of Spongillida is either rare or unique among Demospongiae.
As in other eukaryotes, the apical part of the kinetosome is attached to the plasma membrane by transitional fibres. Above it, the kinetosome transits to the axoneme. The transition zone between the kinetosome and central microtubules is considered to weakly depend on the conditions of flagellum function, so it is evolutionarily conservative (Moestrup 1982, 2000; Melkonian 1982; Andersen et al. 1991; Karpov and Fokin 1995; Karpov 2000, 2016). Aside from spongillids, a short transition zone with transverse plate in larvae is described in Haplosclerida (Sokolova et al. 2019) and distinguishable in dendroceratid Aplysina aerophoba (Nardo, 1833) (Maldonado 2009). Thus, this feature occurs in sponges that are not closely related. A variant of transition zone structure is a long zone without a transverse plate but with an axial granule in the lumen of the kinetosome apex. This was found in also distantly related demosponge taxa (subclasses Keratosa and Verongimorpha, orders Suberitida and Poecilosclerida) and occurred in larval cells of calcarean and homoscleromorph sponges (Gonobobleva 2007; Uriz et al. 2008; Sokolova et al. 2019; personal unpublished data on Hymedesmia irregularis Lundbeck, 1910). This thin structure is rarely clear in published illustrations since it requires good fixation and high-resolution imaging mode. More data are needed to estimate its phylogenetic signal.
Structures of the so-called dark zone above the transversal plate are usually not recognisable in sections because of unsuitable fixation, but this zone is present in all investigated sponge flagella (Pozdnyakov et al. 2017). Previously, we demonstrated that this dark zone is composed of a transition cylinder connected to peripheral doublets and a sheath encircling central microtubules (Sokolova et al. 2019). Here, we add that the dark zone includes Y-linkers connecting the axoneme with the flagellar membrane (seen also in Sokolova et al. 2019, Fig. 2e–h).
Kinetids in adult and larval sponge cells can be associated with the nucleus (Pozdnyakov et al. 2018; Suppl. Table 1). In these cases, the nucleus is pear-shaped and its beak contacts the roots. In the absence of such a link, the nucleus is roundish and usually situated in the basal part of the cell. This character may be prone to homoplasy. We cannot yet conclude which state was initial in larvae of the entire sponge lineage. But we suggest it was more likely the presence of the link at least in demosponge larvae, since their simple ancestral non-striated root is always associated with the nucleus. The link is absent only when a long laminar or tubular root appears. Disconnection of the nucleus and kinetosome therefore correlates with the derived state and probably is a derived state itself.
Thus, we suggest that the spongillid larvae acquired its hollow tubular foot and filamentous lateral train during the evolutionary process, and that these characteristics should be considered as apomorphies. Spongillids share with the proposed demosponge ancestral larva the following features: an accessory centriole, a simple fibrillar root, the absence of a lateral arm, and probably, a kinetosome-nucleus connection.
Comparison of larval cell kinetids in Haplosclerida and Spongillida shows that the haplosclerid kinetid has more traits that have diverged far from the ancestral state of demosponges. While the kinetid modifications in spongillids are the unusual hollow basal foot and long fibrillar bundle, in haplosclerids, they are centriole loss, tubular roots and, in some cases, a complex basal foot and lateral arm. Unlike spongillids, haplosclerids have a disconnected kinetid and nucleus. Thus, kinetid structures of larval cells in these orders differ notably, although they share the transverse plate in the transition zone. Meanwhile, choanocytes of Spongillida and Haplosclerida are related by the absence of a kinetosome-nucleus connection and accessory centriole, and the presence of the transverse plate; this character set distinguishes them from the rest of sponges (Pozdnyakov et al. 2018). The relationships between these orders are still debatable (for details see “Introduction”). While choanocyte kinetid structure can challenge the separation of these orders, the structure of the larval kinetid confirms their distant position.
After metamorphosis, the flagellar vanes are maintained in choanocytes, in spite of resorption of the flagellum (Ivanova 1997b). The newly assembled kinetid of spongillids’ choanocytes retains the transverse plate with the axosome in the transitional zone of flagellum. But it loses its nuclear connection and its permanent accessory centriole, and its MTOC becomes an electron-dense ring around the kinetosome. Thus, the choanocyte kinetid of spongillid adults differs from that in both their larvae and the suggested ancestral choanocyte (Pozdnyakov et al. 2018).
Comparing the inferred larval and choanocyte kinetids of the ancestral sponge reveals similarities: both have a simple basal foot, accessory centriole and no extra cytoskeleton structures. But the ancestral larval cell possessed a striated root, which was replaced by the simple fibrillar root in the Demospongiae lineage. In choanocytes, the simple root apparently emerged in most basal nodes (Pozdnyakov et al. 2018). The kinetid of the supposed ancestral choanocyte was connected to the kinetosome, although it lost this linkage in several lineages (Pozdnyakov et al. 2018, 2020). So, it might also be the same in larval cells. In this case, the main difference between the ancestral kinetid of larvae and adults is the root structure. Generally, kinetids in sponge larvae are prone to strengthen the transverse skeleton—cytoskeletal bundles or lateral arms that have never been encountered in choanocytes. Their longitudinal skeleton (roots) also tends to be longer and stronger. This might result from the different, probably higher, load that larval flagellum faces.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Amano, S., & Hori, I. (1992). Metamorphosis of calcareous sponges I. Ultrastructure of free-swimming larvae. Invertebrate Reproduction and Development, 21(2), 81–90. https://doi.org/10.1080/07924259.1992.9672223.
Amano, S., & Hori, I. (1994). Metamorphosis of a demosponge i. Cells and structure of swimming larva. Invertebrate Reproduction and Development, 25(3), 193–204. https://doi.org/10.1080/07924259.1994.9672386.
Amano, S., & Hori, I. (1996). Transdifferentiation of larval flagellated cells to choanocytes in the metamorphosis of the demosponge Haliclona permollis. Biological Bulletin, 190(2), 161–172. https://doi.org/10.2307/1542536.
Amano, S., & Hori, I. (2001). Metamorphosis of coeloblastula performed by multipotential larval flagellated cells in the calcareous sponge Leucosolenia laxa. Biological Bulletin, 200(1), 20–32. https://doi.org/10.2307/1543082.
Andersen, R. A., Barr, D. J. S., Lynn, D. H., Melkonian, M., Moestrup, & Sleigh, M. A. (1991). Terminology and nomenclature of the cytoskeletal elements associated with the flagellar/ciliary apparatus in protists. Protoplasma, 164, 1–8. https://doi.org/10.1007/BF01320809.
Berquist, P. R. (1978). Sponges. London: Hutchinson.
Borojevic, R. (1969). Etude du développement et de la differentiation cellulaire d’éponges calcaires Calcinées (genres Clathrina et Ascandra). Annales d'Embryologie et de Morphogenèse, 2, 15–36.
Boury-Esnault, N. (1976). Ultrastructure de la larve parenchymella d’Hamigera hamigera (Poecilosclerida). Origine des cellules grises. Cahiers de Biologie Marine, 17, 9–20.
Boury-Esnault, N., Ereskovsky, A., Bézac, C., & Tokina, D. (2003). Larval development in the Homoscleromorpha (Porifera, Demospongiae). Invertebrate Biology, 122(3), 187–202. https://doi.org/10.1111/j.1744-7410.2003.tb00084.x.
Cárdenas, P., Pérez, T., & Boury-Esnault, N. (2012). Sponge systematics facing new challenges. Advances in Marine Biology, 61, 79–209. https://doi.org/10.1016/B978-0-12-387787-1.00010-6.
Dingle, A. D., & Larson, D. E. (1981). Structure and protein composition of the striated flagellar rootlets of some protists. BioSystems, 14, 345–358.
Edgar, R. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research, 32(5), 1792–1797. https://doi.org/10.1093/nar/gkh340.
Efremova, S., Sukhodolskaya, A., & Alekseeva, N. (1988). The different structure of kinetosome rootlet systems in flagellated cells of the larvae and the choanocytes of sponges. In Modern and Perspective Investigations. Porifera and Cnidaria (pp. 22–23). Leningrad: USSR Academy of Sciences, Zoological institute.
Ereskovsky, A. V., & Tokina, D. B. (2004). Morphology and fine structure of the swimming larvae of Ircinia oros (Porifera, Demospongiae, Dictyoceratida). Invertebrate Reproduction and Development, 45(2), 137–150. https://doi.org/10.1080/07924259.2004.9652583.
Ereskovsky, A. V., & Willenz, P. (2008). Larval development in Guancha arnesenae (Porifera, Calcispongiae, Calcinea). Zoomorphology, 127, 175–187. https://doi.org/10.1007/s00435-008-0061-9.
Ereskovsky, A. V., Tokina, D. B., Bezac, C., & Boury-Esnault, N. (2007). Metamorphosis of Cinctoblastula larvae (Homoscleromorpha, Porifera). Journal of Morphology, 268, 518–528. https://doi.org/10.1002/jmor.10506.
Evans, C. W. (1977). The ultrastructure of larvae from the marine sponge Halichondria moorei Bergquist (Porifera, Desmospongiae). Cahiers de Biologie Marine, 18(1), 427–433.
Flammang, P., Demeulenaere, S., & Jangoux, M. (1994). The role of podial secretions in adhesion in two species of sea stars (Echinodermata). Biological Bulletin, 187, 35–47. https://doi.org/10.2307/1542163.
Galissian, M.-F., & Vacelet, J. (1992). Ultrastructure of the oocyte and embryo of the calcified sponge, Petrobiona massiliana (Porifera, Calcarea). Zoomorphology, 112, 133–141.
Gallissian, M.-F. (1983). Etude ultrastructurale du developpement embryonnaire chez Grantia compressa F (Porifera, Calcarea). Archives d’Anatomie Microscopique, 72(1), 59–75.
Gonobobleva, E. (2007). Basal apparatus formation in external flagellated cells of Halisarca dujardini larvae (Demospongiae: Halisarcida) in the course of embryonic development. Porifera Research: Biodiversity, Innovation and Sustainability (pp. 345–351).
Gonobobleva, E., & Ereskovsky, A. (2004). Metamorphosis of the larva of Halisarca dujardini (Demospongiae, Halisarcida). Bulletin de l’Institut Royal des Sciences naturelles de Belgique, Biologie, 74, 101–115.
Gonobobleva, E., & Maldonado, M. (2009). Choanocyte ultrastructure in Halisarca dujardini (Demospongiae, Halisarcida). Journal of Morphology, 270, 615–627. https://doi.org/10.1002/jmor.10709.
Hartman, W. D. (1958). A re-examination of Bidder’s classification of the Calcarea. Systematic Zoology, 7, 55–109. https://doi.org/10.2307/2411971.
Hill, M., Hill, A., Lopez, J., Peterson, K., Pomponi, S., Diaz, M., et al. (2013). Reconstruction of family-level phylogenetic relationships within Demospongiae (Porifera) using nuclear encoded housekeeping genes. PLoS One, 8(1), e50437.
Hooper, J. N. A., & van Soest, R. W. M. (2002). Class Demospongiae Sollas, 1885. In J. N. A. Hooper & R. W. M. van Soest (Eds.), Systema Porifera: A guide to the classification of sponges (pp. 15–18). New York: Kluwer. https://doi.org/10.1007/978-1-4615-0747-5_3.
Ivanova, L. V. (1997a). New data about morphology and metamorphosis of the spongillid larvae (Porifera, Spongillidae). 1. Morphology of the free-swimming larvae. In A. V. Ereskovsky, H. Keupp, & H. R. Kohring (Eds.), Modern problems of Poriferan biology (pp. 55–71). Berlin: Berliner Geowiss Abh, Freie University.
Ivanova, L. V. (1997b). New data about morphology and metamorphosis of the spongillid larvae (Porifera, Spongillidae). 2. The metamorphosis of the spongillid larva. In A. V. Ereskovsky, H. Keupp, & H. R. Kohring (Eds.), Modern problems of Poriferan biology (pp. 73–91). Berlin: Berliner Geowiss Abh, Freie University.
Karpov, S. A. (2000). Flagellate phylogeny: Ultrastructural approach. In J. Green & B. Leadbeater (Eds.), The flagellates (pp. 336–360). London: Taylor and Francis.
Karpov, S. A. (2016). Flagellar apparatus structure of choanoflagellates. Cilia, 5(1), 1–5. https://doi.org/10.1186/s13630-016-0033-5.
Karpov, S. A., & Fokin, S. I. (1995). The structural diversity of flagellar transitional zone in heterotrophic flagellates and other protists. In S. A. Karpov (ed.), The biology of free-living heterotrophic flagellates. Tsitologia (vol. 37, pp. 1038–1052).
Lanna, E., & Klautau, M. (2012). Embryogenesis and larval ultrastructure in Paraleucilla magna (Calcarea, Calcaronea), with remarks on the epilarval trophocyte epithelium (‘placental membrane’). Zoomorphology, 131, 277–292. https://doi.org/10.1007/s00435-012-0160-5.
Lévi, C. (1964). Ultrastructure de la larve parenchymella de démosponge. I: Mycale contarenii. Cahiers de Biologie Marine, 5, 97–104.
Lévi, C., & Lévi, P. (1976). Embryogenese de Chondrosia reniformis (Nardo), demosponge vipare, et transmission des bacteries symbiotiques. Annales des Sciences Naturelles. Zoologie et biologie animale, 18, 367–380.
Leys, S. P., & Degnan, B. M. (2001). Cytological basis of photoresponsive behavior in a sponge larva. Biological Bulletin, 201(3), 323–338. https://doi.org/10.2307/1543611.
Leys, S. P., & Degnan, B. M. (2002). Embryogenesis and metamorphosis in a haplosclerid demosponge: Gastrulation and transdifferentiation of larval ciliated cells to choanocytes. Invertebrate Biology, 121(3), 171–189.
Lynn, D. H., & Small, E. G. (1981). Protist kinetids: Structural conservatism, kinetid structure and ancestral states. BioSystems, 14, 377–385. https://doi.org/10.1016/0303-2647(81)90044-7.
Maddison, W., & Maddison, D. (2019). Mesquite: a modular system for evolutionary analysis. Version 3.61. http://www.mesquiteproject.org
Maldonado, M. (2006). The ecology of the sponge larva. Canadian Journal of Zoology., 84(2), 175–194. https://doi.org/10.1139/Z05-177.
Maldonado, M. (2009). Embryonic development of verongid demosponges supports the independent acquisition of spongin skeletons as an alternative to the siliceous skeleton. Biological Journal of the Linean Society, 97, 427–447. https://doi.org/10.1111/j.1095-8312.2009.01202.x.
Maldonado, M., & Riesgo, A. (2008). Reproductive output in a Mediterranean population of the homosclerophorid Corticium candelabrum (Porifera, Demospongiae), with notes on the ultrastructure and behavior of the larva. Marine Ecology, 29(2), 298–316. https://doi.org/10.1111/j.1439-0485.2008.00244.x.
Maldonado, M., Durfort, M., McCarthy, D. A., & Young, C. M. (2003). The cellular basis of photobehavior in the tufted parenchymella larva of demosponges. Marine Biology, 143(3), 427–441. https://doi.org/10.1007/s00227-003-1100-1.
Manconi, R., & Pronzato, R. (2002). Suborder Spongillina subord. Nov.: Freshwater sponges. In J. N. A. Hooper & R. W. M. van Soest (Eds.), Systema Porifera: A guide to the classification of sponges (pp. 921–1021). New York: Kluwer. https://doi.org/10.1007/978-1-4615-0747-5_97.
Melkonian, M. (1982). Structural and evolutionary aspects of the Flagellar apparatus in Green algae and land plants. Taxon, 31(2), 255–265. https://doi.org/10.2307/1219989.
Moestrup, Ø. (1982). Phycological reviews 7: Flagellar structure in algae: A review, with new observations particularly on the Chrysophyceae, Phaeophyceae (Fucophyceae), Euglenophyceae, and Reckertia. Phycologia, 21(4), 427–528. https://doi.org/10.2216/i0031-8884-21-4-427.1.
Moestrup, Ø. (2000). The Flagellar cytoskeleton: Introduction of general terminology for microtubular Flagellar roots in Protists. In B. S. C. Leadbeater & J. C. Green (Eds.), The flagellates. Systematics association special publications (pp. 69–94). London: Taylor & Francis.
Morrow, C., & Cárdenas, P. (2015). Proposal for a revised classification of the Demospongiae (Porifera). Frontiers in Zoology, 12, 7. https://doi.org/10.1186/s12983-015-0099-8.
Morrow, C. C., Picton, B. E., Erpenbeck, D., Boury-Esnault, N., Maggs, C. A., & Allcock, A. L. (2012). Congruence between nuclear and mitochondrial genes in Demospongiae: A new hypothesis for relationships within the G4 clade (Porifera: Demospongiae). Molecular Phylogenetics and Evolution, 62(1), 174–190. https://doi.org/10.1016/j.ympev.2011.09.016.
Morrow, C. C., Redmond, N. E., Picton, B. E., Thacker, R. W., Collins, A. G., Maggs, C. A., Sigwart, J. D., & Allcock, A. L. (2013). Molecular phylogenies support homoplasy of multiple morphological characters used in the taxonomy of Heteroscleromorpha (Porifera: Demospongiae). Integrative and Comparative Biology, 53(3), 428–446. https://doi.org/10.1093/icb/ict065.
Nielsen, C. (2019). Early animal evolution: A morphologist’s view. Royal Society Open Science, 6, 190638. https://doi.org/10.1098/rsos.190638.
Pozdnyakov, I. R., Sokolova, A. M., Ereskovsky, A. V., & Karpov, S. A. (2017). Kinetid structure of choanoflagellates and choanocytes of sponges does not support their close relationship. Protistology, 11(4), 248–264. https://doi.org/10.21685/1680-0826-2017-11-4-6.
Pozdnyakov, I., Sokolova, A., Ereskovsky, A., & Karpov, S. (2018). Kinetid structure in sponge choanocytes of Spongillida in the light of evolutionary relationships within Demospongiae. Zoological Journal of the Linnean Society, 184(2), 255–272. https://doi.org/10.1093/zoolinnean/zlx109/4905843.
Pozdnyakov, I., Sokolova, A., Ereskovsky, A., & Karpov, S. (2020). The kinetid structure of two Oscarellid sponges (class Homoscleromorpha) unveils plesiomorphies in kinetids of Homoscleromorpha-Calcarea lineage. Invertebrate Biology in press.
Riesgo, A., Taylor, C., & Leys, S. P. (2007). Reproduction in a carnivorous sponge: The significance of the absence of an aquiferous system to the sponge body plan. Evolution and Development, 9(6), 618–631. https://doi.org/10.1111/j.1525-142X.2007.00200.x.
Ronquist, F., & Huelsenbeck, J. (2003). MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19, 1572–1574. https://doi.org/10.1093/bioinformatics/btg180.
Schuster, A., Vargas, S., Knapp, I. S., Pomponi, S. A., Toonen, R. J., Erpenbeck, D., & Wörheide, G. (2018). Divergence times in demosponges (Porifera): First insights from new mitogenomes and the inclusion of fossils in a birth-death clock model. BMC Evolutionary Biology, 18(1), 114. https://doi.org/10.1186/s12862-018-1230-1.
Simion, P., Philippe, H., Baurain, D., Jager, M., Richter, D. J., Di Franco, A., et al. (2017). A large and consistent phylogenomic dataset supports sponges as the sister group to all other animals. Current Biology, 27(7), 958–967. https://doi.org/10.1016/j.cub.2017.02.031.
Sogabe, S., Nakanishi, N., & Degnan, B. (2016). The ontogeny of choanocyte chambers during metamorphosis in the demosponge Amphimedon queenslandica. Evodevo, 7, 6. https://doi.org/10.1186/s13227-016-0042-x.
Sokolova, A. M., Pozdnyakov, I. R., Ereskovsky, A. V., & Karpov, S. A. (2019). Kinetid structure in larval and adult stages of the demosponges Haliclona aquaeductus (Haplosclerida) and Halichondria panicea (Suberitida). Zoomorphology, 138(2), 171–184. https://doi.org/10.1007/s00435-019-00437-5.
Sperling, E., Peterson, K., & Pisani, D. (2009). Phylogenetic-signal dissection of nuclear housekeeping genes supports the paraphyly of sponges and the monophyly of Eumetazoa. Molecular Biology and Evolution, 26, 2261–2274.
Stamatakis, A. (2014). RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics, 30(9), 1312–1313. https://doi.org/10.1093/bioinformatics/btu033.
Stephens, K. M., Ereskovsky, A., Lalor, P., & Mccormack, G. P. (2013). Ultrastructure of the ciliated cells of the free-swimming larva, and sessile stages, of the marine sponge Haliclona indistincta (Demospongiae: Haplosclerida). Journal of Morphology, 274(11), 1263–1276. https://doi.org/10.1002/jmor.20177.
Tamm, S. L., & Tamm, S. (2002). Novel bridge of axon-like processes of epithelial cells in the aboral sense organ of ctenophores. Journal of Morphology, 254, 99–120. https://doi.org/10.1002/jmor.10019.
Uriz, M. J., Turon, X., & Becerro, M. A. (2001). Morphology and ultrastructure of the swimming larvae of Crambe crambe (Demospongiae, Poecilosclerida). Invertebrate Biology, 120(4), 295–307. https://doi.org/10.1111/j.1744-7410.2001.tb00039.x.
Uriz, M. J., Turon, X., & Mariani, S. (2008). Ultrastructure and dispersal potential of sponge larvae: Tufted versus evenly ciliated parenchymellae. Marine Ecology, 29, 280–297. https://doi.org/10.1111/j.1439-0485.2008.00229.x.
Usher, K. M., & Ereskovsky, A. V. (2005). Larval development, ultrastructure and metamorphosis in Chondrilla australiensis Carter, 1873 (Demospongiae, Chondrosida, Chondrillidae). Invertebrate Reproduction and Development, 47(1), 51–62. https://doi.org/10.1080/07924259.2005.9652146.
Van Soest, R., Boury-Esnault, N., Hooper, J., Rützler, K., de Voogd, N., Alvarez, B., Hajdu E, Pisera, A., Manconi, R., Schönberg, C., Klautau, M., Kelly, M., Vacelet, J., Dohrmann, M., Díaz, M.-C., Cárdenas, P., Carballo, J., Ríos, P., Downey, R., & Morrow, C. (2020) World Porifera Database. http://www.marinespecies.org/porifera Accessed on 2020-05-29.
Voigt, O., Wülfing, E., & Wörheide, G. (2012). Molecular phylogenetic evaluation of classification and scenarios of character evolution in calcareous sponges (Porifera, class Calcarea). PLoS One, 7(3), e33417. https://doi.org/10.1371/journal.pone.0033417.
Woollacott, R. M., & Pinto, R. L. (1995). Flagellar basal apparatus and its utility in phylogenetic analyses of the Porifera. Journal of Morphology, 226(3), 247–265. https://doi.org/10.1002/jmor.1052260302.
Acknowledgements
The research was supported by the Russian Foundation for Basic Research (projects nos. 18-04-01314 and 19-34-90084). The work of AMS was conducted under the IDB RAS GBRP № 0088-2019-0005. IP was supported by the ZIN RAS research project АААА-А19-119031200042-9. SK was supported by the ZIN RAS research project AAAA-A19-119020690109-2. We thank Research Resource Center for Molecular and Cell Technologies (RRC MCT) at St. Petersburg State University for access to the EM facilities and the Morphology Service of the Mediterranean Institute of Marine and Terrestrial Biodiversity and Ecology (IMBE). We are grateful to N. Kovalchuk for Lubomirskia larvae collection and fixation and Dr. Barry S. C. Leadbeater for editing the language of the text.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Suppl. Fig. 1
Bayesian phylogenetic tree of the sponges based on the 18S dataset. Node support is indicated as branch labels, scale according to GTR + G + I model distances. (PNG 2995 kb)
Suppl. Fig. 2
Maximum likelihood phylogenetic tree of sponges based on the 18S dataset. Node support is indicated as branch labels, scale according to GTR + G + I model distances. (PNG 2386 kb)
Suppl. Fig. 3
(PNG 127 kb)
Suppl. Table 1
Standard Categorical matrix of morphological characters used in the analysis. Polymorphisms are listed separated by the ‘&’ symbol; uncertain states are indicated by the ‘/’ symbol. Clades included in analysis are highlighted in blue. (DOCX 26 kb)
Rights and permissions
About this article
Cite this article
Sokolova, A.M., Pozdnyakov, I.R., Schepetov, D.M. et al. Kinetid in larval cells of Spongillida (Porifera: Demospongiae): tracing the ancestral traits. Org Divers Evol 20, 669–680 (2020). https://doi.org/10.1007/s13127-020-00460-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-020-00460-1