Abstract
The minute snails of the troglobitic genus Zospeum are known from the southeastern Alps through the Dinarides and from the Pyrenees to the Cantabrian Mountains. The majority of Zospeum has been described from the northernmost region of Southeast Europe, the geographical focus of our study. The taxonomic value of the few available morphological shell characters has been debated for nearly as long as the genus is known. Recent results of genetic studies questioned the established taxonomic system based on morphological characters in the 1970s, with one species (Z. isselianum) appearing to be polyphyletic. Here, we present a comprehensive revision of Zospeum from the Alpine and Dinaride regions, using an integrative approach including genetic methods, morphometry, X-ray micro computer tomography (micro-CT), and SEM. We reveal 25 species and describe 5 new to science. Genetic analysis separated the genus into five clades, strongly challenging the previously valid taxonomic system and revealing several species polyphyletic. The various methods used for morphological characterization often proved useful in separating species showing high incidence of cryptic speciation in the genus. Radular investigation, using SEM, uncovered new insights about the taxonomic value of the configuration of the radular ribbon within Zospeum as well as Zospeum’s dentitional affinity within the Ellobioidea.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Species of the troglobitic genus Zospeum are endemic to Europe and occur in two major geographical areas, i.e., the Central Pyrenees and the Cantabrian Mountains in Spain and in the eastern Alps and the Dinaride karst south to Montenegro (Fig. 1a). In the latter area, the focus of this study, the greatest species richness is known from Slovenia and Croatia.
The identification of Zospeum has posed a major problem since the discovery of the first species from this radiation, Carychium spelaeum Rossmässler, 1838. Several new species were described during the following two decades by Freyer, von Frauenfeld and Hauffen. Von Frauenfeld first remarked upon the broad spectrum of morphological variability, especially rib prominence (Frauenfeld Von 1856). These authors were critically evaluated by Bourguignat in 1856, who not only recognized that these specimens form a new genus based on their subterranean ecology, but who immediately followed the view of the Nouvelle Ecole in further splitting the known taxa to species level.
After the revision by Bourguignat (1856), the intensive interest in the genus of the previous few years waned. The following century was characterized by a relatively slow but steady increase in the number of described taxa, especially in Italy (see Pollonera 1887, 1889, 1905; Stossich 1899; Kuščer 1928; Allegretti 1944; Conci 1956), but to a lesser extent also in the Dinarides (A. J. Wagner 1912; Absolon 1916; Kuščer 1932; Bole 1960). Bole’s (1974) revision of the former Yugoslavian portion of the genus, based on extensive sampling by Bole himself, F. Velkovrh and L. Kuščer, has been up to now the most comprehensive study of the genus. The taxonomic system presented therein has been applied until recently, with a few modifications by R. Slapnik (1991, 1994).
Recent papers (Weigand et al. 2011, 2013), introducing genetic methods, could not replicate this taxonomic system. They found that, among other things, Z. isselianum happens to be polyphyletic, with one lineage clustering with Z. subobesum, while three other lineages formed a monophyletic clade with what is now considered Z. kupitzense, both of which suggest cryptic speciation. This latter clade was assessed further by Jochum et al. (2015c), who matched two of the Z. isselianum lineages with specimens from already known different species using barcoding while further characterizing them morphologically using CT and SEM. Although the current findings question the taxonomic system of Bole (1974) as a whole, previous studies (Weigand et al. 2013; Jochum et al. 2015c) did not have the sample size to address this further.
Newly collected specimens from caves unknown until recently and study of NHMW collection material lead us to doubt this previously-accepted concept. Species were determined that seemed to be intermediate in their character states or similar specimens were found in widely separate caves with no similar records in between. Due to increased exploration by the Croatian Speleological Society, many caves from northern and central Croatia yielded abundant material, lacking concordance to the concept proposed by Bole (1974) (assuming for example, that cryptic speciation plays a role here).
In this work, all available preserved specimens, mainly collected by the Croatian Biospeleological Society, were molecularly assessed to expand the genetic record of the Dinaride radiation. Emphasis is on specimens collected from or near their type localities to fix the use of names. By using an integrative taxonomic approach, we assess the value of various conchological traits in order to match them with the genetic results. Assessing the value of conchological traits is largely achieved by using a sophisticated morphometric analysis of the shells, incorporating landmarks and traditional shell values (i.e., size, shape, lamellar configuration etc.). For the first step, the genetic results were matched with their identification following the traditional morpho-based traits. In the next step, this tree was used to define the major clades and to identify the topology of topotype specimens in the tree. Species were delimited by using several tests to maximize the quality of the results. The distribution patterns of species nested in the clades were then used to evaluate the relative reliability of the subdivision of the genus into species clades. Including the Z. isselianum radula presented in Jochum et al. (2015c), ten radulae of topotypic species are individually assessed here via SEM for the first time for this genus.
Material and methods
Specimens investigated
Species and specimens examined
Zospeum individuals from 99 populations and localities have been imaged and measured within the scope of this work: 602 specimens underwent measurement-based analyses and 625 specimens were subjected to geometric analyses respectively (dry or preserved). All images are documented and stored at the Natural History Museum of Bern (NMBE). Several of these specimens have subsequently been donated to the NMBE. The remaining shells are stored in the respective private collections and official institutions. All records of Dinaride Zospeum species known to the authors are shown in Fig. 1a.
Sampling live Zospeum is always a complicated endeavor: many caves are only accessible to speleologists, the animals usually occur locally (narrow range endemics), and very few cave visitors would recognize a Zospeum (< 2 mm) if they saw one, let alone are prepared to collect living animals for phylogenetic study. All considered, our dataset represents the most comprehensive compilation of Zospeum yet known. The phylogenetically assessed populations, derived in part from Weigand et al. (2013) and new material comprising this study, are summarized in Supplementary Table 1.
Nomenclature of dentition and lamellae
There are three types of lamellae and two types of dentition known in the Zospeum shell. The nomenclature varies between authors, mainly between schemes that name the lamellae according to their position in the aperture (Bole 1974) and schemes that name the lamellae for their position inside the shell (Stummer 1984; Jochum et al. 2015c). For consistency, we mostly follow Bole’s (1974) scheme, naming lamellae and teeth after their respective position in the aperture. Since the lamellae are found in the same section of the aperture, but not necessarily on the same section of the whorl inside the shell, lamellae and teeth are named after their respective position in the aperture (Fig. 2). Of the three possible lamellae, only two can be seen in the aperture: the parietalis and the columellaris. The parietalis appears in the aperture on the parietal side, but continues usually, but not always, onto the columella further in the shell. Subsequently, it has been called “columellaris” (Stummer 1984) or “columellar lamella” (Jochum et al. 2015c, together with “parietal plica” if referring to this lamella in the aperture). The columellaris can be found on the lower part of the columella, both in the aperture and inside the shell. Stummer (1984) and Jochum et al. (2015c) call it “subcolumellaris” and “lower columellar lamella” respectively. The supraparietalis is positioned above the parietalis, half a whorl to one whorl into the shell. It is so far only known in Z. kusceri here. Two teeth can be present in the aperture: the angularis on the parietal side of the aperture, to the right of the parietalis, and the palatalis on the palatal side of the aperture.
This nomenclature is applied to a wide range of pulmonate groups. It is unknown whether these structures are homologous between these groups or simply a result of convergent evolution (Bank and Neubert 2016).
Morphometry
Image acquisition
Each shell was placed on a piece of clay and photographed from different angles using a Leica M205 C stereomicroscope (Leica Microsystems GmbH, Wetzlar, Germany), a Leica DFC425 camera and the IMS Client software (V15Q4; Imagic Bildverarbeitungs AG, Glattbrugg, Switzerland). The latter was also used to take measurements. Measurements were conducted as illustrated in Fig. 2 (top). The measurement scheme was based on Jochum et al. (2015a), Slapnik (1991), and Reichenbach et al. (2012). Whorl number was counted following the method of Kerney et al. (1983). All photos were made by T. Inäbnit if not stated otherwise.
Measurement-based morphometry
A principal component analysis (PCA) was conducted using the measurements made according to Fig. 2. We omitted the Spire angle (SA) since it does not meet the requirements for its inclusion into the PCA. A ratio extractor based on the linear discriminant analysis (LDA) was used to find suitable ratios to further differentiate taxa and clades from each other. The calculations were made in R 3.0.2 (R Core Team 2013) using modified scripts of Baur and Leuenberger (2011).
Geometric morphometry
Tps-files were created in tpsUtil version 1.74 (Rohlf 2017a) using photographs of the frontal view of undamaged specimens. Fourteen Landmarks (Fig. 2 bottom right) were set using tpsDig2w32 (Rohlf 2017b). The resulting tps-file was imported into MorphoJ (Klingenberg 2011) and incorporated for the subsequent analysis. The dataset was standardized using Procrustes Superimposition. A PCA and a Canonical Variance Analysis (CVA) (10′000 permutations) were conducted with the standardized dataset.
Note on the LDA and the CVA
Both the LDA and the CVA, rely on a priori species assignments of all individuals. They were primarily used after the species were defined through other methods (in most cases, genetic analysis) to ascertain additional traits that were overlooked when using different methods.
Genetic analysis
Weigand et al. (2011) and Jochum et al. (2015c) based their studies solely on the topology of one genetic marker, CO1. Weigand et al. (2013) added two additional markers (16S, H3) and recovered a topology slightly different from Weigand et al. (2011), but only applied species delimitation methods to the CO1 sequence. In this study, we added two additional nuclear markers (28S, ITS2) and diversified the markers used for species delimitation (see below).
DNA extraction
Allowing us to retain the shell, our DNA extraction protocol was based on a method initially described in Schizas et al. (1997) and partially modified after Böttger-Schnack and Machida (2011). DNA extraction was conducted on ethanol-preserved individuals. Each specimen was inserted into a 0.2-mL PCR-tube and dried at room temperature. Eight microliters ddH2O and 2 μL 5x PCR-buffer (Promega 5x Colorless GoTaq© Reaction Buffer) were added and the mixture was heated at 94 °C for 2 min. whereby 1.3 μL Proteinase K were then added and the solution was homogenized and then incubated in a thermocycler at 55 °C for 15 min., afterwards at 70 °C for 10 min. The incubation was repeated once. Ten microliters of Gene Releaser (Bioventures Inc.) was then added and the mixture was inserted into a thermocycler with the following protocol: 65 °C for 30 s, 8 °C for 30 s, 65 °C for 1.5 min., 97 °C for 3 min., 8 °C for 1 min., 65 °C for 3 min., 97 °C for 1 min., 65 °C for 1 min., 80 °C for 5 min. The mixture, including the intact shell, was centrifuged for 1 min. using a table centrifuge and the clear phase with the DNA was transferred to another 0.2 mL PCR-tube, where 15 μL of AE-Buffer (DNeasy Kit, Qiagen) was added. The shell was cleaned from the remains of the Gene Releaser chemicals by rinsing with 80% EtOH.
Markers used
Our phylogenetic hypotheses were reconstructed using data from Weigand et al. (2013), which amplified three markers, plus 20 newly sequenced specimens, for which five phylogenetic markers (mitochondrial COI (657 base pairs (bp)), 16S (483 bp) and nuclear 28S (589 bp), H3 (331 bp), and ITS2 (824 bp)), resulting in a length of 2884 bp, were used (see supplementary Table 3).
PCR and sequencing
In contrast to the earlier work of Weigand et al. (2013), the PCR included the following admixture: 2 μL template, 12.5 μL GoTaq (Promega) polymerase, 8.5 μL of nuclease-free water, and 1 μL of both forward and reverse primer (10 μmol) respectively. In cases where the PCR signal was judged too weak, the reaction was repeated using 3 μL template DNA, 3 μL of the previous PCR product, and 5.5 μL of nuclease-free water. The amount of GoTaq and primers stayed the same. The PCR was conducted using the following protocols: For CO1, the admixture was first heated up to 95 °C for 1 min (min), followed by 30 cycles of 30 s (s) at 95 °C, 30 s at 52 °C, and 30 s at 72 °C, finishing with 3 min at 72 °C. For 16S, the protocol started with 2:30 min at 90 °C, followed by 10 cycles of 30 s at 92 °C, 30 s at 44 °C, and 40 s at 72 °C, followed again by 30 s at 92 °C, 40 s at 48 °C, and 40 s at 48 °C. The protocol for 28S started with 1 min at 96 °C, then went into 35 cycles of 30 s at 94 °C, 30 s at 50 °C, and 1 min at 72 °C, finishing with 10 min at 72 °C. The ITS2 protocol started with 1 min at 96 °C, followed by 35 cycles of 30 s at 94 °C, 30 s at 44 °C, and 1 min at 72 °C, ending with 10 min at 72 °C. For H3, the admixture was first heated up to 95 °C for 3 min, followed by 40 cycles of 45 s at 94 °C, 45 s at 50 °C, and 2 min at 72 °C, finishing with 10 min at 72 °C. The protocols for CO1 and H3 could be used for both markers. The PCR products were sequenced at the LGC Genomics GmbH (Berlin, Germany) using their standard protocol.
Alignment optimization and phylogenetic tree reconstruction
In total, 43 Zospeum specimens from the Dinarides and the Alps were used (see supplementary Table 1). The sequences of 23 specimens were extracted from the literature (Weigand et al. 2013; Weigand 2013; Romero et al. 2016). One Carychium tridentatum (Risso, 1826) and eight Zospeum specimens from Spain were used as outgroup. Sequences received from LGC were imported into the Geneious 5.4.7 software (Kearse et al. 2012). The forward and reverse sequences for each gene and individual were combined and edited. For each marker, sequences were aligned in Geneious using the MAFFT multiple sequence alignment plugin version 1.3.6 (based on MAFFT v7.308; Katoh et al. 2002, Katoh and Standley 2013), letting the program choose the appropriate algorithm. The sequence length of each alignment was standardized to the length mentioned above. The alignments were concatenated using the “Concatenate sequences or alignments” function in Geneious.
Topologies were estimated using two different phylogenetic methods: Maximum likelihood (ML) and Bayesian inference (BI). The five markers were set as partitions in both of these methods, using a distinct model for the third codon in protein-coding genes (CO1, H3). The maximum likelihood (ML) topology was estimated using the RAxML 7.2.8 (Stamatakis 2014) plugin of Geneious with the GTR gamma nucleotide model and 1′000 bootstrap replicates. A consensus tree was then reconstructed using the Consensus Tree Builder function in Geneious.
The Bayesian tree was reconstructed with MrBayes 3.2.6 (Huelsenbeck and Ronquist 2001) using the GTR substitution model, invgamma rate variation, a Markov Chain Monte Carlo (MCMC) chain length of 10′000’000 generations, a subsampling frequency of every 4′000 generations, the first 100′000 generations were discarded as burn-in, four heated chains and a chain temperature parameter of 0.2. Calculations were performed on the UBELIx (http://www.id.unibe.ch/hpc), the HPC cluster at the University of Bern.
Data availability
The data matrix and the corresponding trees were deposited in TreeBASE (http://purl.org/phylo/treebase/phylows/study/TB2:S23186). Sequences generated in this study were deposited at GenBank (Accession numbers: MH382953-MH383040).
Species delimitation approaches
Here, three species delimitation methods were used: Automatic Barcoding Gap Discovery (ABGD), Statistical Parsimony networking analysis (SP), and Bayesian Phylogenetics and Phylogeography (BPP). Two (ABGD, SP) are single-gene approaches and were performed with several markers separately. For these methods, we used the alignments created for the phylogenetic tree reconstruction described above.
The ABGD method (Puillandre et al. 2012) segregates DNA sequences based on an automated procedure of barcode gap discovery. Three user-defined input variables are considered: the minimum (Pmin) and maximum intraspecific variability (Pmax), referring to the area where the barcode gap should be detectable, and the minimum gap width (X) which relates to the sensitivity of the method to gap width.
ABGD was conducted with single-gene alignments of the mitochondrial genes CO1 and 16S and, where the amount of sequences was high enough, the nuclear gene ITS2. Those alignments were uploaded to the program’s website (http://wwwabi.snv.jussieu.fr/public/abgd/abgdweb.html) as FASTA-files. The parameters used here (Pmin =0.001; Pmax = 0.1; Steps = 10; X = 1; Nb bins = 20; distance: Jukes-Cantor) mostly correspond to the default options, with the exception of X, which was set down to 1.0 to conform better with the runs performed in Weigand et al. (2013).
SP, which is frequently used to cluster haplotypes within a phylogeographical context (Templeton et al. 1992), was shown to be useful in species delimitation since parsimony networks of different species were shown to be separated (Hart and Sunday 2007).
Single-gene alignments of CO1 and 16S were converted to sequential Nexus files using the Format Converter on the HCV sequence database (https://hcv.lanl.gov/content/sequence/FORMAT_CONVERSION/form.html). These files were then read into TCS 1.21 (Clement et al. 2000) and entities were delimited at 95% connection probability. Clades that were not connected were considered to be species (in accordance with Hart and Sunday 2007).
BPP is a Bayesian approach using reversible-jump Markov Chain Monte Carlo (rjMCMC) and tests the probability of a predetermined classification (Yang and Rannala 2010; Rannala and Yang 2013). The guide trees were based on the previously calculated maximum likelihood (ML) consensus tree, its classification into taxa was varied until all taxa were supported by a posterior probability of 0.95 or higher. For the calculations, we used the full dataset with all five markers, a random seed and a rjMCMC with 100′000 generations, 4000 generations burn-in and a sampling frequency of every five generations. Calculations were performed on the UBELIx (http://www.id.unibe.ch/hpc), the HPC cluster at the University of Bern.
Computer tomography
Computer tomography (CT) was conducted at two different institutes for scanning the Zospeum shells in this study. Some specimens were scanned using the SkyScan 2011 (Bruker MicroCT, Kontich, Belgium) at the Department of Experimental Radiology, Justus-Liebig University Biomedical Research Center Seltersberg (BFS), Giessen, Germany. The Zospeum shells were scanned 185° around their vertical axis in rotation steps of 0.23° at 80 kV tube voltages and 120 μA tube current. Reconstruction was performed using the Feldkamp cone beam reconstruction algorithm. Image resolution was 1.75 μm isotropic voxel side length with a gray scale resolution of 8 bit. Digital images post processing and visualization (maximum intensity projection—MIP, volume compositing, and summed voxel projection) were displayed using the ANALYZE software package (ANALYZE 11.0, Mayo Clinic, Rochester, MN, USA). These images appear in gray visual format here.
The second series of specimens were scanned at the Zoologische Staatssammlung München, Munich, Germany. Scanning was performed with a Phoenix Nanotom m (GE Measurement and Control, Wunstorf, Germany) cone beam CT scanner using a voltage of 80 kV and a current of 325 mA using a tungsten (“Standard”) target. Projection images (1440–1800) were taken during a 360° rotation at a total duration ranging from 72 to 92 min. Voxel size ranged from 0.830 to 1.123 μm. The 16-bit data set generated by reconstruction was cropped and converted to 8 bit using VGStudio MAX 2.2 software (Volume Graphics, Heidelberg, Germany). Further visualization procedures were carried out with Amira 6.4 software (FEI Visualization Sciences Group, Burlington MA, USA), applying manual segmentation for discrimination of external and internal shell structures. Final visualization was enabled using the Volume Rendering module.
Scanning electron microscopy
Shells selected for SEM (scanning electron microscopy) were cleaned from cave encrustations with a tapered dental brush and then mounted on double-sided carbon tabs. The shells were sputtered with gold (1–2 x for 60 s) in the Agar Sputter Coater (Agar Scientific, Stansted, UK) and viewed under high vacuum mode of the Hitachi S-4500 Scanning Electron Microscope (15 kV, probe current 20–100pA) using the secondary electron detector. Photographs were taken with DISS–Digital Image Scanning System 5 (Point Electronic, Halle, Germany). The radulae of ten Zospeum specimens were prepared according to Holznagel (1998), preserved in 96% ethanol and mounted onto a prepared SEM stub. The gold sputtering and imaging procedures were the same as for the shells.
Maps
Maps were constructed using the Natural Earth dataset in QGIS 2.14. In addition to the sites studied here, we extracted occurrence data from Bole (1974; former Yugoslavia), Gittenberger (1982; Austria), Slapnik (1991; Z. alpestre clade in Slovenia), Pezzoli (1992; Italy), Slapnik (1994; isolated karst in eastern Slovenia), and Slapnik and Ozimec (2004; Croatia). This data was used for the overview map and where an agreement with the classification proposed here could be assumed to illustrate a species’ known distribution area. Locality data in Slapnik (1991, 1994) and Slapnik and Ozimec (2004) was given in a 10 × 10 km grid, which is shown via a grid-like appearance in our maps (chiefly Fig. 1a).
Results
Phylogenetic relationships of Zospeum species comprising the Dinaride radiation
Our concatenated trees inferred from an approach using two mitochondrial and three nuclear markers are shown in Fig. 3 (top: BI, bottom: ML).
As already encountered in Weigand et al. (2013), all Spanish taxa remain in a separate clade. The Dinaride group falls into five well-supported clades (here chiefly used to structure the systematic part), which match well with the distribution pattern of the species (Fig. 1b). The relationship between clades is identical in both trees and is, with exception of the sister group relationship of the Z. pretneri clade and the Z. frauenfeldii clade, well-supported.
Morphological results
The interpretation of species clades is based on the genetic results. Species without genetic data are listed separately.
The measurement-based PCA (Supplementary Fig. S1, top) separated the Z. pretneri clade from the Z. obesum clade and the Z. spelaeum clade on the PC1-axis (main components: D1, cjl). The Z. obesum clade is, with the exception of one outlier, separated along the same axis from the Z. alpestre clade (Supplementary Fig. S1).
The geometric PCA (Supplementary Fig. S1, middle) separated the Z. pretneri clade from the Z. obesum clade along the PC1-axis (Shape changes from negative to positive: aperture getting smaller (landmarks 1–6 getting closer together); apex getting more slender (landmark 10 distancing itself from 9 and 11)).
The CVA (Supplementary Fig. S1, bottom) separated the Z. spelaeum clade from the Z. pretneri clade on the CV1-axis (Shape changes from negative to positive: parietalis getting smaller (landmark 14 extending more to the upper left); the palatal side of the aperture getting less shouldered (landmark 2 extending lower); the columellar and basal parts of the aperture getting smaller (landmarks 1, 5, 6 getting to the upper right)), and the aforementioned from the Z. obesum clade on the CV2-axis (Shape changes from negative to positive: aperture getting larger (landmarks 1–6 distancing themselves); apex becoming more blunt (landmarks 9–11 getting closer together)). The Z. alpestre clade can be separated from both the Z. spelaeum clade and the Z. obesum clade by a combination of the CV1- and the CV2-axes.
Systematics
Family Ellobiidae L. Pfeiffer, 1854 (1822), Subfamily Carychiinae Jeffreys, 1830
1886 Zospeidae Brusina, Ueber die Mollusken - Fauna Oesterreich-Ungarns: 48.
Genus Zospeum Bourguignat, 1856
1896 Speozoum Hamann, Europäische Höhlenfauna – Eine Darstellung der in den Höhlen Europas lebenden Tierwelt…:49
Type species
Carychium spelaeum Rossmässler, 1838 (orig. design. Bourguignat 1856).
The arrangement of species follows their position in the cladogram. As the most basal clade in the radiation, the Zospeum spelaeum clade is treated here first. The informal clades without taxonomic rank are based on the main splits in the genetic trees (see Fig. 3) and are chiefly used here as a means of structuring. The placement of species without genetic data in one or another clade is in many cases tentative.
Zospeum spelaeum clade
Distribution (Supplementary Fig. S8)
This clade occurs in the northern Dinarides of northwestern Croatia and Slovenia as well as in the region of Ljubljana, the Julian Alps and the Italian Prealps east of the Brenta River.
Both genetic trees have the same topology (Fig. 3, red part). We follow here the delimitations C-E (Table 1) and accept two species: Z. spelaeum with genetic data from four caves and two specimens from Jama 2 pri Jabljah, which are classified here with Z. costatum due to morphological similarities and geographical proximity to the type locality. The topology within Z. spelaeum is not well-supported in either tree.
Morphology
Shell conical, translucent when fresh, in most cases comparatively taller than other clades; whorl often shouldered; ribs in most cases present, though very variable; aperture taller than wide; parietal shield well-differentiated from the lip, not broad, its margin generally straight, sometimes slightly convex or concave; upper part of the palatal margin of the aperture expanded; parietalis and columellaris present in aperture and well-developed; angularis and palatalis present. Differing from all other clades through the presence of an angularis and a palatalis.
Differing in the LDA from the Z. obesum clade by the ratios aw/D1 (Z. spelaeum clade: 1.389–2.225, Z. obesum clade: (1.718–) 2.2–2.703) and sh/sw (Z. spelaeum clade: 1.338–1.934, Z. obesum clade: 1.208–1.455); from the Z. pretneri clade by the ratios cjl/D1 (Z. spelaeum clade: 1.456–2.075, Z. pretneri clade: 0.837–1.42) and lh/lw (Z. spelaeum clade: 1.01–1.339, Z. pretneri clade: 0.884–1.045).
None of the species could be clearly separated via the measurement-based PCA (Supplementary Fig. S2, top).
The geometric PCA (Supplementary Fig. S2, middle) separated Z. costatum, Z. lamellatum and Z. trebicianum from each other through a combination of the PC1- and PC2-axes.
The CVA (Supplementary Fig. S2, bottom) separated Z. lamellatum from all the other species on the CV1-axis (Shape changes from negative to positive: apex getting more blunt (landmark 10 approach landmarks 9 and 11); parietalis getting smaller (landmark 14 extending more to the upper left)). The CV2-axis separated Z. lautum from the rest (Shape changes from negative to positive: palatal side of the aperture ceasing to stand out (landmark 2 extending more to the left); last whorl decreasing in size (landmarks 7, 8 extending to the right)).
Zospeum spelaeum (Rossmaessler, 1838)
Figures 4a–n, S14a–b, S20a–f, S21a–c
Zospeum spelaeum. a lectotype SMF 158543, Postojnska jama, sh: 1.634 mm. — b1–6 NMBE 553308, Betalov Spodmol, sh: 1.8 mm; c ditto, sh: 1.495 mm. — d NMBE 553306, Betalov Spodmol, sh: 1.8 mm. — e NMBE 553310, Mačkovica jama, sh: 2.39 mm. — f1–2 NMBE 553312, Velika Pasica, sh: 2.055 mm. —(g) NMBE 553311, sh: 1.957 mm; h ditto, sh: 2.07 mm. — i NMBE 553304, Čampari jama, sh: 1.791 mm. — j NMBE 553303, Jama pod križ, sh: 1.782 mm. — k NMBE 553301, Vrlovka, sh: 2.17 mm. — l NMBE 553302, špilja Pećina pod Stržen, sh: 2.03 mm. — m NMBE 553316, Grotte Bac, sh: 2 mm. — n NMBE 554711, Grotta di Boriano. sh: 2.62 mm. — All phot. × 20; a S. Hof, SMF; (b6) M. Kampschulte, G. Martels, University of Giessen
1838 Carychium spelaeum Rossmaessler, Icon., (1) 2 (1/2): 36–37, pl. 49 fig. 661 (shell) [in der Aldelsberger Höhle in Krain an Stalaktiten (Postojnska jama, near Postojna)].
1854 Carychium schmidtii Frauenfeld, Verh. zool-bot. Ges. Wien, Abh., 4 (1): 32, 33, 34, pl. [4] fig. 5 (shell) [Aus der Pasiza-Grotte (F.J. Schmidt leg.; Velika Pasica, near Gornji Ig)].
1855 Carychium pulchellum Freyer, Sitzungsber. mathem.-naturw. Cl. kais. Akad. Wiss. Wien, 15 (1): 20, pl. [1] fig. 4a–d (shell) [In der Grotte am Krimberge bei Laibach zuerst aufgefunden von Anton Kukek (Krimska jama, near Gorenja Brezovica)].
1856 Zospeum aglenum Bourguignat, Rev. Mag. Zool., (2) 8 (11): 507–508 [la caverne de Pasiza, en Carniole (Velika Pasica, near Gornji Ig)].
1856 Zospeum nycteum Bourguignat, Rev. Mag. Zool., (2) 8 (11): 509–510 [la caverne de Pasiza, en Carniole (Velika Pasica, near Gornji Ig)].
1856 Carychium reticulatum Hauffen, Verh. zool.-bot. Ges. Wien, Abh., 6: 623–624, pl. 7 fig. 4 (shell) [In der Grotte Bidou sturm und in einer hinter Laak (Hauffen leg.; Dobruška jama, near Vodice)] (see Hauffen 1856a).
1858 Carychium carinatum Hauffen, Jahresh. Ver. krain. Landes-Mus., 2: 96 [Sie kommt in der Grotte Bidov sturm und in einer hinter Laak vor (Dobruška jama, near Vodice; Communicated at a meeting on 11 June 1856, but published in 1858; clearly reticulatum is meant)].
1889 Zospeum tellinii Pollonera, Bull. Soc. malac. ital., 14 (2): 49–50, pl. 2 fig. 6 (shell) [nelle posature del Natisone nel Friuli... a nord di Cividale (A. Tellini leg., 1 ex.; sieved from the Natisone river north of Cividale)].
1899 Zospeum auritum Stossich, Boll. Soc. adriat. sci. natur. Trieste, 19: 41 + 3 fig. (shell) [Scoperta dal Dott. Moser nella grotta di Corgnale (? Near Lokev, Slovenia)].
1899 Zospeum istrianum Stossich, Boll. Soc. adriat. sci. natur. Trieste, 19: 42 + 3 fig. (shell) [Grotta di San Servolo; rinvenuta dal Dott. Moser (Sveta jama, near Socerb)].
1905 Zospeum lyratum Pollonera, Proteus, 3 (1): 4, fig. 1 (shell) [nella posatura del Natisone, nel Friuli, alquanto a nord di Cividale (A. Tellini leg., 1 ex.; sieved from the Natisone river north of Cividale)].
1905 Zospeum venetum Pollonera, Proteus, 3 (1): 3–4, fig. 2 (shell) [nella posatura del Natisone, nel Friuli, alquanto a nord di Cividale (A. Tellini leg.; sieved from the Natisone river north of Cividale)].
1912 Zospeum alpestre rossmaessleri A.J. Wagner, Verh. kais. königl. zool.-bot. Ges. Wien, 62 (8/9): 257–258 [Johannesgrotte in den Adelsberger Höhlen (Postojnska jama, near Postojna)].
Type specimens
spelaeum: Lectotype (by subsequent designation of Zilch, 1959, Handb. Paläozool., 6 (2, 1): 65, fig. 206) SMF 158543, Rossmaessler leg., October 1835, ± 20 exx. schmidtii: syntype NHMW 71844/1 (imaged by K. Jaksch-Mason 2013). A shell (NHMUK 1991027), mentioned by Jochum et al. (2015b fig. 6G) as a syntype, can not be unambiguously verified as such. NHMW/MO/13032: the label record contains a label stating “carniolica”, which is a manuscript name forwarded to Frauenfeld by Schmidt. Frauenfeld Von (1854: 34) explains that he does not accept this name and names the species “schmidtii” instead. Thus, this specimen is considered a syntype of schmidtii; pulchellum: collection Freyer in Trieste contains no Zospeum specimens (pers. comm. De Mattia 2017), type specimens probably lost; aglenum: replacement name for Zospeum lautum sensu Freyer 1855 non lautum Frauenfeld, 1854; no Zospeum specimens in coll. Freyer in Trieste, type specimens probably lost; nycteum: based on Carychium obesum sensu Freyer (1855: 21, pl. 1 fig. 6a–c “Aus der Pasica-Höhle bei Sonneg in Krain; gesammelt von Herrn Erjavec”) (non Frauenfeld), type specimen lost. reticulatum: no type specimens found in NHMW nor in Ljubljana; carinatum no type specimens found in NHMW nor in Ljubljana; tellinii: MZUT Turin; specimen inaccessible, no information available (pers. comm. Elena Gavetti); auritum: Stossich collection is lost (De Mattia 2004), no type specimens available; istrianum: Stossich collection is lost (De Mattia 2004), no type specimens available; lyratum: MZUT Turin; specimen inaccessible, no information available (pers. comm. Elena Gavetti); venetum: MZUT Turin; specimen inaccessible, no information available (pers. comm. Elena Gavetti); rossmaessleri: syntype NHMW 69394.
Specimens examined
Italy: NMBE 553314/1, Grotta d’Ercole, 14.6.2011, leg. Prodan; NMBE 553315/1, Grotta d’Ercole, 14.6.2011, leg. Prodan; NMBE 553316/1, Grotte Bac, 22.9.2011, leg. Prodan; NMBE 553317/1, Grotte Bac, 22.9.2011, leg. Prodan; NMBE 554704/4, Grotta Valentina, leg. De Mattia; NMBE 554705/2, Grotta dell’Alce, leg. De Mattia; NMBE 554706/2, Grotta delle Gallerie, leg. De Mattia; NMBE 554707/4, Grotta dell’Orso, leg. De Mattia; NMBE 554708/6, Caverna presso Basovizza, leg. De Mattia; NMBE 554710/2, Grotta Bac, leg. De Mattia; NMBE 554711/2, Grotta di Boriano, leg. De Mattia; NMBE 554712/2, Grotta di Padriciano, leg. De Mattia; NMBE 554713/3, Grotta Romana, leg. De Mattia; NMBE 554714/3, Grotta delle Torri di Slivia, leg. De Mattia; NMBE 554715/3, Grotta di Crogole, leg. De Mattia; NMBE 554716/2, Grotta ad E di S. Martino del Carso, leg. De Mattia; NMBE 554717/3, Abisso di Bonetti, leg. De Mattia; NMBE 554718/4, Antro della Biscia Morta, leg. De Mattia; NMBE 554719/1, Grotta delle Radici, leg. De Mattia; NMBE 554721/1, Grotta Rosica, leg. De Mattia; NMBE 554722/3, Grotta Azzurra, leg. De Mattia; NMBE 554723/2, Grotta d’Ercole, leg. De Mattia; NMBE 554724/3, Sorgenti di Vedronza, leg. De Mattia. — Slovenia: NMBE 553306/23, Betalov Spodmol, 4.10.2007, leg. Jochum; NMBE 553307/8, Betalov Spodmol, 4.10.2007, leg. Jochum; NMBE 553308/19, Betalov Spodmol, 4.10.2007, leg. Jochum; NMBE 553309/14, Betalov Spodmol, 4.10.2007, leg. Jochum; NMBE 553311/22, Velika Pasica, 2.9.2010, leg. Jochum; NMBE 553312/3, Velika Pasica, 2.9.2010, leg. Jochum; NMBE 553313/1, Velika Pasica, 2.9.2010, leg. Jochum; NMBE 553310/1, Mačkovica, 26.6.2009, leg. Jochum; NMBE 554396, Hotiške Ponikve, 30.10.2012, leg. Polak; NHMW 71838/2, no further data available; NMBE 554709/4, Jama v Bukovniku, leg. De Mattia; NMBE 554720/3, Miškotova jama v Lokah, leg. De Mattia; NMBE 554725/1, Miškotova jama v Lokah, leg. De Mattia. — Croatia: NMBE 553301/5, Vrlovka, 17.3.2009, leg. Bedek; NMBE 553304/1, Čampari jama, 29.11.2008, leg. Raguz; NMBE 553303/4, Jama pod križ, 27.6.2009, leg. Lukic; NMBE 553302/1, Pećina pod Stržen, 27.6.2009, leg. Bregovic; NMBE 553305/5, Pećina pod Stržen, 27.6.2009, leg. Bilandzija.
Diagnosis
Shell ca. 1.96 mm, transparent, elongate or elongate-conical with an auriform and more or less thickened peristome, bearing two to three apertural barriers; columella with a single lamella.
Measurements (n = 148)
sh: 1.49–2.62 mm (mean: 1.962 ± 0.223 mm); sw: 0.88–1.5 mm (mean: 1.198 ± 0.135 mm); ah: 0.68–1.25 mm (mean: 0.988 ± 0.131 mm); aw: 0.58–1.04 mm (mean: 0.796 ± 0.097 mm); number of whorls: 4.5–6.25 (mean: 5.18 ± 0.306).
Description
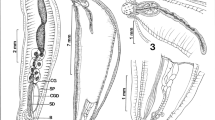
Very variable species. Shell conical, often more slender than other species, translucent when fresh; whorl never well rounded (as, for example, in Zospeum frauenfeldii), usually ovate-conic in form; shell surface usually ribbed, but variable, which has been used to differentiate subspecies in the past (present on the whole whorl as in “Z. s. costatum”, present on the upper part of the whorl as in “Z. s. schmidti” (Fig. 4f–n) or completely absent as in “Z. s. spelaeum” (Fig. 4a–e)), but the spectrum of variation within populations is too broad to establish congruence here; aperture taller than wide, the palatal side usually shouldered; the parietal shield well-differentiated from the lip, its shape either convex or straight; parietalis well-developed in the aperture, extending one whorl into the shell; columellaris visible in the aperture, in some cases (Fig. 4b) disappearing within half a whorl into the shell or continuously weakening (Fig. 4e); a more or less oblique lamella is present above the middle of the short columella; a basal bulge is present just above the umbilicus (Fig. 4f2); angularis in most cases present as a tooth of variable prominence; palatalis usually present. Protoconch covered with pits (Supplementary Figs. S14a–b); interconnected pits on top of the protoconch densely aggregated into rows; pits on the side of the whorl distributed more randomly. Radula: The radula of topotypic Zospeum spelaeum (Postojna; Supplementary Figs. S20a–d) is very different from that of Zospeum spelaeum from Velika Pasica (Supplementary Figs. S20e–f; S21a–c). In topotypic Z. spelaeum (collected by Bole in 1968), the right laterals mostly bear two pointed cusps of uneven length, whereby the left cusp is ¼ longer than the right (Supplementary Fig. S20b). An occasional third, stubby cusp may be present. On the other hand, the equally pointed left laterals are either triscuspid or bicuspid, whereby the median cusp is 1/2 cusp shorter than the longer flanking two cusps of the tricuspid crowns. The crowns of the transitional teeth consist of 5–6 very pointed subequal cusps, which do not extend beyond the edge of the individual, concave basal plates (Supplementary Fig. S20c). The broad marginal teeth are highly and unevenly serrated with very pointed cusps (Supplementary Fig. S20d). The massive, irregular basal plates of the marginals meld into eachother with occasional spaces in between the individual plates. The claw-like crown bears up to 10 pointed cusps of different widths and lengths (Supplementary Fig. S20d). The radular ribbon is long (Supplementary Fig. S20a). Since it folded and ripped upon positioning on the stub, the ends are undecipherable. On the other hand, the radula of Z. spelaeum from Velika Pasica shows multiple rows of obtuse, tricuspid crowns of which the third cusp is a mere nub of an ectocone very close to the top of the basal plate (Supplementary Fig. S21a). The two major cusps are medially grooved and show a wrinkled, marbled-like surface texture. The cusps are melded together and are only partially detached from the middle of the crown. The basal plates are concave and show wing-like prominences (Supplementary Fig. S20f). The obtuse transitional teeth are quadricuspid and sometimes quincuspid of almost equal lengths except the pointed, outermost endocones and ectocones, which are about 1/3–1/2 the length of the preceding, equal-lengthed cusps (Supplementary Fig. S21b). Many cusps are grooved. Each cusp of the transitional teeth is partially detached midway through the crown. The marginal teeth bear 6–7 cusps on each crown of which the furthest endocones and ectocones are sometimes mere stubs. The marginal teeth also show cusp detachment midway from the middle of the crown to the tip of each cusp (Supplementary Fig. S21)c. A median groove is visible on some of the marginal cusps. Laterally, the crowns of the marginal teeth extend beyond the edge of each basal plate by two cusps. The radular ribbon is long and tapered at both ends (Supplementary Fig. S20e).
Differing from Z. costatum by a less expanded upper part of the palatal side of the aperture; differing from Z. lamellatum by its greatly reduced external ribbing and number and size of lamellae and apertural barriers; usually differing from Z. lautum by a slenderer shell, presence of ribs, and its bearing a simple inclined lamella versus the more elaborate 3-tiered lamellar configuration in Z. lautum; differing from Z. trebicianum by its larger size. This species cannot be separated through PCA and LDA from its closest relatives (Z. costatum, Z. lamellatum, Z. lautum, Z. trebicianum).
Distribution (Supplementary Fig. S8)
This species was reported from the region of Ljubljana westwards to the Brenta River in Italy (Bole 1974, Giusti and Pezzoli 1982, Pezzoli 1992, Figs. 4a–h (Slovenia only)), southwards in the region around Trieste (De Mattia 2003, Figs. 4m, n), in Istria (Slapnik and Ozimec 2004, Figs. 4j +l) and on the island of Cres (this study, Fig. 4i). Eastwards, this species was reported from along and just north of the Sava River and to about 14° 00′ E where the Sava River enters the Julian Alps. An isolated record was made from the Vrlovka cave in the northeastern part of the Karlovac region, Croatia (Bole 1974, Fig. 4k).
Zospeum costatum (Freyer, 1855)
Figure 5a–f
a–fZospeum costatum, (a) NHMW 71848, Babja luknja, Goričane, sh: 2.214 mm; (b) ditto, sh: 2.043 mm. — (c1–3) NHMW 71847, Babja luknja, Goričane, sh: 2.08 mm. — (d) NMBE 553383, Jama 2 pri Jabljah, sh: 1.86 mm; (e) ditto, sh: 1.92 mm; (f) ditto, sh: 2.03 mm. —(g–k) Zospeum lamellatum, (g1–3) Lectotype, MCSMNH 35995, Krasnica, sh: 2.059 mm. — (h) MCSMNH 7057, Ukovnik pri sp. Idriji, sh: 1.751 mm; (i) ditto, sh: 1.739 mm; (j) ditto, sh: 1.733 mm; (k) ditto, sh: 1.766 mm. —(l–m) Zospeum lautum, (l1–2) NHMW 71828, Grotte von Klince/Utik (mentioned in Frauenfeld 1856), sh: 1.75 mm. — m NHMW 71834, Mala Bukovije (glatt, No 5 in Frauenfeld 1856), sh: 1.599 mm. —n–rZospeum trebicianum, n NMBE 554702, Grotta di Trebicianum, sh: 1.525 mm; o ditto, sh: 1.466 mm. — p NMBE 554701, Grotta Lazzaro Jerko, sh: 1.639 mm. — q NHMW MO75000-E-46317, Cave at Trebic, sh: 1.63 mm; r sh: 1.66 mm. — All phot. × 20; a, b, q, r P. Albano, NHMW; c1–3, g2–3 B. Ruthensteiner, ZSM; l1, m E. Bochud, NMBE; l2 M. Kampschulte, G. Martels, University of Giessen
1855 Carychium costatum Freyer, Sitzungsber. mathem.-naturw. Cl. kais. Akad. Wiss. Wien, 15 (1): 20, pl. [1] fig. 5a–c (shell) [Von Herrn Ferd. Schmidt in der Grotte bei Goričane unweit des fürstbischöflichen Schlosses Görtschach entdeckt (Babja Luknia, near Goričane)].
1856 Carychium bidentatum Hauffen, Verh. zool.-bot. Ges. Wien, Abh., 6: 701, pl. 7 fig. 5 (shell) [in der Grotte am Glaven vrh “(Hauffen leg., 17 exx.; Ručigajev izvir (Jama pod Ručigajem), near Dobeno)] (see Hauffen 1856b).
Type specimens
costatum: collection Freyer in Trieste contains no Zospeum specimens (pers. comm. De Mattia 2017). bidentatum: no type specimens found neither in NHMW nor in Ljubljana;
Specimens examined
Slovenia: NHMW 71848/2, Babja Luknja, leg. Schmidt & Erjavec; NHMW 71847/1, Babja Luknja, leg. Schmidt & Erjavec; NMBE 553383/8, Jama 2 pri Jabljah, 25.6.2009, leg. Jochum; NHMUK 1939-6-14.504-506, 3 shells, Babja Luknja “loc. class.!”; NHMUK 1935-1-1.280-289, 10 shells, Jama pri Račigajevem mlinu (near Dobeno) (= Hauffen’s orig. Loc. Cerote im “Glaven vrh”), ex Kuščer. According to the Cave registry of the Speleological Assoc. of Slovenia, this cave is most probably #1712 Ručigajev izvir (= Jama pod Račigajem) 46.142393°N 14.537118°E, 374 m.
Diagnosis
Shell ca. 2 mm, transparent, ribbed, with weak spiral lines, elongate-conical with an auriform and more or less thickened peristome, bearing four apertural barriers, two of which continue on the columella as lamellae.
Measurements (n = 10)
sh: 1.86–2.22 mm (mean: 2.002 ± 0.112 mm); sw: 1.2–1.44 mm (mean: 1.298 ± 0.088 mm); ah: 0.96–1.13 mm (mean: 1.026 ± 0.06 mm); aw: 0.83–0.98 mm (mean: 0.896 ± 0.058 mm); number of whorls: 4.75–5.25 (mean: 5.075 ± 0.206).
Description
Shell conical, translucent when fresh; whorls somewhat shouldered, usually stronger than in Z. spelaeum; pronounced equidistantly-spaced axial ribs present on each whorl, though not always covering an entire whorl; weak spiral striae are usually visible (such as in Fig. 5b and e); aperture taller than wide, the upper half of the palatal rim shouldered and expanded; the parietal shield well differentiated from lip, its margin more curved than straight; parietalis well developed in the aperture, extending one whorl into the shell; columellaris visible in aperture, extending ¾ of a whorl into the shell (Fig. 5c2); palatalis always present; angularis sometimes present.
Differing from Z. spelaeum by the generally more expanded upper palatal rim and the thickened basal columellar configuration; differing from Z. lamellatum by its considerably smaller lamellae and dentition; differing from Z. lautum by the marked presence of ribs and presence of a single lamella; differing from Z. trebicianum by its enhanced degree of ribbing and larger size.
Differing in the LDA from Z. lamellatum by the ratios ah/aw (Z. costatum: 1.06–1.19; Z. lamellatum: 1.25–1.35) and sh/lh (Z. costatum: 1.44–1.54; Z. lamellatum: 1.56–1.7); from Z. lautum by the ratio sw/ah (Z. costatum: 1.224–1.306, Z. lautum: 1.351–1.445).
Distribution (Supplementary Fig. S8)
Known from two caves north of Ljubljana: Babja Luknja near Goričane (Fig. 5a–c) and Jama 2 pri Jabljah near Loka pri Mengsu (Fig. 5d–f).
Remarks
Considered a separate species here because of the distinct columellar configuration corroborated by genetic evidence (Weigand et al. 2013).
Zospeum lamellatum Bole, 1974
Figure 5g–k
1974 Zospeum spelaeum lamellatum Bole, Razpr. Slov. Akad. Znan. Umetn., Cl. IV, 17 (5): 276–278, 288, fig. 17d–e (shell), 18, 19 (distribution) [Höhle Krasnica oberhalb Slap na Idrijci, 10 km SO von Tolmin].
Type specimens
A holotype was not selected. We hereby, select MCSMNH 35995/1 (labeled “holotyp”), Krasnica, as lectotype.
Specimens examined
Slovenia: MCSMNH 7057/5, Ukovnik, 21.10.1972.
Diagnosis
Shell ca. 1.8 mm, densely ribbed, transparent, elongate with prominent apertural barriers; columella bears a dense 3-tiered lamella configuration starting at the lower surface of the penultimate whorl and ending with the columellaris as a prominent basal lamella above the umbilicus (in dorsal cutout/CT view).
Measurements (n = 5)
sh: 1.73–2.06 mm (mean: 1.81 ± 0.14 mm); sw: 1–1.2 mm (mean: 1.082 ± 0.075 mm); ah: 0.86–0.95 mm (mean: 0.903 ± 0.033 mm); aw: 0.66–0.72 mm (mean: 0.692 ± 0.022 mm); number of whorls: 5.25–6 (mean: 5.5 ± 0.306).
Description
Shell elongate-conical, translucent when fresh; whorls slightly shouldered; strongly ribbed, though rib density as well as rib prominence can vary considerably within a population (Fig. 5h–k); aperture is taller than wide, the palatal side not or only slightly shouldered; parietal shield well differentiated from the lip, its margin either straight or convex; parietalis and angularis very prominent, usually dominating the aperture; columellaris and palatalis both prominent as well, though to a lesser degree; palatalis and angularis do not extend into the whorl; the columellaris continuing with the same size as in the aperture for at least ½–¾ whorls; parietalis extending somewhat over one whorl into the shell, decreasing in size, though still more pronounced than in any other species (Fig. 5g3).
Differing from all other species through its remarkably large lamellae and teeth.
Differing in the LDA from Z. costatum by the ratios ah/aw (Z. costatum: 1.06–1.19; Z. lamellatum: 1.25–1.35) and sh/lh (Z. costatum: 1.44–1.54; Z. lamellatum: 1.56–1.7); from Z. trebicianum by the ratio sh/lh (Z. lamellatum: 1.563–1.694, Z. trebicianum: 1.368–1.47).
Distribution (Supplementary Fig. S8)
Bole (1974) reported the taxon from four caves in northwestern Slovenia.
Remarks
Due to its morphological distinctness, this taxon is considered an independent species though not yet confirmed via genetic analysis.
Zospeum lautum (Frauenfeld, 1854)
Figure 5l–m
1854 Carychium lautum Frauenfeld, Verh. zool-bot. Ges. Wien, Abh., 4 (1): 32, 33–34, pl. [4] fig. 4 (shell) [Aus der Grotte am Krimberg (F. J. Schmidt leg.; Krimska jama, near Gorenja Brezovica)].
Type specimens
Possible Syntypes ANSP 22529 “Z. lautum Frauen. Ex. Auct.” 4 ex. No type specimens in Vienna; probably lost.
Specimens examined
Slovenia: NHMW 71825/1 Mala Bukovije [= No. 5 in Frauenfeld 1856, leg. Hauffen]; NHMW 71826 Jelenca Grotte [= No. 2 in Frauenfeld 1856, leg. Hauffen]; NHMW 71827 Mlinca [= No. 1 in Frauenfeld 1856, leg. Hauffen]; NHMW 71828/1 [Frauenfeld 1856, Grotte von Klince/Utik leg. Schmid]; NHMW 71830e Sidanka [= No. 6 in Frauenfeld 1856, leg. Hauffen]; NHMW 71834 Mala Bukovije [= No. 5 in Frauenfeld 1856, leg. Hauffen].
Diagnosis
Shell ca. 1.7 mm, transparent, conic-ovate with a more or less auriform and thickened peristome; smooth; short columella with a low-lying, highly placed lamella.
Measurements (n = 2)
sh: 1.59–1.75 mm (mean: 1.675 ± 0.107 mm); sw: 1.17–1.2 mm (mean: 1.187 ± 0.011 mm); ah: 0.82–0.88 mm (mean: 0.85 ± 0.033 mm); aw: 0.73–0.77 mm (mean: 0.752 ± 0.023 mm); number of whorls: 4.75–5.25 (mean: 5 ± 0.353).
Description
Shell conical, translucent when fresh; whorls slightly convex and smooth; aperture somewhat taller than wide; parietal shield well differentiated from the lip, its margin being curved or straight; parietalis and columellaris more or less identical to Z. spelaeum; angularis as well developed as the parietalis; palatalis very weak; generally less slender than most smooth variations of Z. spelaeum, but still falling within that species’ range of variation (see Fig. 4c as an example).
Differing from most others by lacking ribs. Internal aperture-left perspective shows a reduced three-tiered lamella configuration in contrast to the more prominent configuration of Z. lamellatum.
Differing in the LDA from Z. costatum by the ratio sw/ah (Z. lautum: 1.351–1.445, Z. costatum: 1.224–1.306).
Distribution (Supplementary Fig. S8): Known from the region of Ljubljana as well as a few sites in southern Slovenia and the region of Trieste (Bole 1974). Distribution almost completely overlaps with that of Z. spelaeum.
Remarks: Most probably a synonym of spelaeum, but retained here at the species level because DNA data is lacking. Jochum et al. (2015b), Fig. 6C) considered ANSP 22529 to represent the holotype. However, this lot of 4 specimens may well constitute possible syntype material, because there is no information on the collection date and thus, the specimens could have been collected later.
(a–e) Zospeum alpestre, (a1–2) NHMW 75000 (E 12483), Dovja griča, sh: 1.485 mm; (b) ditto, sh: 1.369 mm; (c1–2) ditto, sh: 1.432 mm. — (d1–2) NMBE 553373, Jama pod Mokrico, sh: 1.652 mm. — (e) SMNH 2216, Jama pod Mokrico, sh: 1.53 mm. —(f–i) Zospeum kupitzense, (f) Holotype, SMF 256354, Kupitzklamm, sh: 1.67 mm. — (g1–2) Paratype, NMBE 549730, sh: 1.551 mm; (h) ditto, sh: 1.536 mm. — (i1–2) NMBE 553393, Ložekarjeva jama, sh: 1.657 mm. —(j–k) Zospeum isselianum, (j1–2) Neotype, MCSMNH 37013, Turjeva jama, sh: 1.56 mm. — (k1–2) NMBE 553390, Turjeva jama, sh: 1.419 mm. —(l–p) Zospeum amoenum, (l) Syntype, NHMW 71976, “Juhanča” (Ihanščica). — (m1–2) RS 2037, Ihanščica, sh: 1.412 mm. — (n) RS 59, Potočka zijalka. — (o) NMBE 553378, Konečka zijalka, sh: 1.472 mm. — (p1–2) NMBE 553379, Konečka zijalka, sh: 1.546 mm. — All phot. × 20; (a1–2), (b, c1) E. Bochud, NMBE; (c2, 6e, 6i2, 6j2, 6n, 6o) M. Kampschulte, G. Martels, University of Giessen; (f) S. Hof, SMF; (j1, l M). Ruppel, formerly Goethe University Frankfurt am Main
Zospeum trebicianum Stossich, 1899
Figure 5n–r
1899 Zospeum trebicianum Stossich, Boll. Soc. adriat. sci. natur. Trieste, 19: 41–42 + 2 fig. (shell) [Scoperta dal Prof. Valle nella grotta di Trebich (Grotta di Trebiciano, near Trieste)].
Type specimens
Stossich collection is lost (De Mattia 2003), no type specimens available.
Specimens examined
Italy: NMBE 554701/4, Grotta Lazzaro Jerco, leg. De Mattia; NMBE 554702/2, Grotta di Trebiciano, leg. De Mattia; MP 00695/4, Abisso di Trebiciano, 31.10.2000, leg. Prodan; NHMW MO75000, 2 shells, Höhle bei Trebič (ex. Kuščer/“von Autor”, ex. Edlauer 46,317).
Diagnosis
Shell ca. 1.5 mm, transparent, elongate-conical with an elongated auriform and more or less thickened peristome, bearing two apertural barriers and a long angular parietal shield.
Measurements (n = 6)
sh: 1.46–1.64 mm (mean: 1.517 ± 0.064 mm); sw: 0.99–1.09 mm (mean: 1.045 ± 0.032 mm); ah: 0.78–0.92 mm (mean: 0.835 ± 0.048 mm); aw: 0.68–0.75 mm (mean: 0.699 ± 0.022 mm); number of whorls: 4.5–4.75 (mean: 4.625 ± 0.137).
Description
Shell elongate-conical, translucent when fresh; whorls barely shouldered; ribs usually present at the upper margin of the whorl, but never prominent; aperture taller than wide, the palatal side usually slightly shouldered; parietal shield angular; parietalis present in aperture, though not as well developed as in Z. spelaeum; columellaris not present in the aperture, but usually visible from outside the shell; angularis usually present, though in most cases only weakly developed; palatalis present; differing from Z. spelaeum populations from around Trieste mostly through its smaller size (less than 1.65 mm, though usually even smaller); a CVA of this species and Z. spelaeum from the region of Trieste pointing to two other sets of characters differing from Z. spelaeum: the apex being more obtuse (landmark 10 closer to landmarks 9 and 11; consistent with the average lower number of whorls in this species), and the upper palatal side of the aperture (landmarks 2 and 3) being wider.
Differing from most of the others due to its small size; from Z. lamellatum by the prominently developed lamellae in this species.
Differing in the LDA from Z. lamellatum by the ratio sh/lh (Z. trebicianum: 1.368–1.47, Z. lamellatum: 1.563–1.694).
Distribution (Supplementary Fig. S8)
Only known from the terminal caverns of two caves: Grotta di Trebiciano (Fig. 5n, o, q, r) and Grotta Lazzaro Jerko (Fig. 5p). Both caves are connected via the subterranean river Timavo.
Remarks
The species has been included in the Z. spelaeum clade due to its shell shape (similar to Z. spelaeum), as well as due to the presence of an angularis and a palatalis, which, within Zospeum, are only present in this clade. The species differs from Z. spelaeum also in its ecology: While Z. spelaeum inhabits shallow caves, Z. trebicianum lives only in terminal caverns of deep, mostly vertical caves connected to the subterranean river Timavo, more than 280 m below the surface. There, they live in great numbers on walls covered with loam and sand from the river. The water line of the river can fluctuate several dozen metres. The snails therefore, seem to tolerate periods of submersion (De Mattia 2003, M. Prodan pers. communication 10.2011).
Zospeum alpestre clade
Distribution
This clade is known from the Kamnik-Savinja Alps, the Julian Alps and the Karawanks of Slovenia, northeastern Italy and southeastern Austria (Supplementary Fig. S9).
The BI tree and the ML consensus tree are mostly identical (Fig. 3, blue part), excepting the lack of resolution of the position of Z. isselianum in the Bayesian tree. Here, we retain the classification of Jochum et al. (2015c; delimitation C (see Table 2)) since little additional material (MCSMNH 40651a; RS 2037) was included here and since the phylogenetic trees essentially group the same specimens together. We choose to separate Z. isselianum from Z. amoenum (despite delimitation E) due to the geographical separation and the lack of support in both trees.
Morphology
Shell conical, translucent when fresh; shell size small to medium; shell surface smooth; aperture somewhat taller than wide; parietalis and columellaris usually present. Morphological overlap with the Z. frauenfeldii clade (tends to be larger) and the Z. obesum clade (usually has an expanded last whorl and spiral lines). Differing from the Z. spelaeum clade by the absence of an angularis and a palatalis. Differing from the Z. pretneri clade by the comparatively larger aperture.
Differing in the LDA from the Z. obesum clade by the ratios aw/D1 (Z. alpestre: 1.437–2(–2.202), Z. obesum: (1.718–) 2.2 – 2.703) and sh/lh (Z. alpestre: 1.387–1.739, Z. obesum: 1.215 – 1.448).
Neither the PCAs nor the CVA were able to separate Z. amoenum from other species. The measurement-based PCA (Supplementary Fig. S3, top) is able to separate Z. alpestre from Z. kupitzense and Z. isselianum on the PC1-axis, but not the latter two from each other. The geometric PCA separated Z. isselianum and Z. alpestre on the PC1-axis (Supplementary Fig. S3, middle) and these two from Z. kupitzense on the PC2-axis. The CVA (Supplementary Fig. S3, bottom) is able to separate the three aforementioned species on the CV1-axis.
Zospeum alpestre (Freyer, 1855)
Figure 6a–e
1855 Carychium alpestre Freyer, Sitzungsber. mathem.-naturw. Cl. kais. Akad. Wiss. Wien, 15 (1): 19, pl. [1] fig. 2a–d (shell) [Im Jahre 1854 von Herrn Franz Erjavec in der Höhle Dioja griča, nächst der Veternica-Höhle auf der Velika planina der Steineralpen in Oberkrain aufgefunden (Dovja griča, Kamnik)].
1856 Zospeum nyctozoilum Bourguignat, Rev. Mag. Zool., (2) 8 (11): 513–514 [les cavernes de Dioja-Grica et [sic!] de Veternica, en Carniole” (Dovja griča, Kamnik). [reprint: 15–16, pl. 8 fig. 4–6 (shell; copy from Freyer)]].
1991 Zospeum alpestre bolei Slapnik, Razpr. Slov. Akad. Znan. Umetn., Cl. IV, 32 (1): 54–56, 61–63, fig. 5 + map 1 (distribution), pl. 1 fig. 3a–b, pl. 3 fig. 3 (shell) [Tomaževčeva zijalka, Podvolovljek (J. Bole leg., 18-VI-1964)].
Type specimens
Z. alpestre: collection Freyer in Trieste contains no Zospeum specimens (pers. comm. De Mattia 2017). nyctozoilum: partially based on Carychium alpestre Freyer (partim: pl. 1 fig. 2b, d; Im Jahre 1854 von Herrn Franz Erjavec in der Höhle Dioja griča, nächst der Veternica-Höhle auf der Velika planina der Steineralpen in Oberkrain aufgefunden); bolei: Holotype +32 paratypes (ZRC SAZU 3169) [synonymised by Jochum et al. 2015c].
Specimens examined: Slovenia
NHMW 71817/2, “Juchantza” (Ihanšica), leg. Tusek; NHMW 71815/5; NHMW 75000/E12483/3, Dovja griča, leg. Kuščer. NMBE 553369/1, Jama pod Mokrico, 5.5.2001, leg. Slapnik; NMBE 553370/1, Jama pod Mokrico, 29.8.2008, leg. Slapnik; NMBE 553371/2, Jama pod Mokrico, 18.4.2013, leg. Slapnik; NMBE 553372/3, Jama pod Mokrico, 18.4.2013, leg. Slapnik; NMBE 553373/3, Jama pod Mokrico, 2.10.2007, leg. Jochum; MCSMNH 40651a, Jelenska zijalka, 23.7.2012, leg. Slapnik.
Diagnosis
Shell ca. 1.6 mm, transparent, ovate-conical with a lunate aperture and a singular, obliquely extended lamella. The lamella is frequently turned upwards in its maximal extension within the shell cavity.
Measurements (n = 8)
sh: 1.36–1.71 mm (mean: 1.578 ± 0.107 mm); sw: 1.05–1.2 mm (mean: 1.105 ± 0.051 mm); ah: 0.65–0.84 mm (mean: 0.756 ± 0.058 mm); aw: 0.56–0.68 mm (mean: 0.657 ± 0.048 mm); number of whorls: 4.75–5.5 (mean: 5.346 ± 0.347).
Description
Shell ovate-conical, translucent when fresh; shell surface smooth; whorls convex; aperture lunate; peristome somewhat taller than wide; parietal shield well differentiated from the lip, comparatively thin (see Supplementary Fig. S3, PC2-axis in the geometric PCA and the CV1-axis in the CVA), its margin usually straight; parietalis present in the aperture, though not particularly pronounced; parietalis extends one whorl into the shell, most pronounced half a whorl in, sometimes after increasing considerably (Fig. 6a–c); columellaris starting behind the aperture, sometimes completely absent, extending up to one whorl into the shell, most pronounced half a whorl in. The singular, inclinate, extensive lamella shows a wide variation in the degree of horizontal extension in mature shells, directed upward, almost wing-like, away from the columella.
Differing from Z. kupitzense by the starting point of the columellaris behind the aperture, by its shorter parietal shield and internally, by its oblique and extensive wing-like lamella projecting away from the columella; from Z. isselianum, by its wing-like extension of the inclinate lamella; from Z. amoenum, by the discrete parietalis increasing in size until half a whorl into the shell and the frequent lack of this structure in edentate morphs known within Z. amoenum (Jochum et al. 2015c); differing in the LDA from Z. isselianum by the ratio sh/ah (Z. alpestre: 1.983–2.208, Z. isselianum: 1.737–1.794).
Distribution (Supplementary Fig. S9)
Known from a small area in the Kamnik-Savinja Alps, encompassing the municipalities of Kamnik and Luče.
Zospeum kupitzense A. Stummer, 1984
1984 Zospeum alpestre kupitzense A. Stummer, Heldia, 1 (1): 13–14, pl. 1b fig. 1, 2a-c, 3a–b (shell) [Kupitzklamm südöstlich Eisenkappel, Nordostkarawanken, Kärnten].
2015 Zospeum kupitzense, − Jochum et al., Subterranean Biology 16: 123–165.
Type specimens
Holotype SMF 256354, paratypes SMF 256355/3, NMBE 549730 (ex coll. Subai); NMW, Landesmuseum für Kärnten, coll. Stummer 8682 + 8682a, coll. Falkner 5492/5, coll. Seidl, coll. Stojaspal.
Specimens examined
Slovenia: NMBE 553391/2, Ložekarjeva zijalka, 2.10.2007, leg. Jochum; NMBE 553392/5, Ložekarjeva zijalka, 2.7.1999, leg. Slapnik; NMBE 553393/4, Ložekarjeva zijalka, 12.6.2012, leg. Jochum; NMBE 553394/1, Ložekarjeva zijalka, 12.6.2012, leg. Weigand.
Diagnosis
Shell ca. 1.6 mm, transparent, conical, a long angular parietal shield and a low-lying, robust parietalis extending from the outermost parieto-columellar corner into the aperture, columellaris present in the peristome.
Measurements (n = 17)
sh: 1.44–1.66 mm (mean: 1.569 ± 0.072 mm); sw: 1.02–1.15 mm (mean: 1.104 ± 0.042 mm); ah: 0.72–0.94 mm (mean: 0.836 ± 0.062 mm); aw: 0.62–0.77 mm (mean: 0.701 ± 0.0308 mm); number of whorls: 5–5.75 (mean: 5.365 ± 0.242).
Description
Shell conical, translucent when fresh; convex whorls; shell surface smooth; aperture somewhat taller than wide; angular parietal shield; parietalis present in aperture and slightly more pronounced than in Z. alpestre, extending one whorl into the shell at a more or less constant size keeping on the parietal side of the whorl inside the shell, instead of wandering onto the columella as in most species; columellaris often present in aperture, extending up to one whorl into the shell, being most pronounced half a whorl in; protoconch covered with concentric rows of interconnecting pits on the first three whorls (Supplementary Fig. S14g–h), most densely on the top (sometimes organized into striae) with density decreasing abapically; teleoconch with striae of densely interconnected pits.
Differing from Z. alpestre by the columellaris starting in the aperture and by its parietalis staying on the parietal side of the whorl within the shell; from Z. isselianum, by its much smaller parietal shield; from Z. amoenum, by the parietalis increasing in size until half a whorl into the shell. Differing in the LDA from Z. isselianum by the ratio sw/lh (Z. kupitzense: 0.998–1.145, Z. isselianum: 1.189–1.253).
Distribution (Supplementary Fig. S9)
Known from the Kupitzklamm near Bad Eisenkappel (Carinthia, Austria) and adjacent regions in Slovenia.
Zospeum isselianum Pollonera, 1887
1887 Zospeum isselianum Pollonera, Bull. Soc. malac. ital., 12 (3): 205, pl. 6 fig. 13 (shell) [raccolte nelle posature del Natisone nel Friuli.... a nord di Cividale (A. Tellini leg., 2 exx.; sieved from the Natisone river north of Cividale)].
Type specimens
Syntype MZUT M3232 - heavily deteriorated by Byne’s degradation and unrecognizable. Neotype: MCSMNH 37013 (from Turjeva jama, near Robič) designated by Jochum et al. 2015c.
Specimens examined
Slovenia: NMBE 553389, Turjeva jama, 19.10.2007, leg. Slapnik; NMBE 553390, Turjeva jama, 4.6.2013, leg. Slapnik.
Diagnosis
Shell ca. 1.45 mm, transparent with ovate-conic form, peristome with a strongly developed parietal shield, a parietalis extending into the shell on the columella, a weak columellaris inside the shell.
Measurements (n = 5)
sh: 1.36–1.5 mm (mean: 1.441 ± 0.05 mm); sw: 1.07–1.14 mm (mean: 1.105 ± 0.025 mm); ah: 0.76–0.84 mm (mean: 0.815 ± 0.03 mm); aw: 0.67–0.76 mm (mean: 0.706 ± 0.03 mm); number of whorls: 5.25–5.5 (mean: 5.4 ± 0.137).
Description
Shell conical, translucent when fresh, usually broader than in its relatives; shell surface smooth; aperture somewhat taller than wide; parietal shield well differentiated from the lip, usually broader than in related species; parietalis present in aperture, though not very pronounced, extending less than a whorl into the shell, being most pronounced half a whorl in; columellaris sometimes present in the aperture, sometimes starting just behind, extending up to ¾ a whorl into the shell, being most pronounced ½ a whorl in. Protoconch covered with spiral lines of pits with smooth bands lacking pits in between (Supplementary Fig. S14e); teleoconch with striae of densely interconnected pits (Supplementary Fig. S14f). Radula: Adjacent to the sharply pointed tricuspid rachidian tooth, the lateral teeth of the Z. isselianum (Turjeva jama) radula have long pointed cusps bearing an endocone and an ectocone on both the left and right sides respectively (Supplementary Fig. S18c). These cusps are 1/3 to 1/2 the length of the long mesocone. Laterals to the right of the rachidian tooth have a short, nub-like endocone to the left of the mesocone and a longer ectocone on the right, which is about ½ the length of the mesocone. Grooves run down the length of the mesocones. Some rows have a consistent pattern of pointed tricuspid teeth with both endocones and ectocones measuring about ¼–½ the length of the mesocone (Supplementary Fig. S18a). The marginal teeth of Z. isselianum have 3–4 pointed cusps of uneven length whereby the longest cusps in a given row are about the same length (Supplementary Fig. S18b). The basal plates have wing- or arm-like appendages with conspicuous wart-like prominences on the inner surfaces of these wings (Supplementary Fig. S18a) (lowest row). Martins (1996) reported similar basal plate structures in the radula of Melampus monile (Bruguière, 1789). Despite the folding during preparation, the radular ribbon of Z. isselianum appears shorter and broader, attenuating to a wide triangular base (Fig. not shown).
Differing from most species by its broad parietal shield; from Z. amoenum by the parietalis increasing in size until half a whorl into the shell. Differing in the LDA from Z. alpestre by the ratio sh/ah (Z. isselianum: 1.737–1.794, Z. alpestre: 1.983–2.208); from Z. kupitzense by the ratio sw/lh (Z. isselianum: 1.189–1.253, Z. kupitzense: 0.998–1.145); from Z. amoenum barely by a combination of the ratios ah/lh (Z. isselianum: 0.88–0.926, Z. amoenum: 0.712–0.891) and sw/D1 (Z. isselianum: 2.836–3.09, Z. amoenum: 2.194–3.292).
Distribution (Supplementary Fig. S9)
Originally described from debris collected from the Natisone River in Italy, this species is known from Turjeva jama near Kobarid (Slovenia, neotype locality) (Jochum et al. 2015c) and from the Grotta Nuova di Villanova (Villanova, Italy; Pezzoli 1992). Other Italian reports (Pezzoli 1992) from Grotta di S. Giovanni d’Antro (San Pietro al Natisone) and from Sorgente “Potcouch” (Vedronza) are probably this species as well.
Zospeum amoenum (Frauenfeld, 1856)
1856 Carychium amoenum Frauenfeld, Sitzungsber. mathem.-naturw. Cl. kais. Akad. Wiss. Wien, 19 (1): 82–83, pl. fig. 1, 1a–b (shell) [Ich habe sie selbst in der Pasizagrotte gesammelt, und besitze ausserdem ein Exemplar durch Herrn Schmid aus der Grotte Juhanča (Velika Pasica, near Gornji Ig and Ihanšica, near Ihan respectively)].
Type specimens
There is no type material of amoenum present in NHMW coming from Velika Pasica. The shell NHMW 71976 from the Frauenfeld collection originates from Juhanča (today known as Ihanščica) and thus has to be considered a syntype of C. amoenum. Recent exploration in the Velika Pasica (visited several times) yielded no Z. amoenum at all.
Specimens examined
Slovenia: RS 2037/2, Ihanšica, 29.7.2016, leg. Slapnik; MZRS 0059/1, Potočka zijalka, 8.6.2012, leg. Slapnik; NMBE 553374/1, Konečka zijalka, 2.10.2007, leg. Jochum; NMBE 553375/20, Konečka zijalka, 2.10.2007, leg. Jochum; NMBE 553376/1, Konečka zijalka, 27.6.2008, leg. Slapnik; NMBE 553377/1, Konečka zijalka, 16.8.2008, leg. Slapnik; NMBE 553378/1, Konečka zijalka, 16.8.2008, leg. Slapnik; NMBE 553379/7, Konečka zijalka, 2.10.2007, leg. Jochum; NMBE 553380/1, Konečka zijalka, 27.6.2008, leg. Slapnik; NMBE 553381/2, Jama 2 pri Jabljah, 25.6.2009, leg. Jochum.
Diagnosis
Shell ca. 1.5 mm, transparent with ovate-conic form, sometimes lacking obvious apertural barriers, but often with an obsolete lamella (denticle) in the parietal-columellar region.
Measurements (n = 47)
sh: 1.24–1.78 mm (mean: 1.531 ± 0.132 mm); sw: 0.95–1.24 mm (mean: 1.103 ± 0.073 mm); ah: 0.66–0.89 mm (mean: 0.778 ± 0.05 mm); aw: 0.58–0.76 mm (mean: 0.678 ± 0.033 mm); number of whorls: 4.75–5.75 (mean: 5.245 ± 0.224).
Description
Shell conical, translucent when fresh; shell surface smooth; aperture somewhat taller than wide. Parietal shield well-differentiated from lip, moderately broad to narrow, its margin being straight; parietalis may or may not be present; if present, extending one whorl into the shell and maintaining a constant size; a very weak columellaris sometimes present; protoconch with pits aligned into spiral lines interspersed with irregular zones of smooth bands lacking pits (Konečka zijalka; Supplementary Fig. S14c), though this is not always easily visible (Ihanščica; Jochum et al. 2015c, Fig. 9M–O); teleoconch with striae of densely interconnected pits (Supplementary Fig. S14d). Radula: The obtuse tricuspid teeth of the Z. amoenum radula from Konečka zijalka (L2 in Jochum et al. 2015c) are structurally dense and show a deep median groove down the mesocone (Supplementary Fig. S18d). The transitional teeth bear four cusps of unequal length and width. The endocones are generally semi-detached. One transitional tooth (positioned sideways here) (Supplementary Fig. S18e) shows four wide subequal cusps with the broadest on the right flanked by the second middle cusp bearing furrows on their leftmost edges. The rightmost cusp also has a discrete furrow at its rightmost edge covered in part by crust. The crown of this tooth is very skewed to the right. The radular ribbon completely folded upon positioning onto the SEM stub and thus, we have no complete perspective.
Differing from all other species by the consistent absence of the columellaris and the either very weak or absent parietalis. Barely differing in the LDA from Z. isselianum through a combination of the ratios ah/lh (Z. amoenum: 0.712–0.891, Z. isselianum: 0.88–0.926) and sw/D1 (Z. amoenum: 2.194–3.292, Z. isselianum: 2.836–3.09).
Distribution (Supplementary Fig. S9)
Endemic to Slovenia. Known from four caves east and south from the distribution areas of Z. alpestre and Z. kupitzense: Ihanščica (Ihan, Domžale), Jama 2 pri Jabljah (Loka pri Mengšu, Mengeš), Konečka zijalka (Šmihel nad Mozirjem, Mozirje), and Potočka zijalka (Olševa mountain, Solčava).
Remarks
Likely the locality data to the shells Frauenfeld collected in the Pasica cave got mixed by him or his companion Schmidt. Only Zospeum spelaeum is known from Pasica cave, a relatively small and easily navigable cave.
Zospeum obesum clade
Distribution
Known from the northern Dinarides of Slovenia and Istria (Supplementary Fig. S10).
Within this clade there are two distinct groups (Fig. 3, brown part), which are recognized in the species delimitations B–E (Table 3) as independent species.
Morphology: Shell conical, translucent when fresh; the last few whorls usually slightly to regularly expanded; shell surface often with spiral lines, otherwise smooth; aperture taller than wide, parietal shield not always well- differentiated from the lip; parietalis present, but not very strongly; columellaris beyond the aperture or absent; incidence of morphological overlap with other clades. Differing from the Z. spelaeum clade by the absence of the angularis and the palatalis; differing from the Z. pretneri clade by the shell height and the larger aperture; usually differing (though not always clearly) from the Z. alpestre and the Z. frauenfeldii clade by the expanded last whorl and the presence of spiral lines.
Differing in the LDA from the Z. spelaeum clade by the ratios aw/D1 (Z. obesum: (1.718–)2.2–2.703, Z. spelaeum: 1.389–2.225) and sh/sw (Z. obesum clade: 1.208–1.455, Z. spelaeum clade: 1.338–1.934); from the Z. alpestre clade by the ratios aw/D1 (Z. obesum clade: (1.718–)2.2–2.703; Z. alpestre clade: 1.437–2(−2.202)) and sh/lh (Z. obesum clade: 1.215–1.448, Z. alpestre clade: 1.387–1.739); from the Z. pretneri clade by the ratios lh/D1 (Z. obesum clade: 2.531(outlier) –4.108, Z. pretneri clade: 1.845–2.442), aw/lw (Z. obesum clade: 0.743–0.917, Z. pretneri clade: 0.517–0.698).
The morphometric analyses were constrained by the paucity of specimens of Z. exiguum (n = 4). The PCAs (Supplementary Fig. S4, top and middle) show some overlap between the two species in this clade, though only little in the measurement-based PCA. The CVA (Supplementary Fig. S4, bottom) is able to separate the two species, but the changes in the position of the landmarks (most notably landmarks 1, 4 and 8) are fairly small.
Zospeum obesum (Frauenfeld, 1854)
Figures 7(a–d), S15a–b, S18f–h
(a–d) Zospeum obesum, (a1–2) NMBE 553409, Krška jama, sh: 1.961 mm; (b) ditto, sh: 1.972 mm; (c) ditto, sh: 2.08 mm; (d) ditto, sh: 1.975 mm. —(e–h) Zospeum exiguum, (e) Paratype (?Holotype), NHMW 32008, Križna jama, sh: 1.74 mm. — (f) NHMW 49752, Veliki iz Krkinc jama, sh: 1.812 mm. — (g1–2) NMBE 553384, Križna jama, sh: 1.569 mm. — (h1–2) NMBE 548798, Jama Borušnjak 3, sh: 1.688 mm. —(i–q) Zospeum pretneri, (i1–2) NMBE 553290, Gornja Cerovačka pećina, sh: 1.251 mm; (j) ditto, sh: 1.251 mm. — (k) NMBE 553285, Muda labudova, sh: 1.274 mm; (l) ditto, sh: 1.08 mm. — (m) NMBE 553287, Draženova puhaljka, sh: 1.223 mm; (n) ditto, sh: 1.23 mm; (o1–2) ditto, sh: 1.184 mm. — (p1–2) NMBE 553297, Pećina kod sela Puhari, sh: 1.524 mm; (q) ditto, sh: 1.436 mm. —(r–t) Zospeum tholussum, (r1–2) NMBE 553334, Lukina jama – Trojama, sh: 1.475 mm; (s) ditto, sh: 1.556 mm; — (t) SMF 341633, Lukina jama – Trojama, sh: 1.502 mm. —(u1–4): Zospeum troglobalcanicum, NMBE 553414, Taleža pećina, sh: 1.327 mm. —(v–x) Zospeum manitaense, (v1–3) Holotype, NMBE 554401, Manita peć, sh: 1.292 mm. — (w) NMBE 549731, Paklenica National park, sh: 1.432 mm. — (x) NMBE 553405, Draženova puhaljka, sh: 1.287 mm. — All phot. × 20; (d, g2, j, t M). Kampschulte, G. Martels, University of Giessen; e, f K. Jaksch-Mason, NHMW; (u3–4), (v1) B. Ruthensteiner, ZSM
1854 Carychium obesum Frauenfeld, Verh. zool-bot. Ges. Wien, Abh., 4 (1): 32, 33, 34, pl. [4] fig. 6 (shell) [Grotte von Obergurk (F.J. Schmidt leg.; Krška jama, Gorica)].
Type specimens
Syntypes: NHMW 58043 ex coll. Schmidt 361 [Frauenfeld 1856] syntype; NHMW 71832/3 Obergurk in coll. Frauenfeld syntype; NHMW 71833/2 ex coll. Schmidt [Frauenfeld Von 1856] syntype; NHMW 71835 Obergurk in coll. Frauenfeld syntype.
Specimens examined
Slovenia: NMBE 553284/2, Krška jama, 1.9.2010, leg. Jochum; NMBE 553409/12, Krška jama, 1.9.2010, leg. Jochum.
Diagnosis
Shell ca. 2 mm, ovate-conic in form, with spiral striae, transparent, last whorl expanded, large, oval peristome with a large, often rounded parietal shield, parietalis and columellaris present, the latter only weak.
Measurements (n = 12)
sh: 1.84–2.08 mm (mean: 1.983 ± 0.076 mm); sw: 1.38–1.51 mm (mean: 1.439 ± 0.041 mm); ah: 1.11–1.29 mm (mean: 1.196 ± 0.052 mm); aw: 0.96–1.04 mm (mean: 1.001 ± 0.027 mm); number of whorls: 4.5–5 (mean: 4.813 ± 0.155).
Description
Shell ovate-conical, translucent when fresh; tall compared to most of its congeners; weak spiral lines, and sometimes also weak ribs on the upper part of the whorl, are visible on fresh shells; the last two whorls are rapidly expanded; aperture relatively large, vaguely oval, taller than wide; parietal shield less pronounced than the lip, its margin a continuation of the lip and thus rounded; parietalis present in the aperture, though small compared to the size of the aperture, extending one whorl into the shell; columellaris usually present in the aperture, disappearing after half a whorl into the shell. Protoconch densely covered with striae of interconnected pits (Supplementary Fig. S15a); teleoconch covered with spiral lines, their number increasing with each whorl (Supplementary Fig. S15b). Radula: The tricuspid rachidian tooth of Z. obesum (Krška Jama) is thick and flanked by an equally long endocone and ectocone whereby the endocone does not separate from the mesocone as does the ectocone (Supplementary Fig. S18g). The left lateral teeth bear a small nub-like ectocone but no endocones. The right laterals bear a reduced and tightly attached endocone and a thick, partially disattached ectocone. Both cusps are the same size, reaching half the length of the mesocone. Some of the right laterals have one endocone and one and a half ectocones. The lateral teeth of Z. obesum show no signs of a median groove. The bicuspid tongue-like lateral teeth are rounded and smooth in appearance. Many cusps are broken. Overall, the laterals imply either an aged or stressed individual or a very old part of the radula (Supplementary Fig. S18g). The basal plates supporting the lateral crowns are concave directly under the crown. They are long and sinuous and have a concavity on the lower righthand side, resembling those of Pedipes ovalis (Martins 1996) (Supplementary Fig. S18g). The crown is as wide as the basal plate and does not extend beyond it in width. The transitional teeth show 3–5 subequal cusps of varying width with partially detached endocones and an occasional detached ectocone. The cusps forming the transitional crown are otherwise melded into each other and do not extend further than the edge of the basal plate. The basal plates are concave directly under the crown. The transitional teeth show general wear and tear via erosion and breakage (Supplementary Fig. S18h). The radula ribbon is long and attenuates to both a comparatively pointed anterior and basal end (Supplementary Fig. S18f). The entire radular ribbon of Z. obesum is comparatively broader than that of Z. exiguum.
Differing from Z. exiguum through its larger size; differing from Z. robustum from Markov ponor through its lack of ribs at the top of the whorl. Differs from Z. exiguum through the ratio ah/D1 being over 2.68, though individuals with a ratio close to that number should also be tested with the ratio ah/aw (range: 1.13–1.26), which should allow a unanimous determination in combination with the ah/D1 ratio.
Distribution (Supplementary Fig. S10)
Endemic to central, southern Slovenia, where it is known primarily in the upper parts of the Krka and Kolpa river catchment areas (Bole 1974).
Remarks
Video of CT scans (ESM video) of Z. obesum showing the long intestinal string in relationship to the columella and the inner whorls is viewable via the link. Subsequent investigation of the MHNG 7904 Z. obesum material mentioned in Jochum et al. (2015b) Fig. 6D) clarifies that it can not be syntype material as there is no evidence (i.e., no orthographic similarity) that it originated from Frauenfeld nor evidence that it derived from the type locality. However, there is evidence that it derived from Freyer and subsequently was deposited in the Bourguignat collection in Geneva.
(MP4 3478 kb)
Zospeum exiguum Kuščer, 1932
1932 Zospeum obesum exiguum Kuščer, Arch. Moll., 64 (2): 60–61 [Höhle Križna jama” (= “Kreuzberghöhle”)].
Type specimens
Paratype (if not the holotype (Jochum et al. 2015b Fig. 6h)): NHMW Edlauer 32,008, original label of Kuščer with the number 2010 (2010a being fixed as Holotype in Kuščer 1932) present.
Specimens examined
Slovenia: NHMW Edlauer 49,752/1, Veliki iz Krkinc jama; NMBE 553384/1, Križna jama, 22.6.2009, leg. Jochum. — Croatia: NMBE 548798/1, Borušnjak 3 Jama, 25.6.2009, leg. Bedek; NMBE 548774/1, Borušnjak 3 Jama, 25.6.2009, leg. Jalzic.
Diagnosis
Shell ca. 1.7 mm, transparent, with some spiral striae, with an ovate-conic form, last whorl expanded, large peristome, parietalis and, sometimes, columellaris present, the latter only weak.
Measurements (n = 4)
sh: 1.56–1.82 mm (mean: 1.702 ± 0.102 mm); sw: 1.19–1.33 mm (mean: 1.267 ± 0.056 mm); ah: 0.93–1 mm (mean: 0.961 ± 0.027 mm); aw: 0.79–0.97 mm (mean: 0.886 ± 0.069 mm); number of whorls: 4.25–5.25 (mean: 4.813 ± 0.427).
Description
Shell conical, translucent when fresh; weak spiral striae visible in all populations; smaller than Z. obesum; the last few whorls expanded in those from Slovenia, but not that strongly in Istria; parietalis present, but not very prominent; columellaris present in specimens from Slovenia, visible in the aperture, disappearing half a whorl into the aperture; columellaris completely absent in Istrian material; protoconch densely covered with pits (Supplementary Fig. S15c), some at the top of the whorl aligned into striae; pits disappearing on the teleoconch (Supplementary Fig. S15d); spiral striae never as prevalent on the teleoconch as in Z. obesum. Radula: The radula of Z. exiguum (Križna jama) shows dense rows of strongly hooked, pointed teeth arching over long slender basal plates (Supplementary Fig. S17c). The bicuspid crowns bear semi-detached endocones on the left side of the long, pointed mesocone (Supplementary Fig. S17b). No ectocones are present. The endocones extend about 1/2–3/4 the length of the central tooth. The transitional teeth bear 4 pointed cusps of uneven lengths per crown (lower lefthand side) (Supplementary Fig. S17d). The upper section of the basal plates is concave directly under each crown. The mesocones are very long in this species (Supplementary Fig. S17d) as is the slender radular ribbon (Supplementary Fig. S17a). The radula is tapered at the anterior end while the proximal end is not visible here. The apparently robust, well-formed teeth suggest a healthy Z. exiguum individual in the prime of life.
Differing from Z. obesum by its smaller size; the Istrian population distinguishable from many smooth-shelled species from the Z. frauenfeldii clade by lacking a columellaris. Differing from Z. obesum by the ratio ah/D1 being over 2.65, though individuals with a ratio close to that number should also be tested with the ratio ah/aw (range: 1.02–1.18), which should allow an unambiguous determination in combination with the ah/D1 ratio.
Distribution (Supplementary Fig. S10)
Occurs in the upper parts of the Lubljanica and Kolpa river catchment areas, sometimes together with Z. obesum (Bole 1974). Also occurring at a few sites in Croatia (Slapnik and Ozimec 2004, this study).
Remarks
This species is occasionally found in springs, where it apparently can stay submerged for a period of time (De Mattia 2003).
Zospeum pretneri clade
Distribution
Distributed along the coast of the Adriatic Sea from Istria to southern Montenegro (Supplementary Fig. S11).
Both trees agree on the topology, though the clade containing Z. pretneri and Z. tholussum is poorly supported in both trees (Fig. 3, green part). We follow the delimitations A and C (see Table 4) and accept three species (following Weigand 2013).
Morphology
Shell conical, translucent when fresh; shell height small to medium; shell surface smooth; suture comparatively deep; aperture small and roundish, generally as tall as wide; parietalis starting behind the aperture if present; columellaris seldom present and when only very weak. Differs from all other clades by its small size and the small aperture (in relation to shell size).
Differing in the LDA from the Z. spelaeum clade by the ratios cjl/D1 (Fig. 7m) (Z. pretneri clade: 0.837–1.42, Z. spelaeum clade: 1.456–2.075) and lh/lw (Z. pretneri clade: 0.884–1.045, Z. spelaeum clade: 1.01–1.339); from the Z. obesum clade by the ratios lh/D1 (Z. pretneri clade: 1.845–2.442, Z. obesum clade: 2.531(outlier)–4.108), aw/lw (Z. pretneri clade: 0.517–0.698, Z. obesum clade: 0.743–0.917).
The PCAs (Supplementary Fig. S5 top and middle) are not able to separate the species with more than one specimen in the study. The CVA (Supplementary Fig. S5 bottom) is able to separate Z. manitaense from the other two species, mainly on the CV1-axis, as well as most of Z. tholussum from Z. pretneri on the CV2-axis.
Zospeum pretneri Bole, 1960
1960 Zospeum pretneri Bole, Biol. Vestn., 7: 61, 64, fig. 1a–i (shell) [die Höhle Gornja Cerovačka pećina, ca. 50 m von Eingang (Bole leg., June 1958)].
Type specimens
Holotype MCSMNH 38482
Specimens examined: Croatia: NMBE 553297/2, Pećina kod sela Puhari, 21.4.2009, leg. Bedek; NMBE 553300/1, Pećina kod sela Puhari, 21.4.2009, leg. Bedek; NMBE 553289/1, Gornja Cerovačka pećina, 27.9.2009, leg. Jochum; NMBE 553290/3, Gornja Cerovačka pećina, 27.9.2009, leg. Jochum; NMBE 553288/34, Jamski sustav Kita Gaćešina/Draženova puhaljka, 16.7.2011, leg. Bedek; NMBE 553285/11, Muda Labudova, 2.7.2011, leg. Bedek; NMBE 553286/1, Muda Labudova, 2.7.2011, leg. Bedek; NMBE 553287/2, Munizaba, 26.6.2010, leg. Malenica.
Diagnosis
Shell ca. 1.2 mm, transparent, with a cylindrical to conical form, the protoconch forming a dome-like structure, a small, thick, roundish peristome, nearly without a parietal shield, columella with one lamella (parietalis), rarely two.
Measurements (Crnopac region; n = 38)
sh: 1.13–1.31 mm (mean: 1.223 ± 0.043 mm); sw: 0.73–0.88 mm (mean: 0.789 ± 0.039 mm); ah: 0.43–0.57 mm (mean: 0.5 ± 0.031 mm); aw: 0.4–0.51 mm (mean: 0.456 ± 0.023 mm); number of whorls: 5–5.75 (mean: 5.394 ± 0.18).
Measurements (Istria; n = 3)
sh: 1.43–1.76 mm (mean: 1.571 ± 0.164 mm); sw: 0.96–1.19 mm (mean: 1.04 ± 0.129 mm); ah: 0.67–0.78 mm (mean: 0.715 ± 0.051 mm); aw: 0.56–0.72 mm (mean: 0.632 ± 0.074 mm); number of whorls: 5.25–5.75 (mean: 5.5 ± 0.25).
Description
The shell is slightly conical, sometimes almost cylindrical, translucent when fresh; shell surface smooth; suture deep; aperture small and roundish; the parietal shield thick but narrow, in most cases only weakly differentiated from the rest of the lip; parietalis starting behind the aperture, extending one whorl into the shell, most pronounced half a whorl into the shell; prominence of the parietalis variable (strongly developed (Fig. 7o) to barely present (Fig. 7l)); columellaris sometimes present.
Some shells from the Istrian peninsula (Fig. 7p–q) are preliminarily placed here due to their similar shell shape; differing in their substantially larger size, the parietal shield differentiable from the lip due to its thinness and the complete absence of any lamella.
Radula: The radula of topotypic Z. pretneri (Cerovačka pećina) bears a much reduced tricuspid rachidian tooth with a wing-like basal plate configuration (Supplementary Fig. S19a). The bicuspid lateral teeth are much larger (about 3× the size of the rachidian tooth) with their basal plates bearing irregular modifications and showing different degrees of irregularity. The mesocones are centrally grooved and bear either a semi-detached ectocone or a detached endocone (right laterals) (Supplementary Fig. S19a). Some central teeth are askew and bulge somewhat medially (lower righthand side of Supplementary Fig. S19b). Other parts of the radula clearly show tricuspid teeth with wing-like basal plates for many rows (Supplementary Figs. S19c and d). Elsewhere on the radula, rows of obtuse, medially grooved central teeth are moderately hooked and flanked by 1–2 ectocones (occasionally one larger and one smaller). The semi-detached ectocones are 1/2 the length of the central tooth (Supplementary Fig. S19b). The crowns show a rather gelatinous-like and somewhat malleable texture in contrast to the stiff constitution of those of Z. amoenum from Konecka zijalka. The long radular ribbon has a straight basal edge (not shown here) compared to the attenuated base of the radula of Z. obesum and the wide triangular base of Z. isselianum.
Differing from Z. tholussum by its smaller shell size; differing from Z. manitaense by its slenderer shell, by the undifferentiated parietal shield and that its parietalis is first visible deeper within the shell; differing from Z. troglobalcanicum by its slenderer shell, by the undifferentiated parietal shield and by the presence of a parietalis. Differing in the LDA from Z. manitaense (ignoring the Istrian Z. pretneri specimens) by the ratios sh/lh (Z. pretneri: 1.636–1.831, Z. manitaense: 1.496–1.607) and cjl/D1 (Z. pretneri: 0.837–1.233, Z. manitaense: 1.16–1.401); from Z. troglobalcanicum by the ratios sh/aw (Z. pretneri: 2.331–2.934, Z. troglobalcanicum: 2.09) and sh/lw (Z. pretneri: 1.545–1.818, Z. troglobalcanicum: 1.395).
Distribution (Supplementary Fig. S11)
Known chiefly from the Crnopac Mountain south of Gračac, where it is known to occur in five caves (Gornja Cerovačka pećina (Fig. 7i–j), Muda Labudova (Fig. 7 k–l), Munizaba, Jamski sustav Kita Gaćešina/Draženova puhaljka (Fig. 7m–o). Slapnik and Ozimec (2004) also reported Z. pretneri from Pčelina špilja, Mogorić, and from Slovačka jama, Veliki Lubenovac. The latter site lies close to the type locality of Z. tholussum (Lukina jama/Trojama) and might represent this species. Similar shells were found in Pećina kod sela Puhari (Opatija, Istria peninsula, Croatia; Fig. 7p–q).
Zospeum tholussum Weigand, 2013
Figure 7r–t
2013 Zospeum tholussum Weigand, Subterranean Biology, 11: 47–51, fig. 2–3, 4A [the Lukina Jama – Trojama cave system situated in the Velebit mountain range of Croatia.... in an unnamed large chamber at 980 m depth (leg. 31.07.2010, J. Bedek)].
Type specimens
Holotype and eight paratypes: SMF 341633
Specimens examined
Croatia: NMBE 553334/7, Lukina jama – Trojama, 29.7.2010, leg. Miculinic; NMBE 553333/1, Lukina jama – Trojama, 29.7.2010, leg. Bedek; NMBE 553332/1 Lukina jama – Trojama, 26.10.2007, leg. Bedek.
Diagnosis
Shell ca. 1.5 mm, transparent, with a conical form, the protoconch forming a dome-like structure, a small, thick, roundish peristome, nearly without a parietal shield, columella with one lamella (parietalis).
Measurements (n = 12)
sh: 1.42–1.6 mm (mean: 1.512 ± 0.054 mm); sw: 0.92–1.09 mm (mean: 1.009 ± 0.058 mm); ah: 0.58–0.75 mm (mean: 0.658 ± 0.042 mm); aw: 0.48–0.65 mm (mean: 0.575 ± 0.051 mm); number of whorls: 5.25–6 (5.417 ± 0.222).
Description
Shell conical, translucent when fresh; shell surface smooth; suture deep; the first one or two whorls often as wide as or wider than the whorls beneath; aperture roundish, the parietal shield thick and narrow, but better differentiated from the rest of the lip than in Z. pretneri; parietalis absent in the aperture, starting further within the shell, staying small (Fig. 7t; Fig. 3 in Weigand 2013); columellaris present, though very weak and not visible in the aperture.
Differing from Z. pretneri by its smaller size; differing from Z. manitaense by its slenderer shell, by the undifferentiated parietal shield and by its parietalis appearing further within the shell; differing from Z. troglobalcanicum by its slenderer shell, by the undifferentiated parietal shield and by the presence of a parietalis. Differing in the LDA from Z. troglobalcanicum through the ratios sh/lw (Z. tholussum: 1.505–1.771, Z. troglobalcanicum: 1.395) and sh/aw (Z. tholussum: 2.378–2.992, Z. troglobalcanicum: 2.09).
Distribution (Supplementary Fig. S11)
Only known from the Lukina jama/Trojama cave system, where it has been found at 980 m depth.
Zospeum manitaense Inäbnit, Jochum & Neubert n. sp.
Figure 7v–x
Type specimens
Holotype: NMBE 554401/1, Manita peć, 25.7.2012, leg. Lukić; Paratype: NMBE 548813/4, Manita peć, 25.7.2012, leg. Lukić; NMBE 548794/1, Manita peć, 25.7.2012, leg. Drazina; NMBE 553406/1, Manita peć, 1.10.2008, leg. Cukusic.
Specimens examined
Croatia: NMBE 553408/2, Manita peć, 1.9.2012, leg. Miculinic; NMBE 549731/2, Paklenica National Park, 1980, leg. Kreissl; NMBE 553405/1, Jamski sustav Kita Gaćešina/Draženova puhaljka, 16.7.2011, leg. Bedek; NMBE 553407/1, Jamski sustav Kita Gaćešina/Draženova puhaljka, 16.7.2011, leg. Bedek.
Diagnosis
Shell ca. 1.3 mm, transparent, conical, peristome roundish, the parietal shield sometimes well defined, columella with a parietalis.
Measurements (n = 12)
sh: 1.24–1.44 mm (mean: 1.33 ± 0.055 mm); sw: 0.86–1.02 mm (mean: 0.94 ± 0.044 mm); ah: 0.57–0.67 mm (mean: 0.617 ± 0.027 mm); aw: 0.49–0.64 mm (mean: 0.562 ± 0.038 mm); number of whorls: 4.75–5.25 (mean: 5.104 ± 0.198).
Description
Shell conical, translucent when fresh; shell surface smooth; suture deep; aperture somewhat roundish; parietal shield well-differentiated from the rest of the lip in width and degree of thinness, with a fairly straight margin; a weak parietalis usually present in the aperture, extending one whorl into the aperture at a constant size; columellaris absent.
Differing from Z. pretneri and Z. tholussum by its broader shell, by the differentiated parietal shield and the complete lack of a parietalis; differing from Z. troglobalcanicum by the lack of a parietalis. Differing from Z. pretneri and Z. tholussum in the CVA by its more obtuse apex (Landmark 10 being lower in relation to landmarks 9 and 11). Differing in the LDA from Z. pretneri (ignoring the Istrian Z. pretneri specimens) by the ratios sh/lh (Z. manitaense: 1.496–1.607, Z. pretneri: 1.636–1.831) and cjl/D1 (Z. manitaense: 1.16–1.401, Z. pretneri: 0.837–1.233); from Z. troglobalcanicum by the ratios lh/cjl (Z. manitaense: 1.63–1.907, Z. troglobalcanicum: 1.584) and sw/lh (Z. manitaense: 1.023–1.156, Z. troglobalcanicum: 1.207).
Etymology
Named after the cave Manita peć, the type locality.
Distribution (Supplementary Fig. S11)
This species was reported from one or two caves within Paklenica National Park and from one cave on the Crnopac Mountain (Jamski sustav Kita Gaćešina/Draženova puhaljka), where it co-occurs with Z. pretneri.
Zospeum troglobalcanicum Absolon, 1916
Figure 7u
1916 Zospeum troglobalcanicum Absolon, Zlatá Praha, 33 (48): 586, textfig. [Benetina Pecina, a cave above Zatokou Slanskou = Zatoka Slanska??].
Type specimens
not located, the current allocation of the collection Absolon is unknown to the authors.
Specimens examined
Bosnia and Herzegovina: NMBE 553414/1, Taleža pećina, 9.8.2010.
Diagnosis
Shell ca. 1.3 mm, transparent, with a globose-conical form, peristome with a well-defined parietal shield, lamellae missing completely.
Measurements (n = 1)
sh: 1.327 mm; sw: 1.028 mm; ah: 0.64 mm; aw: 0.635 mm; number of whorls: 5.25.
Description
Shell conical, sometimes almost globose, translucent when fresh; suture deep; aperture somewhat roundish; parietal shield clearly differentiated from the rest of the lip, straight; no lamellae present, but an undetermined fold present on the columella of the specimen from Taleža pećina.
Differing from Z. pretneri and Z. tholussum by its broader shell, by the differentiated parietal shield and the complete lack of a parietalis; differing from Z. manitaense by the lack of a parietalis. Differing in the LDA from Z. pretneri by the ratios sh/aw (Z. troglobalcanicum: 2.09, Z. pretneri: 2.331–2.934) and sh/lw (Z. troglobalcanicum: 1.395, Z. pretneri: 1.545–1.818); from Z. tholussum by the ratios sh/lw (Z. troglobalcanicum: 1.395, Z. tholussum: 1.505–1.771) and sh/aw (Z. troglobalcanicum: 2.09, Z. tholussum: 2.378–2.992); from Z. manitaense by the ratios lh/cjl (Z. troglobalcanicum: 1.584, Z. manitaense: 1.63–1.907) and sw/lh (Z. troglobalcanicum: 1.207, Z. manitaense: 1.023–1.156).
Distribution (Supplementary Fig. S11)
Described from Benetina pećina (Grebci, Bosnia-Herzegovina). Also known from Taleža pećina near Trebinje.
Remarks
We identified the single shell from Taleža pećina with Z. troglobalcanicum because of its overall resemblance with the figure supplied by Absolon along with the original description. Gittenberger (1975) reported shells by this name from two caves in the region of Cetinje (Montenegro), Cetinjska pećina and Lipska pećina. These populations were reported to have ribs on the uppermost part of the whorls (not visible in Absolon), some of which continued as vertical bands further down the teleoconch whorls. We have not seen these specimens, nor did we have fresh specimens from Montenegrin caves. These shells could potentially constitute a separate species.
Zospeum frauenfeldii clade
Distribution
Known from the northern Dinarides, with genetic records extending from southern Slovenia southwards to the Ličko-senjska region in Croatia and the region of Sanski Most in northern Bosnia (Supplementary Fig. S12). It has been reported from the isolated karst regions in eastern Slovenia (Slapnik 1994).
The resolved topologies of both the Bayesian tree and the Consensus tree are mostly identical (Fig. 3, violet part). The Bayesian tree was not able to resolve the base of the Z. frauenfeldii clade, leaving four clades (1. Two specimens from Tounjčica, 2. A specimen from Jopićeva špilja, 3. Z. frauenfeldii & Z. bucculentum, 4. Z. pagodulum & Z. robustum) with unkown relationship affinity. The ML Consensus tree was able to resolve the base of the clade, though the support threshold was set at only 20%. The consensus tree was not able to position the specimen from Hrustovača špilja. Support values within this clade are often very low in both trees.
The species delimitations (Table 5) differ considerably from each other. Here we follow the delimitation of BPP (Table 5: F), as it is the delimitation method that is the most congruent with both trees and the only one which included all studied specimens and all markers.
Morphology
Shell conical, translucent when fresh; shell surface ribbed, smooth and everything in between; aperture taller than wide, parietal shield generally well-differentiated from the lip; parietalis present in the aperture; columellaris generally starting behind the aperture, continuing for approximately half a whorl into the shell, sometimes completely absent. Can not be reliably separated from the Z. alpestre clade and the Z. obesum clade; tends to be taller than the former and does not usually have the expanded last whorl seen in most specimens of the latter. Differs from the Z. pretneri clade by its larger size and larger aperture in relation to shell size. Differs from the Z. spelaeum clade by the absence of the angularis and (in most cases) the palatalis.
Both PCAs, as well as the CVA (Supplementary Fig. S6), were not able to separate any of the species defined by the genetic analyses here. None of the LDAs conducted in this clade were able to clearly distinguish between any of the accepted species.
Zospeum frauenfeldii (Freyer, 1855)
(a–e) Zospeum frauenfeldii, (a1–2) NMBE 553388, Podpeška Jama, sh: 1.982 mm. — (b1–2) NHMW 71882, Podpeška Jama, sh: 1.986 mm. — (c1–2) Paratype of Z. frauenfeldii osolei, MCSMNH 6835, Osoletova jama, sh: 2.01 mm; (d1–2) Paratype of Z. frauenfeldii osolei, ditto, sh: 1.805 mm. — (e1–2) NMBE 548795, Hrustovača špilja, sh: 1.494 mm. —(f–j) Zospeum subobesum, (f1–2) Syntype, MCSMNH 2083, Tounjčica, sh: 1.718 mm. — (g1–2) NMBE 553326, Tounjčica, sh: 1.655 mm. — (h) NMBE 553323, Sustav Đula Medvedica, sh: 1.637 mm. — (i) NMBE 553322, Jama ispod heliodroma, sh: 1.35 mm. — (j1–2) NMBE 553328, Jopićeva špilja, sh: 1.885 mm. —(k–o) Zospeum bucculentum, (k) NMBE 553387, Zvonečka II, sh: 1.62 mm. — (l) NMBE 553344, Jama Đot, sh: 1.546 mm. — (m1–2) Holotype, NMBE 548793, Pivnica špilja, sh: 1.89 mm. — (n1–2) NMBE 548788, Vrelić špilja, sh: 2.053 mm. — (o1–2) NMBE 548814, Jama na Škrilama, sh: 1.997 mm. —All phot. × 20; (b1) E. Bochud, NMBE; (b2), (g2) M. Kampschulte, G. Martels, University of Giessen; (d2) B. Ruthensteiner, ZSM
1855 Carychium frauenfeldii Freyer, Sitzungsber. mathem.-naturw. Cl. kais. Akad. Wiss. Wien, 15 (1): 19, pl. [1] fig. 3a–c (shell) [Im Jahre 1853 von den Herren Franz und Matthias Erjavec zuerst in der Grotte zu Podpeč bei Guttenfeld in Unterkrain gesammelt (Podpeška jama, Dobrepolje); dann von den Herren Skubic und Franz Erjavec in der Grotte bei Duplice nächst Weichselberg in Unterkrain aufgefunden].
1994 Zospeum frauenfeldi osolei Slapnik, Razpr. Slov. Akad. Znan. Umetn., Cl. IV, 35 (13): 312–313, map 6 (distribution), pl. 6 fig. a–c, pl. 7 fig. a (shell) [Osoletova jama in Dešen nad Moravčami].
Type specimens
frauenfeldii: collection Freyer in Trieste contains no Zospeum specimens (pers. comm. De Mattia 2017). osolei: Holotype MCSMNH 6835/1 & paratypes MCSMNH 6835/2–10; ZRC SAZU.
Specimens examined: Slovenia: NMBE 553388/3, Podpeška jama, 23.6.2009, leg. Jochum; NHMW/MO/13031, Podlon cave. — Bosnia and Herzegovina: NMBE 553385/2, Hrustovača špilja, 27.3.2012, leg. Lukic; NMBE 553386/2. Hrustovača špilja, 27.3.2012, leg. Ozimec; NMBE 553387/1, Hrustovača špilja, 27.3.2012, leg. Ozimec; NMBE 548795/2, Hrustovača špilja, 27.3.2012, leg. Cukovic.
Diagnosis
Shell ca. 1.8 mm, conical, transparent, sometimes ribbed, peristome roundish with a straight parietal shield, columella with a parietalis and a weak columellaris.
Measurements (n = 18): sh: 1.45–2.22 mm (mean: 1.776 ± 0.24 mm); sw: 0.99–1.33 mm (mean: 1.171 ± 0.12 mm); ah: 0.77–1.08 mm (mean: 0.9 ± 0.097 mm); aw: 0.64–0.89 mm (mean: 0.741 ± 0.075 mm); number of whorls: 4.75–5.5 (mean: 5.139 ± 0.246).
Description
Shell conical, translucent when fresh; two morphologically very different, but genetically related forms are present:
1) “Typical” Z. frauenfeldii (Fig. 8a–d): relatively tall, its shell surface strongly ribbed; aperture taller than wide; parietal shield sometimes well differentiated from the lip, sometimes not; its margin being straight or convex; parietalis present in the aperture and fairly prominent, extending one whorl into the shell, has a constant size throughout; columellaris starting either just behind the aperture or further within the shell, quickly declining in size, is very weak ¾ a whorl into the shell. Z. frauenfeldii osolei Slapnik 1994 (Fig. 8c, d) is preliminarily classified as a synonym here. It differs from the nominate form through it’s smaller size, it’s more pronounced columellaris reaching one whorl into the shell, its very weak palatalis and it’s, on average, higher rib density. These traits vary either in this taxon or in the nominate species. We are currently averse to subdividing species, given the somewhat chaotic and incomplete taxonomic situation of the genus at the moment and are hesitant to elevate Z. f. osolei to species level (which might be done if genetic data in future studies supports it).
2) Z. frauenfeldii from northern Bosnia (Fig. 8e): small; shell surface smooth; aperture taller than wide; parietal shield well- differentiated from the lip, its margin straight; parietalis present in the aperture, but relatively weak; columellaris starting behind the aperture.
Protoconch densely covered with striae consisting of interconnected pits (Supplementary Fig. S16e); second whorl lacks pits but has discrete ribs and spiral lines forming a reticulate pattern (Supplementary Fig. S16f); teleoconch strongly costate (Supplementary Fig. S16g). Radula: The tricuspid rachidian tooth of Z. frauenfeldii (Podpeška jama) has an obtuse mesocone flanked by a shorter endocone and an ectocone ¾ the length of the mesocone (Supplementary Fig. S17f). The lateral teeth are highly irregular (Supplementary Fig. S17f). Adjacent to the rachidian tooth, the right lateral teeth show multivariate forms (i.e., the mesocone bearing two subequal ectocones and a stubby endocone) with those on the left of the rachidian tooth, bearing two cusps with an additional nub-like endocone on the left side of the mesocone in some rows (Supplementary Fig. S17f). Superficial median grooves are visible on some of the mesocones. Other rows of laterals show a sharply pointed bicuspid tooth bearing a shorter left endocone obliquely extending out from the longer mesocone, measuring about 1/3 the length of the mesocone (Supplementary Fig. S17g). The basal plates are concave their entire length. The strongly-pointed laterals show distinct grooves all down the mesocone with lesser degrees of groove distinctness in others. Grooves down the median section of certain cusps and an uneven wrinkly texture is detectable at the base of the crowns of the marginal teeth of Z. frauenfeldii (Supplementary Fig. S17e). The crowns are broad comb-like structures of varying numbers of pointed, but obtuse cusps (maximal 8), of unequal lengths supported by broad, concave basal plates (Supplementary Fig. S17e). The crowns, are in some cases, one ectocone broader than the tops of the respective basal plate. The radular ribbon is long and broader than that of Z. exiguum (Supplementary Fig. S17h). The folded anterior end appears to be tapered while the opposite end suggests a triangular configuration.
Differing from other forms with ribbed shells by its larger size; not really separable from other smooth species within the Z. frauenfeldii clade (such as Z. bucculentum, Z. pagodulum, Z. robustum).
Distribution (Supplementary Fig. S12)
Mainly distributed to the south and southeast of Ljubljana, with a few sites to the east (Bole 1974; Slapnik 1994). A genetically similar form occurs in northern Bosnia, near Sanski Most.
Zospeum subobesum Bole, 1974
Figures 8f–j, S16c–d, S16h, S21d–f
1974 Zospeum subobesum Bole, Razpr. Slov. Akad. Znan. Umetn., Cl. IV, 17 (5): 278–279, 288, fig. 20a–d (shell), 13 (distribution) [Höhle Tounjčica bei Tounj, 5 km NO von Ogulin].
Type specimens
a holotype was not selected; syntypes MCSMNH 2083/5, Tounjčica, 26.10.1962.
Specimens examined
Slovenia: NHMW 75000/E/00012/7. Spring by Zaga on the left Kulpa bank; NHMW 75000/E/49.251/2, Tounjčica; NMBE 553322/1, Jama ispod heliodroma, 5.12.2009, leg. Lukic; NMBE 553323/2, Sustav Đula Medvedica, 5.9.2009, leg. Bedek; NMBE 553324/1, Špiljski sustav Jopićeva špilja, 9.4.2012, leg. Cvitanovic; NMBE 553325/2, Špiljski sustav Jopićeva špilja, 9.4.2012, Cvitanovic; NMBE 553326/1, Tounjčica, 26.9.2009, leg. Jochum; NMBE 553327/3, Tounjčica, 26.9.2009, leg. Jochum; NMBE 553328/6, Špiljski sustav Jopićeva špilja, 21.4.2011, leg. Komerici; NMBE 553329/1, Špiljski sustav Jopićeva špilja, 21.4.2011, leg. Komerici; NMBE 553330/3, Špiljski sustav Jopićeva špilja, 21.4.2011, leg. Komerici; NMBE 553331/2, Špiljski sustav Jopićeva špilja, 21.4.2011, leg. Komerici.
Diagnosis
Shell ca. 1.7 mm, ovate-conic, transparent, incised suture, last whorl sometimes expanded, peristome often with a large parietal shield, columella with an especially weak parietalis.
Measurements (n = 21)
sh: 1.35–1.89 mm (mean: 1.701 ± 0.127 mm); sw: 0.99–1.35 mm (mean: 1.203 ± 0.091 mm); ah: 0.67–1.09 mm (mean: 0.943 ± 0.102 mm); aw: 0.67–0.97 mm (mean: 0.812 ± 0.069 mm); number of whorls: 4.25–5.25 (4.845 ± 0.301).
Description
Shell ovate-conic, translucent when fresh; shell surface either smooth or with ribs on the uppermost part of the spire; suture deep; last whorl sometimes expanded; aperture as tall as wide or somewhat taller than wide; the parietal shield well-differentiated from the lip, its margin usually straight; a small parietalis present in the aperture, extending one whorl into the shell, remaining as weak as in the aperture; columellaris completely absent; protoconch covered with pits, some interconnecting at intervals (Supplementary Fig. S16c); teleoconch covered with spiral striae consisting of densely interconnected pits (Supplementary Fig. S16d), those getting weaker and fewer towards the aperture. Junction of penultimate and ultimate whorl briefly costate (Supplementary Fig. S16h). Radula: Since the radular ribbon of Z. subobesum did not position well onto the SEM stub, we have fewer views of the radula of this species. The crowns of the pointed transitional teeth are quincuspid, whereby the outermost ectocone is often a mere stub extending beyond the edge of the concave basal plate (Supplementary Fig. S21f). The central cusp is long and pointed, flanked directly by a seemingly detached, shorter ectocone. The deeply cut cusps, forming the crown, are all joined together at the base but detached in their overall appearance as they extend forward over the concave, and partially wing-like, basal plates. The serrated crowns of the marginal teeth appear as broad strips of moderately subequal cusps (Supplementary Fig. S21e). Each crown bears 11–13 mixed obtuse and pointed cusps, divided from each other by an almost equally narrow, cusp-wide space. The basal plates are broad and massive with thick prominences at their edges. The radular ribbon is broad and tapered at its anterior end. Although transposed on top of the back section of the radula, the base of the radula appears to be straight rather than tapered (Supplementary Fig. S21d).
Differing conchologically from all other species in the clade by its small parietalis and by the lack of a columellaris.
Distribution (Supplementary Fig. S12): This species has been genetically confirmed for two caves in Croatia: Tounjčica (Tounj, Karlovac; Fig. 8f, g) and Jopićeva špilja (Krnjak, Karlovac; Figs. 8j). The specimens from Sustav Đula Medvedica (Fig. 8h) and Jama ispod heliodroma (both from Ogulin, Karlovac, Croatia; Fig. 8i) are also tentatively classified with this species.
Zospeum bucculentum Inäbnit, Jochum & Neubert n. sp.
Figure 8k–o
Type specimens
Holotype NMBE 548793, Pivnica špilja, 9.3.2009, leg. Rade; paratypes: NMBE 553339/1, Pivnica špilja, 5.6.2009, leg. Cvitanovic; NMBE 553346/1, Pivnica špilja, 18.3.2009, leg. Cvitanovic; NMBE 553340/3, Pivnica špilja, 18.3.2009, leg. Lukic; NMBE 553351/1 (ex AJC 2306), Pivnica špilja, 18.3.2009; leg. Rade.
Specimens examined
Slovenia: NMBE 548796/3, Pivnica špilja, 5.6.2009, leg. Cvitanovic; NMBE 548814/4, Jama na Škrilama, 20.3.2009, leg. Ozimec; NMBE 548788/2, Vrelić špilja, 12.9.2009, leg. Cvitanovic; NMBE 553338/1, Vrelić špilja, 12.9.2009, leg. Komericki; NMBE 553341/6, Zvonečka II, 17.9.2009, leg. Rade; NMBE 553342/1, Zvonečka II, 19.3.2009, leg. Rade; NMBE 553347/10, Zvonečka II, 6.6.2009, leg. Lukic; NMBE553349/4, Zvonečka II, 19.3.2009, leg. Cvitanovic; NMBE 553350/1, Zvonečka II, 19.3.2009, leg. Rade; NMBE 553343/1, Jama Đot, 6.6.2009, leg. Ozimec; NMBE 553344/2, Jama Đot, 6.6.2009, Cvitanovic; NMBE 553345/1, Jama Đot, 6.6.2009, leg. Ozimec; NMBE 553348/1, Jama Đot, 20.3.2009, leg. Bedek; NMBE 548801, Jama na Škrilama, 20.3.2009, leg. Ozimec & Rade; NMBE 548806, Vrelic špilja, 12.9.2009, leg. Cvitanovic.
Diagnosis
Shell ca. 1.8 mm, conic, transparent, peristome roundish to oval, with a straight or rounded parietal shield, columella with a parietalis and a weak columellaris.
Measurements (n = 41): sh: 1.54–2.06 mm (mean: 1.808 ± 0.122 mm); sw: 1–1.4 mm (mean: 1.186 ± 0.066 mm); ah: 0.73–1.09 mm (mean: 0.947 ± 0.062 mm); aw: 0.7–0.86 mm (mean: 0.773 ± 0.037 mm); number of whorls: 4.75–4.75 (mean: 5.146 ± 0.216).
Description
Shell conical, translucent when fresh; on average, greatly variable, taller and more slender than its relatives (especially in the north of its range and close to that of Z. robustum); whorl often slightly shouldered, sometimes with an obtuse keel; aperture taller than wide, especially pronounced in shells of the northernmost cave (Pivnica špilja), but hardly pronounced in shells from Jama na Škrilama; parietal shield well-differentiated from the lip, its margin usually slightly convex; parietalis present in the aperture with varying prominence, its size increasing until reaching its maximum at half a whorl into the shell; columellaris either present in the aperture, starting just behind the aperture or absent; if present, rapidly decreasing in size within half a whorl; columella sometimes thickened from just below the parietalis down almost to the base, forming a frequently subdivided bump incorporating the columellaris (when present).
Not really separable from other smooth-shelled species within the Z. frauenfeldii clade (such as Z. frauenfeldii, Z. pagodulum, Z. robustum), though generally larger, with a more variable whorl and aperture shape.
Etymology
Named for the large aperture frequently observed in specimens from the Locus typicus (lat.: bucculentus: having a large mouth).
Distribution (Supplementary Fig. S12)
Endemic to the northwestern part of the Karlovac region, Croatia. Genetic records are from three caves: Pivnica špilja (Fig. 8m), Jama na Škrilama (Fig. 8o) and Vrelić špilja (Fig. 8n). Non-molecularly-assessed specimens from Zvonečka II (Fig. 8 k) and Jama Đot (Fig. 8l) are morphologically considered to belong to this species.
Zospeum pagodulum Inäbnit, Jochum & Neubert n. sp.
Figure 9a–e
Type specimens
Holotype NMBE 548815, Kučka jama, 23.4.2009, leg. Bedek; Paratypes NMBE 553415/4, Kučka jama, 23.4.2009, leg. Bedek; NMBE 553362/1, Kučka jama, 23.4.2009, leg. Bedek.
Specimens examined
Croatia: NMBE 548789/1, Grnjača špilja, 24.6.2009, leg. Bedek; NMBE 553361/1, Pećina kod sela Puhari, 21.4.2009, leg. Bedek; NMBE 553359/2, Proljetna jama, 28.6.2009, leg. Lukic; NMBE 553357/1, Jama ispod Tominićevog brega, 25.4.2009, leg. Lukic; NMBE 553358/1, Jama ispod Tominićevog brega, 25.4.2009, leg. Bilandzija; NMBE 553363/5, Jama ispod Tominićevog brega, 25.4.2009, leg. Lukic; NMBE 553360/1, Lučićka špilja, 7.6.2008, leg. Kljakovic; NMBE 548807/1, Grnjača špilja, 24.6.2009, leg. Bedek.
Diagnosis
Shell ca. 1.8 mm, conic, transparent, sometimes ribbed, peristome roundish with a straight parietal shield, columella with a parietalis and a weak columellaris.
Measurements (n = 18): sh: 1.61–1.99 mm (mean: 1.764 ± 0.089 mm); sw: 1.08–1.26 mm (mean: 1.167 ± 0.049 mm); ah: 0.8–1.01 mm (mean: 0.924 ± 0.059 mm); aw: 0.66–0.81 mm (mean: 0.747 ± 0.038 mm); number of whorls: 4.75–5.5 (mean: 5.056 ± 0.22).
Description
Shell conical, translucent when fresh; shell usually smooth, but a ribbed population occurs in Grnjača špilja; aperture taller than wide; parietal shield well-differentiated from the lip, its margin usually straight, sometimes convex; parietalis present in the aperture, though comparatively weak; columellaris starting behind the aperture, usually weak.
Not really separable from other smooth-shelled species within the Z. frauenfeldii clade (such as Z. frauenfeldii, Z. bucculentum, Z. robustum); whorl usually less shouldered than in Z. bucculentum. Both species demonstrate considerable variation.
Etymology
Named for the pagoda-like shell shape of the species in general and the holotype in particular.
Distribution (Supplementary Fig. S12)
Endemic to the Croatian Primorje-Gorski Kotar region, where it is known primarily from five caves on the Istrian peninsula: Kučka jama (Fig. 9a), Grnjača špilja (Fig. 9e), Proljetna jama, Pećina kod sela Puhari (Fig. 9c; together with Z. pretneri) and Jama ispod Tominićevog brega (Fig. 9b). An empty shell from Lučićka špilja near Delnice (Fig. 9d) is morphologically assigned to this species.
(a–e) Z. pagodulum, (a1–2) Holotype, NMBE 548815, Kučka jama, sh: 1.982 mm. — (b) NMBE 553363, Jama ispod Tominićevog brega, sh: 1.759 mm. — (c) NMBE 553361, Pećina kod sela Puhari, sh: 1.781 mm. — (d) NMBE 553360, Lučićka špilja, sh: 1.613 mm. — (e1–2) NMBE 548789, Grnjača špilja, sh: 1.765 mm. —(f–n) Z. robustum, (f1–2) NMBE 553536, Tonkovića špilja, sh: 1.826 mm. — (g) NMBE 554397, Tonkovića špilja, sh: 1.52 mm. —(h) NMBE 553368, Špilja pod Mačkovom dragom, sh: 1.562 mm. — (i) Holotype, NMBE 548791, Markov ponor, sh: 2.15 mm. — (j1–2) NMBE 553352, Budina špilja, sh: 1.826 mm. — (k1–2) NMBE 553534, Vrlovka, sh: 1.727 mm. — (l) NMBE 553367, Vrlovka, sh: 1.595 mm. — (m) NMBE 554399, Židovske kuće, sh: 1.51 mm. — (n) NMBE 554400, Pušina jama, sh: 1.61 mm. — All phot. × 20
Zospeum robustum Inäbnit, Jochum & Neubert n. sp.
Figure 9f–n
Type specimens
Holotype NMBE 548791, Markov ponor, 20.8.2011, leg. Raguz; paratypes: NMBE 548777/1, Markov ponor, 20.8.2011, leg. Raguz; NMBE 548778/1, Markov ponor, 20.8.2011, leg. Raguz; NMBE 548779/1, Markov ponor, 20.8.2011, leg. Raguz; NMBE 548780/1, Markov ponor, 20.8.2011, leg. Raguz; NMBE 548781/1, Markov ponor, 20.8.2011, leg. Raguz; NMBE 548782/1, Markov ponor, 20.8.2011, leg. Raguz; NMBE 548783/1, Markov ponor, 20.8.2011, leg. Raguz; NMBE 548784/1, Markov ponor, 20.8.2011, leg. Raguz; NMBE 548785/1, Markov ponor, 20.8.2011, leg. Raguz; NMBE 548786/1, Markov ponor, 20.8.2011, leg. Raguz; NMBE 548787/1, Markov ponor, 20.8.2011, leg. Raguz.
Specimens examined
Croatia: NMBE 548797/1, Budina špilja, 29.1.2011, leg. Bregovic; NMBE 553352/4, Budina špilja, 11.6.2011, leg. Raguz; NMBE 553353/3, Budina špilja, 29.1.2011, leg. Bilandzija; NMBE 553355/1, Budina špilja, 24.7.2007, leg. Pavlec; NMBE 548790/1, Ostrvička špilja, 12.6.2011, leg. Cukovic; NMBE 553354/1, Ostrvička špilja, 12.6.2011, leg. Bilandzija; NMBE 553356/1, 29.7.2010, leg. Bedek; NMBE 548773/1, Budina špilja, 29.1.2011, leg. Bregovic; NMBE 548809/1, Ostrvička špilja, 22.8.2009, leg. Lukic; NMBE 553534, Vrlovka, 7.6.2009, leg. Lukic; NMBE 553367/16, Vrlovka, 7.6.2009, leg. Lukic; NMBE 548799/7, Vrlovka, 19.3.2009, leg. Bedek; NMBE 553364/1, Vrlovka, 19.3.2009, leg. Bedek; NMBE 553365/10, Vrlovka, 6.6.2009, leg. Bedek; NMBE 553366/1, Vrlovka, 17.3.2009, leg. Bedek; NMBE 548776/1, Vrlovka, 17.9.2009, leg. Cvitanovic; RS 2210a/1, Vrlovka, 2.12.2015, leg. Ozimec; NMBE 554399/1, Židovske kuće, 12.4.2008, leg. Kljaković; NMBE 554400/1, Pušina jama, 20.4.2008, Pavlek; NMBE 553536, Tonkovića špilja, 24.7.2011, leg. Bedek; NMBE 554397/1, Tonkovića špilja, 24.7.2011, leg. Bedek; NMBE 554398/1, Tonkovića špilja, 24.7.2011, leg. Bedek; NMBE 553368/1, Špilja pod Mačkovom dragom, 11.9.2009, leg. Lukic.
Diagnosis
Shell ca. 1.6 mm, ovate-conic, transparent, last whorl sometimes expanded, peristome roundish, sometimes quite massive, with a straight parietal shield, columella with a parietalis and a weak columellaris.
Measurements (n = 51)
sh: 1.45–2.15 mm (mean: 1.649 ± 0.124 mm); sw: 0.99–1.54 mm (mean: 1.108 ± 0.0911 mm); ah: 0.76–1.32 mm (mean: 0.863 ± 0.083 mm); aw: 0.62–1.13 mm (0.738 ± 0.073 mm); number of whorls: 4.75–6 (mean: 5.025 ± 0.231).
Description
Shell ovate-conical, translucent when fresh; shell either smooth or ribbed, either at the uppermost part of the whorl or the entire whorl; shell shape variable: specimens from Markov ponor resembling Z. obesum, specimens from Tonkovića špilja resembling Z. frauenfeldii, others resemble more Z. pagodulum and Z. bucculentum; aperture taller than wide; parietal shield differentiated from the lip, its margin either straight or, more rarely, concave; parietalis in most cases present in the aperture, though often fairly small, increasing in size for half a whorl; a weak columellaris usually starting behind the aperture; columellaris completely absent in ribbed populations.
Smaller, smooth forms (e.g., Budina špilja, Vlovka) not really separable from other smooth-shelled species within the Z. frauenfeldii clade (such as Z. frauenfeldii, Z. bucculentum, Z. pagodulum); tentatively differing from Z. bucculentum by its smaller size and its less shouldered whorls; the form from Markov ponor differs from Z. obesum by the presence of ribs on the upper part of the whorl; the form from Tonkovića špilja and Špilja pod Mačkovom dragom differ from Z. frauenfeldii and Z. pagodulum by their lack of a columellaris.
Etymology
Named for the solid, robust shell of the Holotype (lat.: robustum: robust)
Distribution (Supplementary Fig. S12)
Occurs in Croatia in three disjunct areas: 1) The Lika-Senj region, for which we have genetic records for two caves (Budina špilja (Fig. 9j) and Markov ponor (Fig. 9i)). Two additional populations are tentatively assigned to this species, including Ostrvička špilja and the upper part of the Lukina jama-Trojama cave system (see Fig. 4C in Weigand 2013)); 2) Two caves north of Ogulin, one with a genetic record (Tonkovića špilja (Fig. 9f, g)) and one without (Špilja pod Mačkovom dragom (Fig. 9h)); 3) Three caves in and around the Žumberak Mountains (Vrlovka (Fig. 9k, l), Pušina jama (Fig. 9n) and Židovske kuće (Fig. 9m)).
Remarks
The three disjunct areas are reflected in the phylogenetic trees, albeit with low node support. Although future studies might find them to be independent species, our sample size does not allow definite conclusions.
Not assigned to any of the clades
Included here are the species without genetic data that could not be unequivocally assigned to any of the genetic clades.
Zospeum globosum Kuščer, 1928
(a–h) Zospeum globosum, (a) Syntype, NHMW 49117, Covelo del Rio Malo, sh: 1.617 mm; (b) Syntype, ditto, sh: 1.63 mm. — (c1–2) Syntype, NHMW 75000, Covelo del Rio Malo, sh: 1.521 mm. — (d) NMBE 554730, Buso del Sasso, sh: 1.28 mm; (e) ditto, sh: 1.53 mm. — (f) NMBE 554726, Bucco del Budrio, sh: 1.62 mm. — (g1–2) NMBE 549728, Funtani di Nalmase, sh: 1.436 mm. — (h1–2) NHMW 111543, Cavernetta di Vobarno, sh: 1.741 mm. — (i–j) Zospeum clathratum, (i1–2) Holotype, NMBE 553535, Špilja Mandelaja, sh: 1.594 mm. — (j) Paratype, NMBE 553298, Špilja Mandelaja, sh: 1.643 mm. —(k–p) Zospeum kusceri, (k) Syntype, NHMW 69380, Tončetova jama, sh: 1.77 mm; (l1–2) Syntype, ditto, sh: 1.74 mm; (m) Syntype, ditto, sh: 1.79 mm. — (n) NMBE 553400, Jama SDB, sh: 1.815 mm; (o) ditto, sh: 1.618 mm. — (p) NMBE 548792, Jama pod Boljunskim dolom, sh: 1.902 mm. —(q–s) Zospeum freyeri, (q) CSRSASA 30007, Vratnica, sh: 1.554 mm; (r1–3) ditto, sh: 1.497 mm; (s1–2) ditto, sh: 1.508 mm. —(t–v) Zospeum likanum, (t1–2) Paratype, CSRSASA 1234, Gornja Cerovačka pećina, sh: 1.622 mm; (u) Paratype, ditto, sh: 1.65 mm; (v) Paratype, ditto, sh: 1.739 mm. — Figs. 201–205: Zospeum shells of unknown identity, (e1–2) NMBE 553291, Velja peć, sh: 1.665 mm; (x) ditto, sh: 1.732 mm; (y) ditto, sh: 1.757 mm. — (z) NMBE 553294, Utopišće. — (aa) NMBE 553293, Kali pećina. — All phot. × 20; (a–c1) S. M. Schnedl, NHMW; (c2, h2, l2, v) M. Kampschulte, G. Martels, University of Giessen; (h1) E. Bochud, NMBE; (k, l1, m) P. Albano, NHMW; (r2 + 3) B. Ruthensteiner, ZSM
1928 Zospeum globosum Kuščer, Studi trent. Sci. nat., 9 (2): 185–187, 1 figure (shell) [Questa specie cavernicola fu scoperta dal mio amico coleotterologo E. Pretner (Trieste) nel 1917 durante le battaglie sull’altipiano di Folgaria in una grotta dell’altipiano, presso il villaggio di Piccoli (45° 55¾ N, 28° 55¾ E Greenw. alt. s. m. ca. 950 m.) (on page 187 the name of the cave is mentioned: “Covelo di Rio Malo”)].
1944 Zospeum cariadeghensis Allegretti, Le Grotte d’Italia, (2) 5: 48–49, 50, pl. 1 fig. 2 (shell), pl. 2 fig. 1–2 [nel Buco del Budrio, n. 71 Lo. (Altipiano di Cariàdeghe), e nella grotta Omber Calamor, n. 64 Lo. (Serle – Brescia) (Allegretti leg.)].
1944 Zospeum cariadeghensis var. turriculatum Allegretti, Le Grotte d’Italia, (2) 5: 49–50, 51, pl. 1 fig. 3 (shell) [Trovato in un paio di insenature rappresentate da due basi di camino, in fondo al Legondol del Rigù, n. 201 Lo. (M. Dragone – Brescia) (Allegretti leg.)].
1956 Zospeum allegrettii Conci, Premier Congr. Intern. Spéléol., 3 (3): 278–280, fig. 7–10 (shell), 13 (distribution) [nel Buso de la Rana N. 40 V., cavità che si apre a 350 m, presso Monte di Malo (provincia di Vicenza) (Bozzini, Conci, Galvagni & Tamanini leg., 28–29 December 1952)].
1956 Zospeum galvagnii Conci, Premier Congr. Intern. Spéléol., 3 (3): 278, fig. 306 (shell), 11–12 (tracks), 13 (distribution) [Grotta del Calgeron N. 244 V.T., cavità che si apre a 450 m, presso Grigno in Valsugana (provincia di Trento) (Conci, Galvagni, Perini & Tamanini leg., 1952 + 1953)].
Type specimens
Z. globosum: syntypes NHMW/E 12469/5 = NHMW-MO-75000, Grotte bei Piccoli, Plateau von Lavarone, coll. Edlauer ex Kuščer; syntypes NHMW/E 49117, from the type locality ex coll. Edlauer ex Kuščer; probable syntypes NHMW 69379, Grotte bei Piccoli, Plateau von Lavarone, ex coll. Oberwimmer. Z. cariadeghensis: a holotype was not selected. Z. turriculatum: a holotype was not selected. Z. allegrettii + Z. galvagnii: no type specimens present in the museums of Verona, Genua, Milano, Torino, Trento. Holotypes for both taxa were not selected.
Specimens examined
Italy: NHMW 111543/3, Cavernetta di Vobarno, leg. Pezzoli; NMBE 549728/20, Funtani di Nalmase, 8.9.1970, leg. Pezzoli; NMBE549729/3, Funtani di Nalmase, 8.10.1997, leg. Nardi; GN 428/3, Buco del Budrio, 22.1.1988, leg. Ferrari & Piva; GN 429/7, Spluga Carpene, 7.5.1983, leg. Ferrari & Piva; GN 430/2, Büs Pra de Rent, 22.1.1988, leg. Ferrari & Piva; GN 431/3, Grotta d’Inverno, 27.3.1984, leg. Ferrari & Piva; GN 432/6, Buso del Sasso, 14.5.1988, leg. Ferrari & Piva.
Diagnosis
Shell ca. 1.5 mm, transparent, with a globose to conical form, peristome roundish, with a straight parietal shield, columella with a parietalis and a weak columellaris.
Measurements (n = 38)
sh: 1.22–1.75 mm (mean: 1.538 ± 0.107 mm); sw: 0.91–1.31 mm (mean: 1.097 ± 0.093 mm); ah: 0.64–0.93 mm (mean: 0.821 ± 0.076 mm); aw: 0.63–0.9 mm (mean: 0.741 ± 0.072 mm); number of whorls: 4.5–6.5 (mean: 5.191 ± 0.383).
Description
Shell conical to conical-globose, transparent when fresh; last 1–1½ whorls usually expanded, suture. shallow; shell shape inter- and intraspecifically very variable, ranging from globose to turriculate, prominence of last whorl variable; parietalis present in the aperture extending one whorl into the shell while maintaining a constant size; columellaris usually, but not always, present in the aperture, its size attenuating considerably to a varying degree half a whorl into the shell, (disappearing in Covelo di Rio Malo (Fig. 10c1), considerably weaker in specimens from Cavernetta di Vobarno (Fig. 10h2)).
Distribution (Supplementary Fig. S13)
Reported from the Italian Provinces of Brescia, Trento, Verona and Vicenza (Pezzoli 1992).
Remarks
Giusti and Pezzoli (1982) and Pezzoli (1992) synonymized Z. cariadeghense Allegretti 1944, Z. cariadeghense var. turriculatum Allegretti 1944, Z. galvagnii Conci 1956 and Z. allegrettii Conci 1956 with Z. globosum without giving any justification. This classification is retained here for the following reasons: (1) There is some variation within populations concerning shell shape. The population in Buso del Sasso (Fig. 10d, e), for instance, shows both globose and non-globose individuals. Shell shape is thus, an insufficient character to separate Z. globosum from the taxa mentioned above. (2) Topotypic material of Z. c. turriculatum (Fig. 9.5–8 in Pezzoli 1992) displays a wide range of variation in shape that overlaps with topotypic Z. cariadeghense and therefore, cannot be considered an independent species. (3) Topotypic Z. allegrettii (Fig. 15.1–4 in Pezzoli 1992) is very similar to topotypic Z. cariadeghense (Fig. 10f; Fig. 9.1–2 in Pezzoli 1992). The same is true for topotypic material of Z. galvagnii (Fig. 11.1–4 in Pezzoli 1992). Both PCA analyses (Supplementary Fig. S7, top & middle) were unable to separate distinct clusters. The CVA (Supplementary Fig. S7, bottom) separated populations from west of Lake Garda from those to the east, as well as, the eastern caves from each other. However, the relevant shape changes are minimal and the sample sizes usually small. Hence, we can not confidently split Z. globosum into several species. A more comprehensive study on the Z. globosum clade, including genetic data, would be beneficial (Nardi 2015), especially since the samples available to us were too old for genetic analyses.
Zospeum clathratum Inäbnit, Jochum & Neubert n. sp.
Figure 10i–j
Type specimens
Holotype: NMBE 553535, Špilja Mandelaja, 10.9.2009, leg. Bedek; Paratypes: 553299/2, Špilja Mandelaja, 10.9.2009, leg. Bedek; NMBE 553298/2, Špilja Mandelaja, 10.9.2009, leg. Lukic;
Diagnosis
Shell ca. 1.6 mm, conical, transparent, with both ribs and strong spiral striae, peristome roundish, with a straight parietal shield, columella with a parietalis and a weak columellaris.
Measurements (n = 5)
sh: 1.49–1.65 mm (mean: 1.58 ± 0.064 mm); sw: 0.95–1.15 mm (mean: 1.052 ± 0.077 mm); ah: 0.8–0.94 mm (mean: 0.871 ± 0.057 mm); aw: 0.66–0.71 mm (mean: 0.695 ± 0.018); number of whorls: 4.5–5 (mean: 4.75 ± 0.204).
Description
Shell conical, translucent when fresh; the entire teleoconch costate, especially in the upper part of the whorl; ribs in the lower part of the whorl usually as strong as the spiral striae; strong spiral striae, in fresh specimens always more pronounced than, e.g., in Z. obesum; aperture taller than wide; parietal shield well-differentiated from the lip, its margin straight; parietalis present in the aperture, though not very pronounced, extending one whorl into the shell, maintaining a constant size; columellaris either present in the aperture or starting just beyond, though not extending far into the shell.
Differing from all other species by its pronounced grid-like microsculpture, an entirely unique character.
Etymology
Named after its grid-like surface structure (Latin: clathratus, −a, −um = covered by a grid).
Distribution (Supplementary Fig. S13)
Only known from the Špilja Mandelaja close to Oštarjie (Ogulin, Karlovačka, Croatia).
Zospeum kusceri A.J. Wagner, 1912
Figure 10k–p
1912 Zospeum frauenfeldi kusceri A.J. Wagner, Verh. kais. königl. zool.-bot. Ges. Wien, 62 (8/9): 257 [Kačna jama bei Divača, Tončetova jama, Grotte Inceria bei Markovina in der Umgebung von Triest].
Type specimens
syntypes NHMW N. 69,380/3. Tončetova jama.
Specimens examined
Slovenia: NMBE 554703/6, Štefakova pečina, leg. De Mattia. — Croatia: NMBE 548792/1, Jama pod Boljunskim dolom, 26.6.2009, leg. Bedek; NMBE 553397/11, Jama SDB, 26.6.2009, Jalzic; NMBE 553400/18, Jama SDB, 26.6.2009, leg. Lukic; NMBE 553404/1, Jama SDB, 26.6.2009, leg. Bilandzjia; NMBE 553396/1, Klanski ponor, 2.4.2011, leg. Đud; NMBE 553395/1, Borušnjak 3 Jama, 25.6.2009, leg. Lukic; NMBE 553398/1, Borušnjak 3 Jama, 25.9.2009, leg. Jalzic; NMBE 553401/4, Borušnjak 3 Jama, 25.6.2009, leg. Lukic; NMBE 553402/1, Vrelo, 7.7.2007, leg. Bilandzija; NMBE 553403/1, Vrelo, 7.7.2007, leg. Lukic; NMBE 553399/2, Jama pod križ, 27.6.2009, leg. Lukic.
Diagnosis
Shell ca. 1.7 mm, conic, transparent, upper section of teleoconch whorls moderately costate, peristome roundish, with a straight parietal shield, columella with a parietalis and a columellaris, both being prominent in the aperture.
Measurements (n = 47): sh: 1.53–1.91 mm (mean: 1.698 ± 0.083 mm); sw: 1.06–1.21 mm (mean: 1.135 ± 0.036 mm); ah: 0.77–0.97 mm (mean: 0.871 ± 0.043 mm); aw: 0.65–0.84 mm (mean: 0.759 ± 0.036 mm); number of whorls: 5–5.75 (mean: 5.356 ± 0.2).
Description
Shell conical, translucent when fresh; upper section of teleoconch whorls moderately costate; aperture roundish, somewhat taller than wide; parietal shield well separated from the lip; parietalis pronounced in the aperture, extending one whorl into the shell at a constant size; columellaris present, reaching nearly as far into the shell as the parietalis with a similarly constant size; a supraparietalis present (Fig. 10L2, 10 N), starting half a whorl into the shell and ending after half a whorl.
Distribution (Supplementary Fig. S13)
Known from eastern Istria and Primorje-Gorski kotar in Croatia (Slapnik & Osimec 2004). Widely distributed in Slovenia, primarily in the southwestern parts, with some sites east and northeast of Ljubljana (Bole 1974; Slapnik 1994).
Zospeum freyeri (F.J. Schmidt, 1849)
Figure 10q–s
1849 Pupa freyeri F.J. Schmidt, Laibacher Zeitung, Illyrischen Blatte, April: 154 [Laschitz (Vratnica cave, near Velike Lašče)].
1855 Carychium freyeri Freyer, Sitzungsber. mathem.-naturw. Cl. kais. Akad. Wiss. Wien, 15 (1): 18–19, pl. fig. 1a–c (shell = holotype) [unweit des Einganges in der Bratenea-Grotte entdeckt, in welche sich der Bratenea-Bach bei Grosslaschitz in Unterkrain ergiesst, dessen unterirdisches Flussbett ich 385 Schritte weit verfolgen konnte (Freyer leg., 29-08-1848, 1 ex.)].
Type specimens
Not in Ljubljana fide Slapnik; not found in the von Frauenfeld collection in Vienna (specimen collected by Erjavec from 1853).
Specimens examined
Slovenia: MCSMNH 30007/15, Vratnica.
Diagnosis
Shell ca. 1.5 mm, conic, transparent, peristome roundish, with a straight parietal shield, columella with a parietalis and a weak columellaris.
Measurements (n = 14)
sh: 1.44–1.68 mm (mean: 1.543 ± 0.075 mm); sw: 1.04–1.17 mm (mean: 1.092 ± 0.046 mm); ah: 0.75–0.88 mm (mean: 0.807 ± 0.038 mm); aw: 0.66–0.8 mm (mean: 0.716 ± 0.042 mm); number of whorls: 4.75–5.25 (5.117 ± 0.186).
Description
Shell conical, translucent when fresh; shell surface smooth; aperture somewhat taller than wide; parietal shield well-differentiated from lip, its margin more or less straight; parietalis present in the aperture, though not very pronounced, extending one whorl into the shell at a constant size; columellaris either present in the aperture or starting just behind it, disappearing after half a whorl (Fig. 10r2 and 3); morphologically indistinguishable from Z. likanum.
Distribution (Supplementary Fig. S13)
Only known from the Vratnica cave, close to Velike Lašče (central Slovenia).
Zospeum likanum Bole, 1960
Figure 10t–v
1960 Zospeum likanum Bole, Biol. Vestn., 7: 61–62, 64, fig. 2a–i (shell) [die Höhle Gornja Cerovačka pećina (Bole leg., June 1958)].
Type specimens
Holotype: MCSMNH 1237, Gornja Cerovačka pećina, 6.1958, Bole; paratypes. MCSMNH 1234, Gornja Cerovačka pećina, 6.1958, Bole.
Diagnosis
Shell ca. 1.7 mm, conic, transparent, peristome roundish, with a straight parietal shield, columella with a parietalis and a weak columellaris.
Measurements (n = 11)
sh: 1.56–1.77 mm (mean: 1.668 ± 0.064 mm); sw: 1.1–1.27 mm (mean: 1.183 ± 0.044 mm); ah: 0.79–0.94 mm (mean: 0.882 ± 0.044 mm); aw: 0.71–0.84 mm (mean: 0.793 ± 0.047 mm); number of whorls: 4.75–5.25 (5.045 ± 0.218).
Description
Shell conical, translucent when fresh; shell surface smooth; aperture somewhat taller than wide; parietal shield well-differentiated from the lip, its margin straight; parietalis present in the aperture, though not very pronounced, extending one whorl into the shell, broadest half a whorl in; columellaris present, starting just behind the aperture and extending half to ¾ a whorl into the shell, most pronounced close to the aperture (Fig. 10(v)); morphologically indistinguishable from Z. freyeri.
Distribution (Supplementary Fig. S13)
Known from the region around Gračac (Zadar, Croatia).
Unassigned Individuals
We left the following specimens without further classification:
Three specimens (NMBE 553291; Fig. 10w–y) from Velja peć (Nikšič, Montenegro), which are morphologically similar to Z. pagodulum, Z. robustum or Bosnian Z. frauenfeldii, though the columellaris is considerably weaker in these specimens than is usually the case in these species. We avoided assigning it its own species status or designating it as part of another species due to lack of distinctive morphological characters separating it from similar taxa, the paucity of available specimens and the relatively great geographical distance to populations with similar morphology.
One fragment of the upper part of the shell (NMBE 553294; Fig. 10z) from Utopišće (Proložac, Splitsko-dalmatinska, Croatia) might well represent a potentially undescribed species with a globose shell. Our material is too scanty to properly diagnose or compare it to other species.
Several fragments from the same individual (NMBE 553293; Fig. 10aa), found in Kali pećina (Grebci, southern Herzegovina), might belong to Z. troglobalcanicum because it was found close to the type locality of Z. troglobalcanicum (both located close to Grebci) and because it bears congruent morphological characters such as a naked columella and the strongly increasing teleoconch whorls, indicating that the complete shell was as similarly broad as the Z. troglobalcanicum specimen in our dataset. The shape of the apex is, however, not in agreement with the figures depicting Z. troglobalcanicum (Absolon 1916; Bole 1974, Fig. 3h; this work Fig. 10u), as the protoconch here is expanded in a way that can be found in other species from the Z. pretneri clade (most similar to Z. manitaense), while it is not expanded in the figures presented above.
Three specimens similar to Z. frauenfeldii (NMBE 553382) were found in the same sample as seven Z. costatum (NMBE 553383) and two Z. amoenum (NMBE 553381) specimens from Jama 2 pri Jabljah (Loka pri Mengšu, Slovenia). Z. frauenfeldii has never been found in that cave nor in that region but might have previously been accidentally added to the sample (Bole 1974). This could well be substantiated by the finding of three specimens (NMBE 553295) that are very similar to the aforementioned Z. amoenum individuals in a sample of Z. frauenfeldii (NMBE 553388) from Podpeška jama. We remark that NMBE 553295 could also represent the “Z. isselianum” specimens Bole (1974) reported from this cave.
Discussion
Our molecular phylogenetic analysis contradicts the conventional morphology-based system proposed by Bole (1974) and modified by Slapnik (1991, 1994) for Dinaridic Zospeum species (see Table 6). The conflict may be due to the following: (1) frequent homoplasy in character states previously thought to be taxonomically significant, (2) rapid morphological changes in certain lineages, (3) cryptic speciation not discernible by morphology-based methods due to the general paucity of available morphological characters and d) hybridization processes. The most striking example is Z. isselianum as defined by Bole (1974), which was found to be highly polyphyletic in our genetic study. Except for the Z. spelaeum clade, individuals of Z. isselianum appeared in each of the major clades. In some cases, conspecific with specimens with a clearly distinct morphology (such as in Z. pagodulum and in Z. robustum).
Phylogenetic analysis
The topologies for both trees are identical (Fig. 3) in which five main clades are recovered in the phylogenetic reconstructions. The relationships between these clades are mostly well supported and correspond with those reported in Weigand et al. (2013). Not well supported is the sister group relationship between the Z. pretneri clade and the Z. frauenfeldii clade, which was also recovered and well supported in Weigand et al. (2013).
For the Z. spelaeum clade, the genetic material is, with the exception of the specimen from Hotiške ponikve, identical to that used in Weigand et al. (2013). The results in this clade are identical to those reported by Weigand et al. (2013): Two species were recovered: one from the Alpine foothills north of Ljubljana, the second, distributed from the northern Dinarides just south of Ljubljana to the region of Trieste. We use Z. costatum here for the first species, which remained nameless in Weigand et al. (2013), due to morphological similarities to topotypic specimens and geographic proximity to the type locality. The second species is referred here, as in Weigand et al. (2013), Z. spelaeum. In the past, Z. spelaeum has been subdivided into several subspecies (following Bole 1974) based on the presence of ribs. Our results do not support such a subdivision, as no clearly supported lineages could be recovered in the phylogeny.
The Z. alpestre clade (Fig. 3, blue part), endemic to the southeastern Alps (Supplementary Fig. S9), splits into four species: Z. alpestre, Z. amoenum, Z. isselianum and Z. kupitzense. These results corroborate the findings of Weigand et al. (2013) and Jochum et al. (2015c) however, the topology of the trees is different. While the trees of Weigand et al. (2013) and Jochum et al. (2015c) recover Z. isselianum and Z. kupitzense as sister species, with Z. amoenum as their sister species and Z. alpestre positioned at the base of the clade, we found that the clade splits into two lineages. One lineage contains Z. alpestre and Z. kupitzense, the other, which is not supported in the Bayesian tree, Z. amoenum and Z. isselianum. We suspect that this change in the topology was caused by the addition of two new specimens (Z. alpestre from Jelenska zijalka and Z. amoenum from Ihanščica).
The Z. obesum clade (Fig. 3, brown part), currently known from southern Slovenia and Istria (Supplementary Fig. S10), was divided into two clearly separated species as already shown by Weigand et al. (2013): Z. obesum and Z. exiguum. A specimen previously determined morphologically as Z. isselianum from Istria was found to cluster among Z. exiguum.
The Z. pretneri clade (Fig. 3, green part), distributed close to the coast of the Adriatic Sea (Supplementary Fig. S11), splits into two main lineages. The first one is not well-supported in the Bayesian tree and contains Z. pretneri and Z. tholussum, which were found to be closely related by Weigand et al. (2013). The second lineage comprises one species (Z. manitaense) that was previously determined morphologically as either Z. isselianum or Z. amoenum.
The Z. frauenfeldii clade (Fig. 3, violet part), chiefly distributed in the Dinarides (Supplementary Fig. S12), splits into three main lineages, whose ancestral relationships are not resolved in the Bayesian tree and not significantly resolved in the Consensus tree. The first lineage contains two species: Z. frauenfeldii and Z. bucculentum. The topology within Z. bucculentum is identical in both trees while the three caves yielding genetic material happen to be geographically proximal to each other. This instance, as well as the fact that it was recognized as different from Z. frauenfeldii in five out of six species delimitation runs (Table 5), convinces us to disregard the fairly low node support and consider it an independent species. The individuals, classified as Z. bucculentum here, were formerly classified morphologically as Z. isselianum as was a specimen from northern Bosnia, which we classified here with Z. frauenfeldii. Though found far from the type locality, the Bosnian specimen aligned with Z. frauenfeldii on the Bayesian tree in sync with the results of the BPP run. Its association with Z. frauenfeldii is, however, not supported by the Consensus tree. This designation, should therefore, be considered preliminary as the population might also be an independent species. The second main lineage includes one species, Z. subobesum. The specimen from Jopićeva špilja is classified along with Z. subobesum, as it was in Weigand et al. (2013), even though their association is not supported in the Bayesian tree and only very weakly supported in the Consensus tree. Most of the species delimitation runs including these sequences support this classification, as well as the observed morphological similarities (very small parietalis, missing columellaris). The third main lineage consists of two species, Z. pagodulum and Z. robustum. Z. pagodulum showed high node support values and was recovered as different from Z. robustum in four out of six species delimitation runs (Table 5), leading us to consider it an independent species. The remaining specimens of the Z. frauenfeldii clade, originating from three distinct geographical regions, were united into Z. robustum. The topology within this species is identical in both trees separating the three geographic regions but, it is badly supported. We thus, refrain from subdividing this species any further. All species in the third main lineage were initially classified as Z. isselianum. Z. pagodulum from Grnjača špilja and Z. robustum from Tonkovića špilja were previously designated as Z. frauenfeldii while Z. robustum from Markov ponor was previously classified as Z. obesum.
Morphometry
As already emphasized in Jochum et al. (2015b), Zospeum harbors a wide spectrum of variation of conventional morphological characters (i.e., lamella configuration and superficial ribbing) within and between populations. In this study, we also observed (chiefly within the Z. frauenfeldii clade) that some of these characters can stay constant across several species. Moreover, these characters reliably separated species on occasions in which the uniqueness of a stable configuration persisted throughout a species such as in the prime example of Z. kusceri. Morphological characters were also useful in separating species based on certain traits such as within the Z. isselianum clade.
Overall, the morphometrical methods (measurement-based PCA: 602 individuals; Geometric PCA and CVA: 625 individuals) yielded mixed results. At the clade level, both PCAs and the CVA (Supplementary Fig. S1) largely separated both the Z. pretneri clade and the Z. obesum clade from the others with little overlap. In comparison, the other clades were divided much less consistently across the three methods, whereby the CVA proved to be the most effective. Successful was also the LDA, which separated four out of ten pairs. In applying LDA and CVA, complications arose if a predefined classification was not possible (i.e., for cryptic species or caves without genetic data) or if errors in the classification were made.
The realignment of the classification system of the Z. spelaeum clade contrasted considerably to that proposed by Bole (1974). In concurrence with De Mattia (2003), we found that superficial ribbing within populations is too variable to be used as a character for separating taxa. Our experience here showed that superficial ribbing, together with the genetic data, supported the lumping of the subspecies Z. s. spelaeum, Z. s. schmidti and most of “Z. s. costatum” into one monotypic species, Z. spelaeum. Because Z. lamellatum is clearly morphologically distinct (slender shell, strongly enlarged lamellae and prominent dentition), it is recognized here as an independent species. Specimens from the “Z. s. schmidti” population of Jama 2 pri Jabljah have a similar apertural shape (strongly shouldered palatal side) as the topotypic material of Z. costatum (whose type locality is only 12.46 km away). Therefore, we treat them together as an independent species. Z. lautum is retained here mainly due to lack of specimens both in the morphological and the molecular part of this study. The few specimens we had did not differ significantly in their morphology. Their distinctness in the CVA (Supplementary Fig. S2, bottom) could be attributed to the insufficient sample size. Z. trebicianum, a seldom recognized name that some authors unofficially considered to be a synonym of Z. spelaeum (De Mattia 2003), is considered here as an independent species due to differences in morphology and ecology to local Z. spelaeum populations. Morphometry was only marginally useful to separate species in this clade, especially for Z. spelaeum. The geometric PCA, the CVA and the LDA were able to separate species with fewer specimens (Z. costatum, Z. lamellatum, Z. lautum, T. trebicianum).
The treatment of the Z. alpestre clade mostly follows that proposed in Jochum et al. (2015c). The only exception is that we attributed the name Z. amoenum to their lineage “L2” due to the placement in the genetic trees of a specimen from Ihanščica, the same cave from which the broken syntype of Z. amoenum derived. As Jochum et al. (2015c) emphasized, there is substantial variation in the few known morphological characters, making it difficult to separate the genetically derived species. Our results suggest a possible separation based on:
-
a)
The development and position of the parietalis within the shell (such as remaining attached to the parietal side of the whorl in Z. kupitzense, increasing in size until half a whorl into the shell in Z. alpestre or maintaining a constant size (and being occasionally absent) in Z. amoenum);
-
b)
The prominence or presence of the columellaris (though this varies within Z. alpestre). The geometric PCA and the CVA were able to separate all species except for Z. amoenum from each other. The LDA separated all other species from Z. isselianum, but none of the other species from each other.
Z. obesum and Z. exiguum are morphologically fairly similar, which is at odds with their clear genetic separation. Their main differences seem to be the larger shell height of Z. obesum, some differences in the microstructure (see above) and in the CVA, which, however, is based on only minor changes in the landmarks 1, 4 and 8.
The status of Z. tholussum is currently unclear. Morphologically, it is essentially a bigger and somewhat wider version of Z. pretneri (to which it wasn’t compared in its original description (Weigand 2013)), which agrees well with the placement of the CO1 sequence in our tree. Since our species delimitation methods using CO1 sequences separate these two taxa, we retained the classification of Weigand (2013). However, more research using additional markers is necessary. The status of the Z. pretneri population from Pećina kod sela Puhari is unclear. Though it has a seemingly distinct morphology, we chose not to consider it an independent species without genetic data. Z. manitaense was found to be morphologically (such as in the CVA; Supplementary Fig. S5, bottom) distinct from the other species and in the genetic record. A few specimens were found together with Z. pretneri in Jamski sustav Kita Gaćešina, where both species were differentiable with the LDA. Z. troglobalcanicum is aligned within this clade based on the single specimen from Taleža pećina that clustered close to Z. manitaense in the PCAs and in the CVA, as well as on the specimens figured in Absolon (1916) and Gittenberger (1975). Gittenberger (1975) reported Z. troglobalcanicum from Cetinje, Montenegro. His designation is retained here since our study lacked material from this southern Balkan region. We remark that the population he addressed might well belong to a separate, undescribed species.
The configuration of the Z. frauenfeldii clade is currently chaotic and requires additional investigation. Several forms treated here as Z. frauenfeldii are taxonomically still ambiguous. This has much to do with the fact that a smooth form occurring in northern Bosnia with an ambiguous position in the Consensus tree could not be separated using the species delimitation method BPP although it could be clearly geographically separated. A separation to species level is very probable, but we currently lack the data to consider it with confidence. Z. frauenfeldii osolei is synonymized here with Z. frauenfeldii despite lack of genetic data, although the few consistent morphological differences might indicate that it merits independent species status after further investigation. Many species in this clade (such as the smooth form of Z. frauenfeldii, Z. bucculentum, Z. pagodulum, Z. robustum) include forms with similar morphologies that were previously classified with the catch-all species, Z. isselianum. These forms are characterized by a smooth shell, a smallish to medium-sized parietalis in the aperture and a columellaris that starts just behind the aperture, extending half a whorl into the shell and which are usually impossible to tell apart. Three of the species that contain these forms (Z. frauenfeldii, Z. pagodulum, Z. robustum) also include populations that differ considerably from them by having ribs (Z. frauenfeldii, Z. pagodulum) and/or by differing in shell shape (Z. frauenfeldii, Z. robustum). The confusion in the traditional morphology is reflected in the morphometry: neither the PCAs nor the LDA were able to separate lineages and the CVA was only able to somewhat separate Z. subobesum from the rest (Supplementary Fig. S6). It is currently impossible to describe species in this group without phylogenetic support.
Species without genetic material were sometimes assigned to the genetically defined clades. That is, if this classification was unequivocally possible on a morphological basis (such as Z. lamellatum, Z. trebicianum, Z. troglobalcanicum). We refrained from assigning five species without genetic record (Z. globosum, Z. kusceri, Z. clathratum, Z. freyeri, Z. likanum) to clades since an unambiguous morphological classification was not possible. Though the changes in the position of the landmarks are fairly minimal, Z. globosum (occurring in the Italian Alpine foothills) could include several species as indicated by our CVA (Supplementary Fig. S7). More research, including genetic data, is necessary to determine the definite status of this species as well as its ancestral relationship to all other species. Z. kusceri is remarkable for its definability through a set of traditionally used characters that are highly stable across its range. Although it might belong to the Z. frauenfeldii clade, as it resembles Z. frauenfeldii and related species in shell shape, we were not able to extract DNA from the preserved specimen at our disposal and thus, can not verify it. Although Z. clathratum is described here as a new species despite the lack of genetic data, we are confident that the unique combination of ribs on the top of the whorls of the teleoconch, some of which extend weakly further down the whorl, and the presence of strong, clearly visible spiral lines (not present in other species occurring in that region), are criteria enough to separate this taxon at the species level. Moreover, the grid-like structure resulting from the combination of spiral lines and weak ribs is unique for Zospeum. Z. clathratum is only known from one cave south of Ogulin. Z. freyeri was last applied as a taxon name by Bourguignat (1856) and has been completely ignored by subsequent authors following Frauenfeld Von’s (1856) assessment, that too little was known about this species to regard it as valid. Its type locality (Vratnica cave, near Velike Lašče) appears in the list of caves recorded by Bole (1974) for Z. isselianum. We found that Z. freyeri was reliably described (see Schmidt 1849) and resembles other forms that were previously classified as Z. isselianum (e.g., Z. robustum sp. n.) in Bole (1974). A future classification within the Z. frauenfeldii clade is probable although classification within the Z. obesum clade could be possible as well (i.e., the configuration of the lamellae could point to either clade, as is the case for geographic proximity), especially since a specimen previously morphologically determined as Z. isselianum was found to be conspecific with Z. exiguum (see above). Z. likanum, a species only known from its type locality (Gornja Cerovačka pećina near Gračac), which is about 200 km away from the type locality of Z. freyeri, is indistinguishable in the LDA and in its configuration of lamellae from Z. freyeri.
SEM of shells and radulae
Jochum et al. (2015c) used SEM to detect microstructural differences on the surface of the shell to differentiate species of Z. amoenum, Z. isselianum and Z. kupitzense. In this study, we describe the superficial microsculpture of six additional species (Z. spelaeum, Z. obesum, Z. exiguum, Z. globosum, Z. frauenfeldii, Z. subobesum). Our results show that shell microstructure is useful for separating species within genetic clades (e.g., Z. isselianum and Z. kupitzense; Z. obesum and Z. exiguum; Z. subobesum and Z. frauenfeldii). We were however, not able to detect consistent differences between these clades. The variation within species is not well known since Z. amoenum is the only species for which more than one specimen was scanned. This specific case might suggest that there is indeed variation in between caves (pits organized into distinct bands on the protoconch in shells from Konečka zijalka (Supplementary Fig. S14c), a character state, which is not evident in those from Ihanščica (see Jochum et al. 2015c)). Homoplasy is another possibility demonstrated by similar patterns of microstructure on the protoconch of Z. isselianum and Z. subobesum (pits mostly organized into bands, whereby the space between them lacks pits (as in Z. isselianum) or has considerably fewer pits (as in Z. subobesum).
Investigation of the tiny, fragile Zospeum radula was accomplished by Bole (1974), Giusti (1975), Jochum (2011), and Jochum et al. (2015). Although Bole (1974) dexterously dissected the radulae of Z. exiguum, Z. frauenfeldii, Z. kusceri, Z. obesum, and, what he considered as Z. spelaeum spelaeum, Z. spelaeum costatum, and Z. spelaeum schmidtii. He did not have access to SEM and thus concluded that the radula was of little value in differentiating species. Giusti (1975) on the other hand, first described the tricuspid crown he encountered in Z. tellini (syn. of Z. spelaeum) as bearing a symmetrical central tooth (mesocone) flanked on each side by a shorter tooth (endocone and ectocone) accompanied by a series of extremely irregular lateral teeth and a subsequent series of marginal teeth. He laboured in trying to find a consistent pattern, remarking that the teeth varied much from zone to zone on the same radular ribbon. Martins (2007) later defined the additional cusps (endocones and ectocones) in the lateral and marginal teeth of the carychiid radula as “Carychium-type” because though they appear like they may be detached from the crown, they remain connected to it. These cusps are not subdivisions of the crown. It wasn’t until Jochum (2011) and Jochum et al. (2015a) that SEM was used for the first time on the Zospeum radula to explore its potential use in species differentiation.
Our study of radulae from diverse Dinaride species of Zospeum shows a wide spectrum of morphological variation of radular teeth within rows on the same radula as well as within species. Although our investigation suggests potential ecological phenotypisation within Zospeum, it does not provide enough information from which to extract taxonomic significance. Moreover, due to the meager sample size, our study does not provide enough evidence to corroborate Bole’s (1974) understanding that the radula has little taxonomic value in separating Zospeum species. Considering that we have studied only nine different Zospeum radulae here, they appear to align into four different ribbon morphologies irrespective of species characteristics and genetic grouping: (1) very long; tapered to obtuse ends (Z. exiguum, Z. spelaeum), (2) long and broad; tapered anterior end with attenuated triangular base (Z. frauenfeldii) (3) short and broad; attenuated with triangular base (Z. isselianum) or (4) very long; tapered anterior end with straight-edged base (Z. pretneri, Z. subobesum). Since dentitional form strongly differentiated between individuals and the tooth alignment varied considerably from row to row, a potential pattern for species differentiation could not be recognized. Moreover, although the Carychium-like cusps defined by Martins (2007) are clearly seen in most all the teeth, we can not specifically differentiate certain rows of the Zospeum radula from similar ellobioid forms in Martins’ vast works (1996, 2007). We however, remark that it is a paradigm homologous structure clearly demonstrating affinity to other known ellobioid relatives presented in Martins (1996, 2007). For example, the radula of Z. exiguum (Križna jama) (Supplementary Fig. S17b) with its array of strongly arched and hook-like lateral teeth and long slender basal plates is homologous to that of the marine ellobiid species Pedipes dohrni D’Ailly, 1896 (Martins 2007 fig. 148) and Marinula tristanensis Connolly, 1915 (Martins 2007, fig. 150). Moreover, the lateral and marginal teeth of Z. isselianum show strong affinity to those of Leuconopsis manningi Martins 1996 (Martins 2007, fig. 152). Jochum et al. (2015c) reported grooves on the cusps of topotypic Z. isselianum (Turjeva jama) and the newly classified, Z. amoenum here (L2 in Weigand et al. 2013) from Konečka zijalka. Jochum (2015) initially considered these median grooves a structural modification intrinsic to the radulae of Z. isselianum and the population (L2) from Konecka zijalka. In this study, we found that more or less deep grooves run the length of the median section of many rachidian, lateral and marginal teeth in at least six additional species. Martins (1996) remarks a “weak medial depression” on the central tooth of the radula of Melampus monile (Bruguière, 1789) and at the posterior edge of the central tooth of Melampus coffeus (Linnaeus, 1758). For Zospeum here, these grooves may indicate an ecologically induced structural modification (i.e., adaptation) in response to the fine nuances of diet, substrate composition and grain in certain Dinaride caves (Hickman 1980; Luchtel et al. 1997) whereby, the depth of these grooves may indicate nuance gradients or potentially individual age.
Conclusion
In this integrative study, 25 species of Zospeum were described, 16 of which were detectable using genetic data and seven yet without genetic data. Several morphometric methods were adapted for this group, though their applicability was found insufficient. Established for the group by Jochum et al. (2015), computer tomography (CT) and SEM for shells and radulae was widely applied to supplement traditional methods for interpreting morphology. Our resulting classification scheme differs substantially from that established by Bole (1974), which up to now, with but a few modifications, defined the standard for Dinaridic Zospeum. By using contemporary techniques to analyze and reinterpret historical understanding, we hereby present a new interpretion for the genus Zospeum.
Many of the newly discovered species, however, can still not be differentiated morphologically, rendering it difficult to treat forms with minimum distinctness (such as Z. pretneri from Pećina kod sela Puhari) and lacking genetic data. Although we uncovered new and remarkable information about the Zospeum radula and its affinity within the broader context of the Ellobioidea, the radula is not useful for species discrimination nor is there any overlap with the genetic clades revealed in this study. Increased sampling efforts within Zospeum’s southernmost distributional range are paramount for deeper understanding of the phylogenetic and phylogeographic picture of this enigmatic Dinaride radiation.
Outlook
Although we have established a framework based on genetic data, there are several issues still to be addressed in future studies: (a) to conduct genetic studies on known species still lacking genetic data or forms that might represent independent species (such as Z. pretneri from Pećina kod sela Puhari), but which we were not able to conclusively separate; (b) to conduct a denser genetic sampling in the known distribution range of the genus in order to recover a more complete picture of the species present; (c) to explore, as in most cases, insufficiently known distribution boundaries of the genus, which might well be substantially larger than currently known; and (d) to conduct further morphological studies, such as investigating the cellular and biochemical level via state-of-the-art histological techniques.
Abbreviations
- Shell characters:
-
(see Fig. 2)
- AH:
-
Aperture height
- AW:
-
Aperture width
- CJL:
-
Length of columellar junction between shell and aperture
- D1:
-
Diameter of first whorl
- LH:
-
Height of last whorl
- LW:
-
Width of last whorl
- SA:
-
Spire angle
- SH:
-
Shell height
- SW:
-
Shell width
- AJC:
-
Adrienne Jochum Collection, Kelkheim, Germany
- ANSP:
-
Academy of Natural Sciences, Philadelphia, Pa., USA
- NHMUK:
-
Natural History Museum, London, UK
- GN:
-
Gianbattista Nardi Collection, Gussago, Brescia, Italy
- MCSMNH:
-
Malacological Collection of the Slovenian Museum of Natural History (former CSR SASA, MZBI and SMNH) Ljubljana, Slovenia
- MCSNTS:
-
Museum of Natural History of Trieste, Trieste, Italy
- NHMW:
-
Naturhistorisches Museum Wien, Wien, Austria
- NMBE:
-
Naturhistorisches Museum der Burgergemeinde Bern, Bern, Switzerland
- RS:
-
Rajko Slapnik Collection, Kamnik, Slovenia
- SMF:
-
Senckenberg Forschungsinstitut und Naturmuseum, Frankfurt am Main, Germany
- WdM:
-
Willy De Mattia Collection, Muggia, Italy
- MP:
-
Massimo Prodan Collection, Trieste, Italy
- ZRC SAZU:
-
The Slovenian Academy of Sciences and Arts (encompasses Jovan Hadži Biological Institute (BIJH)) Ljubljana, Slovenia
- ZSM:
-
Zoologische Staatssammlung München
References
Absolon, K. (1916). Z výzkumných cest po krasech Balkánu. O balkánské temnostní zvířeně. Zlatá Praha, 33(48), 574–576 (49): 586–588; (50): 597–600; (51): 609–612; (52): 622–624.
Allegretti, C. (1944). Primo Contributo alla Conoscenza della Speleofauna Malacologica della Lombardia. Le Grotte d’Italia, 5(2), 48–56.
Bank, R. A., & Neubert, E. (2016). Notes on Enidae, 7. Revision of the Enidae of Iran, with special reference to the collection of Jacques de Morgan (Gastropoda: Pulmonata). Vita Malacologica, 14, 1–84.
Baur, H., & Leuenberger, C. (2011). Analysis of ratios in multivariate morphometry. Systematic Biology, 60, 813–825.
Bole, J. (1960). Novi vrsti iz rodu Zospeum Bourg. (Gastropoda). Biološki Vestnik, 7, 61–64.
Bole, J. (1974). Rod Zospeum Bourguignat 1865 (Gastropoda, Ellobiidae) v Jugoslawiji. Razprave Slovenska Academija znanosti in Umetnosti, Cl. IV, 17(5), 249–291.
Böttger-Schnack, R., & Machida, R. J. (2011). Comparison of morphological and molecular traits for species identification and taxonomic grouping of oncaeid copepods. Hydrobiologia, 666(1), 111–125.
Bourguignat, J. R. (1856). Aménités Malacologiques. § LI. Du genre Zospeum. Revue et Magasin de Zoologie pure et appliquée, 8(2), 499–516.
Bruguière, J. G. (1789). 1789–1792. Encyclopédie méthodique. Histoire naturelle des vers. Panckouke, Paris, 1, 1–344 [1789]; 2: 345–758 [1792].
Brusina, S. (1886). Ueber die Mollusken - Fauna Oesterreich-Ungarns. Mittheilungen des Naturwissenschaftlichen Vereins für Steiermark, Abhandlungen, 22, 29–56.
Clement, M., Posada, D., & Crandall, K. A. (2000). TCS: a computer program to estimate gene genealogies. Molecular Ecology, 9, 1657–1659.
Conci, C. (1956). Nuovi rinvenimenti di Molluschi troglobi del genere Zospeum in caverne delle Prealpi Trentine e Venete (Italia settentrionale). Premier Congrès International de Spéléologie, 3(3), 275–281.
Connolly, M. (1915). Notes on South African Mollusca. Annals of the South African Museum, 13, 108–109.
D’Ailly, A. (1896). Contributions à la connaissance des mollusques terrestres et d'eau douce de Kaméroun. Bihang till Kungliga Svenska vetenskaps-akademiens handlingar, bd 22, 4(2), 118.
De Mattia, W. (2003). I molluschi ipogei del Carso Triestino (Friuli-Venezia Giulia, Italia) (Gastropoda: Prosobranchia, basommatophora, styllommatophora; Bivalvia: Pterioida). Check-list delle specie, tassonomia, sistematica, ecologia e Biogeografia. Atti del museo civico di storia natural di Trieste, 50, 89–218.
De Mattia, W. (2004). Pseudochondrula seductilis (Rossmässler, 1837) in Italia (Mollusca:Stylommatophora: Enidae). Bolletino Malacologico, 40(9–12), 104–108.
Frauenfeld Von, G. (1854). Über einen bisher verkannten Laufkäfer beschrieben von L. Miller; und einen neuen augenlosen üsselkäfer, beschrieben von F. Schmidt; ferner einige von Schmidt in Schischka neu entdeckte Höhlentiere. Verhandlungen des Zoologisch-Botanischen Vereins in Wien, 4, 23–34.
Frauenfeld Von, G. (1856). Die Gattung Carychium. Sitzungsberichte der mathematisch-natur-wissenschaftlichen Classe der aiserlichen Akademie der Wissenschaften, 19, 70–93.
Freyer, H. (1855). Über neu endeckte Conchylien aus den Geschlechtern Carychium und Pterocera. Sitzungsberichte der mathematisch-naturwissenschaftlichen Classe der kaiserlichen Akademie der Wissenschaften, 5, 18–23.
Gittenberger, E. (1975). Cave snails found in southern Crna Gora. Glasnik Republ. Zavoda zast. Prirode. Prirodnjackog muzeja, 8, 21–37 Titograd.
Gittenberger, E. (1982). Nachweis der Höhlenschnecke Zospeum alpestre (FREYER, 1855) in der Hafnerhöhle, Karawanken – Kärnten. Carinthia II, 172, 351–354.
Giusti, F. (1975). Prime indagini anatomiche sul genere Zospeum (Pulmonata, Basommatophora). Notulae malacologicae XXI. Conchiglie, 11(3/4), 53–64.
Giusti, F., & Pezzoli, E. (1982). Mollusci cavernicoli italiani (Notulae malacologicae. XXVII). Lavori della Società italiana di Biogeografia, 7, 431–450.
Hamann, O. (1896). Europäische Höhlenfauna–Eine Darstellung der in den Höhlen Europas lebenden Tierwelt mit besonderer Berücksichtigung der Höhlenfauna Krains. Costenoble, Jena 375 pp.
Hart, M. W., & Sunday, J. (2007). Things fall apart: biological species form unconnected parsimony networks. Biology Letters, 3, 509–512.
Hauffen, H. (1856a). Ueber ein neues Carychium. Verhandlungen des Zoologisch-Botanischen Vereins in Wien, 6, 623–624.
Hauffen, H. (1856b). Zwei neue Schnecken. Verhandlungen des Zoologisch-Botanischen Vereins in Wien, 6, 701–702.
Hauffen, H. (1858). Beiträge zur Grottenkunde Krain’s. Jahresheft des Vereines des krainischen Landes-Museums, 2, 96.
Hickman, C. S. (1980). Gastropod radulae and the assessment of form in evolutionary paleontology. Paleobiology, 6(3), 276–294.
Holznagel, W. A. (1998). Research note: a nondestructive method for cleaning gastropod radulae from frozen, alcohol-fixed, or dried material. American Malacological Bulletin, 14, 181–183.
Huelsenbeck, J. B., & Ronquist, F. (2001). MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics, 17, 754–755.
Jeffreys, J. G. (1830). A synopsis of the testaceous pneumonobranchous Mollusca of Great Britain. Transactions of the Linnean Society of London., 16, 323–392.
Jochum, A. (2011). Evolution and diversity of the troglobitic Carychiidae - a morphological and phylogenetic investigation of the terrestrial ellobiiod genera, Carychium and Zospeum. The Malacologist, 57, 16–18.
Jochum, A. (2015). Contributions to the Carychiidae (Gastropoda, Ellobioidea): new perspectives in carychiid taxonomy. [DPhil thesis]. Bern, Switzerland: University of Bern. 169 pp.
Jochum, A., de Winter, A. J., Weigand, A. M., Gómez, B., & Prieto, C. (2015a). Two new species of Zospeum Bourguignat, 1856 from the Basque-Cantabrian Mountains, Northern Spain (Eupulmonata, Ellobioidea, Carychiidae). ZooKeys, 483, 81–96.
Jochum, A., Prozorova, L., Sharyi-ool, M., & Páll-Gergely, B. (2015b). A new member of troglobitic Carychiidae, Koreozospeum nodongense gen. Et sp. N. (Gastropoda, Eupulmonata, Ellobioidea) is described from Korea. ZooKeys, 517, 39–57.
Jochum, A., Slapnik, R., Klussmann-Kolb, A., Páll-Gergely, B., Kampschulte, M., Martels, G., Vrabec, M., Nesselhauf, C., & Weigand, A. M. (2015c). Groping through the black box of variability: An integrative taxonomic and nomenclatural re-evaluation of Zospeum isselianum Pollonera, 1887 and allied species using new imaging technology (Nano-CT, SEM), conchological, histological and molecular data (Ellobioidea, Carychiidae). Subterranean Biology, 16, 123–165.
Katoh, K., & Standley, D. M. (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution, 30, 772–780.
Katoh, K., Misawa, K., Kuma, K., & Myata, T. (2002). MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acid Research, 30, 3059–3066.
Kearse, M., Moir, R., Wilson, A., Stones-Havas, S., Cheung, M., Sturrock, S., Buxton, S., Cooper, A., Markowitz, S., Duran, C., Thierer, T., Ashton, B., Mentjies, P., & Drummond, A. (2012). Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics, 28, 1647–1649.
Kerney, M. P., Cameron, R. A. D., & Jungbluth, J. H. (1983). Die Landschnecken Nord- und Mitteleuropas. Ein Bestimmungsbuch für Biologen und Naturfreunde, 1–384 Taf. 1–24. Hamburg, Berlin. (Parey).
Klingenberg, C. P. (2011). MorphoJ: an integrated software package for geometric morphometrics. Molecular Ecology Resources, 11, 353–357.
Kuščer, L. (1928). Primo contributo alla fauna malacologica cavernicola della Venezia Tridentina. Studi Trentini di Scienze Naturali, 9(2), 185–187.
Kuščer, L. (1932). Höhlen- und Quellenschnecken aus dem Flussgebiet der Ljubljanica. Archiv für Molluskenkunde, 64(2), 60–61.
Linnaeus, C. (1758). Systema naturae per regna tria naturae, secundum classes, ordines, genera, species cum characteribus, differentiis, synonymis, locis, 10th ed. Laurentii Salvii, Holmiae, vol. 1: 824 pp.
Luchtel, D. L., Martin, A. W., Deyrup-Olsen, I., & Boer, H. H. (1997). Gastropoda: Pulmonata. In F. W. Harrison & A. J. Kohn (Eds.), Microscopic Anatomy of Invertebrates (Vol. 6B, pp. 459–718). Mollusca II: Wiley-Liss, Inc., New York 828 pp.
Martins, A. M. F. (1996). Anatomy and systematics of the Western Atlantic Ellobiidae (Gastropoda: Pulmonata). Malacologia, 37(2), 163–332.
Martins, A. M. F. (2007). Morphological and anatomical diversity within Ellobiidae (Gastropoda, Pulmonata, Archaeopulmonata). Vita Malacologica, 4, 1–28.
Nardi, G. (2015). Gli endemiti della fauna malacologica Bresciana. Natura Bresciana, 39, 57–93.
Pezzoli, E. (1992). Il genere Zospeum Bourguignat, 1856 in Italia (Gastropoda Polmonata Basommatophora). Censimento delle stazioni ad oggi segnalate. Natura Bresciana, 27, 123–169.
Pfeiffer, L. (1854). Synopsis Auriculaceorum. Malakozoologische Blätter, 1(5), 145–156 [august 1854]. Cassel (Theodor Fischer).
Pollonera, C. (1887). Note malacologiche. I. Molluschi della Valle del Natisone (Friuli). Bullettino della Società Malacologica Italiana, 12(1886), 204–208.
Pollonera, C. (1889). Note malacologiche - Un nuovo Zospeum italiano - Acme italiane del gruppo della costulatae - Vitrina stabilei e V. majori- La Xerophila maritima. Bollettino Società Malacologica Italiana, 14, 50–64.
Pollonera, C. (1905). I Zospeum italiani. Proteus Rivista di Biologia Sotterranea Bologna, 3, 2–4.
Puillandre, N., Lambert, A., Brouillet, S., & Achaz, G. (2012). ABGD, automatic barcode gap discovery for primary species delimitation. Molecular Ecology, 21, 1864–1877.
R Core Team. (2013). R: a language and environment for statistical computing. Vienna, Austria: R Foundation for statistical Computing. URL: http://www.R-project.org/. (Downloaded on the 20.2.2014)
Rannala, B., & Yang, Z. (2013). Improved reversible jump algorithms for Bayesian species delimitation. Genetics, 194, 245–253.
Reichenbach, F., Baur, H., & Neubert, E. (2012). Sexual dimorphism in shells of Cochlostoma septemspirale (Caenogastropoda, Cyclophoroidea, Diplommatinidae, Cochlostomatinae). ZooKeys, 208, 1–16.
Risso, A. (1826). Histoire naturelle des principales productions de l’Europe méridionale et particulièrement de celles des environs de Nice et des Alpes Maritimes. Tome quatrième. – Paris (F.G. Levrault): pp. 439, 12 plates. [8 November].
Rohlf, F. J. (2017a). tps Utility program version 1.74. Stony brook: Department of Ecology and Evolution, State University of New York. http://life.bio.sunysb.edu/morph/. Accessed 24 April 2017
Rohlf, F. J. (2017b). tpsDig 2 version 2.30. Stony Brook: Department of Ecology and Evolution, State University of New York. http://life.bio.sunysb.edu/morph/. Accessed 24 April 2017
Romero, P. E., Pfenninger, M., Kano, Y., & Klussmann-Kolb, A. (2016). Molecular phylogeny of the Ellobiidae (Gastropoda: Panpulmonata) supports independent terrestrial invasions. Molecular Phylogenetics and Evolution, 97, 43–54.
Rossmässler, E. A. (1838). Iconographie der Land- und Süsswasser-Mollusken, mit vorzüglicher Berücksichtigung der europäischen noch nicht abgebildeten Arten. 1: 2 (3/4) (p. 46). Dresden, Leipzig: Arnoldische Buchhandlung.
Schizas, N. V., Street, G. T., Coull, B. C., Chandler, G. T., & Quattro, J. M. (1997). An efficient DNA extraction method for small metazoans. Molecular Marine Biology and Biotechnology, 6(4), 381.
Schmidt, F. J. (1849). Besuch der Sele‘er Grotte, der Berg-Ruine Friedrichsstein bei Gottschee und der Grotten von Podpeč, Kompolje und Laschitz im August 1848. Illyrisches Blatt, 39, 153–154.
Slapnik, R. (1991). Distribution of Zospeum alpestre (Freyer 1855), Z. isselianum Pollonera, 1886 and Z. alpestre bolei ssp. n. (Gastropoda, Carychiidae) and their variability in the caves in Kamnik and Savinja Alps. Razprave − Slovenska akademia znanosti in umetnosti. Razred za naravoslovne vede, 32, 3–73.
Slapnik, R. (1994). Razširjenost rodu Zospeum Bourguignat 1856 (Gastropoda, Pulmonata, Carychiidae) v osamelem krasu vzhodne Slovenjie. Razprave − Slovenska akademija znanosti in umetnosti. Razred za naravoslovne vede, 35(5), 297–335.
Slapnik, R., & Ozimec, R. (2004). Distribution of the genus Zospeum Bourguignat, 1856 (Gastropoda, Pulmonata, Ellobiidae) in Croatia. Natura Croatica, 13(2), 115–135.
Stamatakis, A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics, 30, 1312–1313.
Stossich, A. (1899). Contribuzione alla fauna malacologica terrestre e fluviatile del territorio di Trieste ed in parte delle località contermini. Bollettino della Società adriatica di scienze naturali in Trieste, 19, 17–51.
Stummer, A. (1984). Eine neue Unterart der Höhlenschnecke Zospeum alpestre (Freyer) aus der Kupitzklamm bei Eisenkappel, Kärnten (Basommatophora: Ellobiidae). Heldia, 1(1), 13–14.
Templeton, A. R., Crandall, K. A., & Sing, C. F. (1992). A cladistic analysis of phenotypic associations with haplotypes inferred from restriction endonuclease mapping and DNA sequence data. III. Cladogram estimation. Genetics, 132, 619–633.
Wagner, A. J. (1912). Beschreibungen neuer Land- und Süsswasserschnecken aus Südösterreich, Croatien und Bosnien. Verhandlungen des Zoologisch-Botanischen Vereins in Wien, 62, 246–260.
Weigand, A. M. (2013). New Zospeum species (Gastropoda, Ellobioidea, Carychiidae) from 980m depth in the Lukina Jama–Trojama cave system (Velebit Mts., Croatia). Subterranean Biology, 11, 45–53.
Weigand, A. M., Jochum, A., Pfenninger, M., Steinke, D., & Klussmann-Kolb, A. (2011). A new approach to an old conundrum—DNA barcoding sheds new light on phenotypic plasticity and morphological stasis in microsnails (Gastropoda, Pulmonata, Carychiidae). Molecular Ecology Resources, 11, 255–265.
Weigand, A. M., Jochum, A., Slapnik, R., Schnitzler, J., Zarza, E., & Klussmann-Kolb, A. (2013). Evolution of microgastropods (Ellobioidea, Carychiidae): integrating taxonomic, phylogenetic and evolutionary hypotheses. BMC Evolutionary Biology, 13, 1–23.
Yang, Z., & Rannala, B. (2010). Bayesian species delimitation using multilocus sequence data. Proceedings of the National Academy of Sciences, 107, 9264–9269.
Acknowledgements
We are grateful to the collection managers and curators, who generously gave access to the collections under their care: Anita Eschner (Naturhistorisches Museum, Vienna), Ronald Janssen (Research Institute Senckenberg, Frankfurt am Main), Jonathan Ablett (Natural History Museum, London), and Matjaž Kuntner (Center for Scientific Research of the Slovenian Academy of Sciences and Arts, Ljubljana). We especially thank those who patiently imaged the type material including Katharina Jaksch-Mason (NHMW), Paulo Albano (NHMW), Sara-Maria Schnedl (NHMW), Sigrid Hof (SMF), and Estée Bochud (NMBE). We are also indebted to those who generously sent us precious specimens from their private collections including Massimo Prodan, Willy De Mattia, and Gianbattista Nardi. For many insightful discussions and support, we especially thank Anita Eschner, Ruud Bank, Alexander Weigand, Beat Pfarrer, and Massimo Prodan. We acknowledge Tonći Rađa for his kind assistance interpreting Croatian cave names and locality data. For material and GPS collection data, we thank Roman Ozimec and the Croatian Biospeleological Society as well as Sara-Maria Schnedl for her help in translating Italian texts. We gratefully acknowledge Thomas Neubauer who kindly transported borrowed material back and forth to Vienna. We also thank Manfred Ruppel (ret. Goethe University Frankfurt) for his insights and help with the SEM and Annette Klussmann-Kolb for her generous support. For creation of the video of Z. obesum, we thank Jean Martels and Henning Heckmann (Justus Liebig University Computer Center, Giessen). This work was made possible in part by grants to AJ from the Malacological Society of London for SEM resources and from the SYNTHESYS Project http://www.synthesys.info/, which enabled AJ to spend weeks on end studying the NHMW collection. The SYNTHESYS Project is financed by the European Community Research Infrastructure Action under the FP7 “Capacities” Program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Figure S1
Overall morphometric analyses. From top to bottom: measurement-based PCA, geometric PCA, CVA. (PNG 789 kb)
High Resolution Image
(TIF 7811 kb)
Supplementary Figure S2
Morphometric analyses of the Z. spelaeum clade. From top to bottom: measurement-based PCA, geometric PCA, CVA. (PNG 537 kb)
High Resolution Image
(TIF 6203 kb)
Supplementary Figure S3
Morphometric results of the Z. alpestre clade. From top to bottom: measurement-based PCA, geometric PCA, CVA. (PNG 471 kb)
High Resolution Image
(TIF 5776 kb)
Supplementary Figure S4
Morphometric results of the Z. obesum clade. From top to bottom: measurement-based PCA, geometric PCA, CVA. (PNG 355 kb)
High Resolution Image
(TIF 5292 kb)
Supplementary Figure S5
Morphometric results of the Z. pretneri clade. From top to bottom: measurement-based PCA, geometric PCA, CVA. (PNG 523 kb)
High Resolution Image
(TIF 6493 kb)
Supplementary Figure S6
Morphometric results of the Z. frauenfeldii clade. From top to bottom: measurement-based PCA, geometric PCA, CVA. (PNG 546 kb)
High Resolution Image
(TIF 8475 kb)
Supplementary Figure S7
Morphometric results of Z. globosum, sorted by populations. From top to bottom: measurement-based PCA, geometric PCA, CVA. (PNG 525 kb)
High Resolution Image
(TIF 6412 kb)
Supplementary Figure S8
Distribution of species within the Z. spelaeum clade. (PNG 1276 kb)
High Resolution Image
(TIF 2132 kb)
Supplementary Figure S9
Distribution of species within the Z. alpestre clade. (PNG 1027 kb)
High Resolution Image
(TIF 1896 kb)
Supplementary Figure S10
Distribution of species within the Z. obesum clade. (PNG 909 kb)
High Resolution Image
(TIF 1693 kb)
Supplementary Figure S11
Distribution of species within the Z. pretneri clade. (PNG 1584 kb)
High Resolution Image
(TIF 2414 kb)
Supplementary Figure S12
Distribution of species within the Z. frauenfeldii clade. (PNG 2445 kb)
High Resolution Image
(TIF 28018 kb)
Supplementary Figure S13
Distribution of species that were not assigned to any clade and of specimens with unknown identity. (PNG 2715 kb)
High Resolution Image
(TIF 3674 kb)
Supplementary Figure S14
Scanning electron micrographs (SEM) showing superficial microstructure on Zospeum shells. (a) Zospeum speleaum, CSRSASA 3983ª, Skocjanske jama, pitting on protoconch. — (b) Zospeum spelaeum, MCSMNH 31286, Mačkovica jama, Interconnected rows of pitting on protoconch. — (c) Zospeum amoenum, MCSMNH 21675, Konečka zijalka, rows of pits interspersed with irregular smooth zones on protoconch; (d) ditto, interconnected rows of pits on teleoconch. — (e) Zospeum isselianum, MCSMNH 37013, Turjeva jama, distinct regular rows of pits interspersed by regular non-pitted zones on protoconch; (f) ditto, distinct spiral striae of interconnected pits and raised revolving lines of microstructure on teleoconch. — (g) Zospeum kupitzense, SMNH 3291, Ložekarjeva jama, pitting on protoconch; (h) ditto, incised suture and more or less regular bands of interconnected pits on teleoconch. — Magnification varies for each perspective, see scale bars; all Figs taken by M. Ruppel, formerly Goethe University Frankfurt am Main. (PNG 2642 kb)
High Resolution Image
(TIF 14590 kb)
Supplementary Figure S15
Scanning electron micrographs (SEM) showing superficial microstructure on Zospeum shells. (a) Zospeum obesum, NMBE 553409, Krška jama, interconnected pits on protoconch; (b) ditto, distinct spiral striae on teleoconch. — (c) Zospeum exiguum, MCSMNH 39012, Križna jama, protoconch with dense pitting and organic debris; (d) ditto, distinct spiral striae on the penultimate whorl; — Magnification varies for each perspective, see scale bars; all Figs taken by M. Ruppel, formerly Goethe University Frankfurt am Main. (PNG 1210 kb)
High Resolution Image
(TIF 7633 kb)
Supplementary Figure S16
Scanning electron micrographs (SEM) showing superficial microstructure on Zospeum shells. (a) Zospeum globosum, NHMW 111543, Cavernetta di Vobarno, pitted protoconch; (b) ditto, sprial striae of interconnected pits and eroded rib-like microstructure on teleoconch. — (c) Zospeum subobesum, NMBE 553326, Tounjčica Cave, pitting on protoconch; (d) ditto, spiral striae of interconnected pits on teleoconch. — (e) Zospeum frauenfeldii, NMBE 553388, Podpeška jama, protoconch showing dense microstructure of interconnected pits; (f) ditto, revolving lines of reticulate structure on 2nd whorl; (g) ditto, pronounced ribbing on teleoconch. — (h) Zospeum subobesum, NMBE 553326, Tounjčica Cave, teleoconch showing partial costate structure at junction of penultimate and ultimate whorls. — Magnification varies for each perspective, see scale bars; all Figs taken by M. Ruppel, formerly Goethe University Frankfurt am Main. (PNG 2205 kb)
High Resolution Image
(TIF 14249 kb)
Supplementary Figure S17
Scanning electron micrographs (SEM) showing microstructure on Zospeum radulae. (a) Zospeum exiguum, NMBE 553384, Križna jama, long, tapered-type of radular ribbon; (b) ditto, bicuspid crowns bearing semi-detached endocones; (c) ditto, overview showing tooth and basal plate alignment; (d) ditto, biscuspid laterals (right side) and transitional teeth bearing four-pointed cusps (left side). — (e) Zospeum frauenfeldii, NMBE 553388, Podpeška jama, serrated marginal crowns atop of concave basal plates; (f) ditto, rachidian tooth (middle column) flanked by irregular lateral teeth; (g) ditto, row of lateral teeth showing distinct grooves down the mesocone; (h) ditto, tapered-type radular ribbon with attenuated triangular base. — Magnification varies for each perspective, see scale bars; all Figs taken by M. Ruppel, formerly Goethe University Frankfurt am Main. (PNG 3140 kb)
High Resolution Image
(TIF 10240 kb)
Supplementary Figure S18
Scanning electron micrographs (SEM) showing microstructure on Zospeum radulae. (a) Zospeum isselianum, NMBE 553389, Turjeva jama, tricuspid lateral teeth; (b) ditto, marginal teeth; (c) ditto, rachidian and lateral teeth. — (d–e) Zospeum amoenum, NMBE 553377, Konečka zijalka, crowns showing mesocones with deep median grooves and semi-detached endocones. — (f) Zospeum obesum, NMBE 553409, Krška jama, very long, tapered-type ribbon with pointed or obtuse ends; (g) ditto, stressed and broken lateral crowns; (h) ditto, worn crowns of transitional teeth. — Magnification varies for each perspective, see scale bars; all Figs taken by M. Ruppel, formerly Goethe University Frankfurt am Main. (PNG 3123 kb)
High Resolution Image
(TIF 14250 kb)
Supplementary Figure S19
Scanning electron micrographs (SEM) showing microstructure on Zospeum radulae. (a) Zospeum pretneri, NMBE 553290, Gornja Cerovačka pećina, tiny rachidian tooth and wing-like basal plates; (b) ditto, centrally grooved and partially bulged crowns of lateral teeth; (c) ditto, tricuspid teeth with wing-like basal plates; (d) ditto, consecutive rows of tricuspid crowns and wing-like basal plates. — (e) Zospeum kupitzense, NMBE 553393, Ložekarjeva jama, very long, anteriorly-tapered ribbon with straight-edged-type base; (f) ditto, long and sharply pointed transitional crowns. — Magnification varies for each perspective, see scale bars; all Figs taken by M. Ruppel, formerly Goethe University Frankfurt am Main. (PNG 2407 kb)
High Resolution Image
(TIF 11274 kb)
Supplementary Figure S20
Scanning electron micrographs (SEM) showing microstructure on Zospeum radulae. (a) Zospeum spelaeum, MCSMNH 36841a, Postojnska jama, long and tapered-type ribbon with obtuse ends; (b) ditto, right lateral teeth; (c) ditto, transitional teeth; (d) ditto, marginal teeth. — (e) Zospeum spelaeum, NMBE 553311, Velika Pasica, very long and tapered-type ribbon with pointed or obtuse ends; (f) ditto, overview of middle radular section and concave basal plates. — Magnification varies for each perspective, see scale bars; all Figs taken by M. Ruppel, formerly Goethe University Frankfurt am Main. (PNG 2405 kb)
High Resolution Image
(TIF 11240 kb)
Supplementary Figure S21
Scanning electron micrographs (SEM) showing microstructure on Zospeum radulae. (a) Zospeum spelaeum, NMBE 553311, Velika Pasica, grooved and wrinkled, marble-like surface texture; (b) ditto, grooved transitional teeth; (c) ditto, marginal teeth. — (d) Zospeum subobesum, NMBE 553326, Tounjčica, very long, anteriorly-tapered ribbon with straight-edged-type base; (e) ditto, marginal teeth; (f) ditto, transitional teeth. — Magnification varies for each perspective, see scale bars; all Figs taken by M. Ruppel, formerly Goethe University Frankfurt am Main. (PNG 2317 kb)
High Resolution Image
(TIF 11083 kb)
ESM 1
(DOC 306 kb)
Rights and permissions
About this article
Cite this article
Inäbnit, T., Jochum, A., Kampschulte, M. et al. An integrative taxonomic study reveals carychiid microsnails of the troglobitic genus Zospeum in the Eastern and Dinaric Alps (Gastropoda, Ellobioidea, Carychiinae). Org Divers Evol 19, 135–177 (2019). https://doi.org/10.1007/s13127-019-00400-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-019-00400-8