Abstract
The diet of the fossil cave bears (Ursus spelaeus group) has been debated extensively. Thought traditionally to be herbivorous, more recent studies have proposed more meat in the cave bear diet. To test this, the mandibular morphology of cave bears was analysed using 3D geometric morphometrics and compared to that of extant Ursidae. Landmarks for 3D digitisation of the mandible were chosen to reflect functional morphology relating to the temporalis and masseter muscles. Extant and extinct Pleistocene Ursidae were digitised with a MicroScribe G2. Generalised Procrustes superimposition was performed, and data were allometrically and phylogenetically corrected. Principal component analysis (PCA), two-block partial least squares analysis (2B-PLS), regression analysis and discriminant function analysis were performed. PCA and 2B-PLS differentiate between known dietary niches in extant Ursidae. The lineage of the cave bear runs parallel to that of the panda (Ailuropoda melanoleuca) in morphospace, implying the development of morphological adaptations for eating foliage. A regression of shape onto foliage content in the diet and a discriminant function analysis also indicate that the cave bear diet consisted primarily of foliage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There are eight extant species of Ursidae, spread over five genera (Ursus, Helarctos, Tremarctos, Ailuropoda and Melursus), with diets ranging from folivory to carnivory (Tables 1 and 2). Two species of cave bear arose during the Pleistocene: Ursus deningeri and Ursus spelaeus. The former has been interpreted as the ancestor of the latter (Bon et al. 2008; Hänni et al. 1994; Rossi and Santi 2001). Their distribution extended from northwest Spain to the Urals, and from Belgium and the Harz region of Germany to Italy and Greece, and to the Crimea (Enloe et al. 2000; Grandal-d’Anglade and Vidal Romaní 1997; Kosintsev 2007). The later species, U. spelaeus, became extinct in Eurasia during the late Quaternary, approximately 27,800 calibrated years before 1950 A.D. (cal. yr. BP) (Pacher and Stuart 2008). Here and throughout, U. spelaeus and cave bear will be used to include the entire group, including proposed species, subspecies and haplotypes (Baca et al. 2012; Rabeder and Hofreiter 2004; Rabeder et al. 2004; Stiller et al. 2014), but excluding U. deningeri.
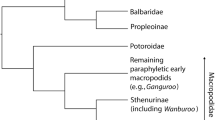
There is no real consensus on the phylogeny of the whole Ursidae family, including fossil species. For example, the position of Ailuropoda is assumed to be within Ursidae by some (e.g., Bininda-Emonds et al. 1999; Flynn and Nedbal 1998; Talbot and Shields 1996; Wozencraft 1989) and a separate family by others (Tagle et al. 1986; Zhang and Shi 1991); also, the number of subfamilies within Ursidae varies from three to five (e.g., McLellan and Reiner 1994; Pilgrim 1932; Quilès 2002). According to recent taxonomic classifications, however, Ursidae are subdivided in four subfamilies: the extant Ursinae, Ailuropodinae and Tremarctinae (Fulton and Strobeck 2006) and the fossil Hemicyoninae (Ginsburg and Morales 1998). Here, we use the phylogeny as shown in Fig. 1. Not much is known of the evolution of Ailuropodinae from Ursavus. A number of teeth have been found in China dating to the Miocene, which have been assigned to the species Ailurarctos lufengensis (Hunt 2004). Rare Pleistocene specimens have either been assigned to Ailuropoda microta or Ailuropoda melanoleuca (Hunt 2004). Short-faced ursids (Tremartinae) first appear about 7 Ma in North America as the genus Plionarctos, which does not have a premasseteric fossa, and is very rare (Hunt 1999). Tremarctos first appears in North America at about 2.5 Ma and may have evolved directly from Plionarctos (Hunt 1999). Tremarctos subsequently reached South America, where it is still present as Tremarctos ornatus.
The genus Ursus evolved from Ursavus, but its exact ancestor is unknown due to the time gap between the last record of Ursavus and the earliest Ursus (Hunt 2004). The genus Ursus is first known from a species from the late Pliocene of Europe: Ursus minimus (Martin 1989; McLellan and Reiner 1994). U. minimus migrated to Asia and became Ursus abtrusus in North America (Hunt 1999), which later evolved into Ursus americanus (McLellan and Reiner 1994). In Asia, U. minimus evolved into the Asiatic black bear (Ursus thibetanus) and the lineage leading to the sloth and Malayan sun bears (McLellan and Reiner 1994). The Etruscan bear (Ursus etruscus) evolved from U. minimus in Europe and possibly gave rise to both the brown bear (Ursus arctos) and U. deningeri, followed by U. spelaeus (Azzaroli 1983; Bon et al. 2008; Hänni et al. 1994; Martin 1989; McLellan and Reiner 1994; Rossi and Santi 2001). A population of U. arctos evolved into the polar bear (Ursus maritimus) around 1 Ma (Bininda-Emonds et al. 1999; Cahill et al. 2013).
There is no consensus on the diet of cave bears, and the topic is still intensively debated. Cave bears have been hypothesised by most authors to have been primarily herbivorous (Bocherens et al. 1997, 1994, 2011, 2014; Christiansen 2007c; Meloro et al. 2008, 2015a; Rabeder et al. 2010; Raia 2004; Stiner et al. 1998), based on the morphology of their dentition and isotope analyses. Other analyses using 2D geometric morphometrics (Figueirido et al. 2009; Meloro 2011) as well as taphonomical (Rabal-Garcés et al. 2012) and isotope analyses (Richards et al. 2008; Robu et al. 2013) have suggested that cave bears included a substantial amount of meat in their diet. It has even been proposed that the end-Pleistocene extinction of large herbivores represented a decrease in large prey/carrion for the cave bear and contributed to its extinction (Hilderbrand et al. 1996).
Previous morphological and morphometric studies (Christiansen 2008; Figueirido et al. 2012; Kupczik and Stynder 2012; Oldfield et al. 2012; Prevosti et al. 2012; Sacco and Van Valkenburgh 2004; Stynder and Kupczik 2013) have shown a strong correlation between feeding ecology and craniodental morphology for the family Ursidae. Two- (Figueirido et al. 2009; Seetah et al. 2012) and three-dimensional (van Heteren et al. 2009, 2012, 2014) geometric morphometrics have been used to infer the feeding ecology of the cave bear from mandible and skull morphology. The results of Figueirido et al. (2009) suggest that craniodental and mandibular morphology of extant faunivorous and herbivorous bears differs, with omnivorous bears intermediate between the two, and with U. spelaeus resembling extant omnivores. The results of van Heteren et al. (2009, 2014), however, indicate that the shape of the mandible and skull in extant members of the genus Ursus shows a gradient from carnivory to herbivory, with U. spelaeus at the herbivorous extreme. Both studies were based on the genus Ursus only, which has a rather limited dietary range.
Thus far, morphometrical analyses of ursid masticatory morphology have been based on principal component analysis (PCA), which is a statistical tool in exploratory data analysis that identifies patterns in the data without any a priori assumptions. This is a very useful method for getting a general idea of how the data are structured, but when looking at specific research questions, more information can be extracted from the data if they are explored in relation to relevant additional data, such as dietary information. Here, we aim to interpret ursid mandible morphology in relation to diet and through evolutionary time, with a particular focus on the cave bear. The present study includes all bears to include a broader range of possible bear diets, including that of the herbivorous panda (A. melanoleuca). We will do so, first, by objectively exploring the morphology data using PCA and, then, by specifically analysing the relationship between morphology and dietary variables using a two-block partial least squares analysis (2B-PLS), which identifies directions in morphospace related to diet. We hypothesise that the cave bear will show morphological similarities with its congeners, but will display a clear departure in morphospace towards a more herbivorous morphology. We also hypothesise that mandibular shape is related to the percentage of foliage in the diet. If confirmed, this will allow us to predict the amount of foliage in fossil U. spelaeus.
Material and methods
To test the dietary hypotheses of cave bears, a sample of 103 bear specimens, including 13 fossil U. spelaeus, were collected (see Supplementary Information 1). Adult mandibles were measured with a MicroScribe G2 desktop digitising system (Immersion Corporation, San Jose, CA, USA). When both hemimandibles were present, the more complete was chosen for digitisation. Landmarks were chosen to reflect functional aspects of the mandibular corpus (Fig. 2 and Table 3).
Mandibles of Ursus spelaeus (a, b) and Tremarctos ornatus (c) and showing the position of the masseteric fossa (MF) and the premasseteric fossa (PMF). Positions of the landmarks on a mandible of Ursus spelaeus on the lingual side (a) and the labial side (b). The landmarks are described in Table 3. Not to scale. Part A of the figure also displays the biomechanics. The resistance (Fout) at the carnassial of the lower jaw depends on the muscular input force (Fin), the angle of insertion of the muscle onto the jaw (α) and the ratio of in-lever arm or moment arm (Li) to out-lever arm (Lo). Angle of insertion of jaw muscles changes during jaw closing and determines the moment arm of the muscular input force (Mi). Mass pertaining to the masseter, Temp pertaining to the temporalis. Li Mass and Mi Mass happen to be the same in this diagram
Using MorphoJ version 1.06d (Klingenberg 2010a), raw 3D coordinates were scaled, rotated and translated by Procrustes superimposition. MorphoJ uses a full Procrustes fit and projects the data onto a Euclidean space tangent to shape space by orthogonal projection (Klingenberg 2010b). Reflection was used to make left and right specimens comparable. Specimens were aligned by principal axes, although the choice of alignment does not influence the statistical results (Klingenberg 2010b).
Shape associated with size (i.e., allometry), however, is still present in the data at this point. Taking this into account is important in view of the amount of body size variation within the group (Rabeder et al. 2008). To counteract the effects of allometry, so that (as near as possible) only shape is compared, a pooled regression analysis within species of the Procrustes coordinates onto log centroid size (Klingenberg 2010c) was performed. This analysis, used previously in both traditional and geometric morphometrics (Bruner and Costantini 2009; Mutsvangwa et al. 2010; Pierce et al. 1994; van Heteren et al. 2009; van Heteren et al. 2014), assumes that the allometry of the different species has the same exponent (slope) but different coefficients (intercepts). Supplementary Information 2 verifies the validity of these assumptions for the present dataset.
A composite phylogenetic tree of the taxa involved was created, based on topologies of Bininda-Emonds et al. (1999), Hofreiter et al. (2002) and Krause et al. (2008) (Fig. 1). The regression residuals were, subsequently, tested for the presence of a phylogenetic signal using the function physignal of the R package geomorph, using 10,000 iterations (Adams 2014). The morphological variation was then partitioned into a phylogenetic component, a component explained by diet and any unexplained variation using the methodology of Desdevises et al. (2003). To avoid the problem of pseudo-replication, the input data were based on averaged species as well as regions in the case of brown bears. Morphological variation was represented by the first four PCs, and the phylogenetic information was represented by the first five principal coordinates of the principal coordinate matrix computed from the phylogenetic distance matrix. A PCA on dietary percentages was performed to remove collinearity in the data and concentrate variation in the first few PCs. Only the first four axes were used to retain enough degrees of freedom for the analysis. The four PCos and the four dietary PCs both explain more than 99.9 % of the variance.
Differences between species were assessed in shape space by performing PCA. The landmark configurations of thirteen fossil U. spelaeus and 90 extant bear specimens were subjected to PCA. Regression residuals of the Procrustes coordinates, regressed onto log centroid size, served as the dependent variables. The alternative form space (based on the regression residuals plus log centroid size) is presented in Supplementary Information 3. To take into account the effects of phylogeny on the regression residuals for the PCA, a second PCA was performed. This second PCA used regression residuals calculated with the function pgls.Ives in the R package phytools (Revell 2012), which takes phylogeny into account, based on the work of Ives et al. (2007).
2B-PLS was performed in MorphoJ on the Procrustes coordinates in block 1 and standardised dietary percentages of the extant species (Table 1) in block 2. A permutation test with 10,000 repetitions was performed. The permutation test is performed by randomly varying the association between variables and the regression residuals (Rohlf and Corti 2000). The permutation test assumes a null hypothesis of complete independence between the two blocks of variables (Klingenberg 2010a). The position of U. spelaeus was projected into the PLS morphospace by regressing the PLS scores of the extants onto the PC scores of the first four PCs and substituting the PC scores of U. spelaeus in the resultant regression equations, which gives the estimated PLS scores of U. spelaeus in the extants’ morphospace. The nexus file of the phylogenetic tree was imported into MorphoJ and mapped onto the 2B-PLS results. Scatter plots of the taxon means of the PLS scores, with the phylogeny superimposed according to the reconstructed ancestral values, were interpreted in terms of evolutionary pathways.
To confirm the 2B-PLS results, a second regression between PC scores and foliage content of the diet was performed in PASW 17.0.3 (SPSS Inc. 2009). To determine the most appropriate variables, stepwise linear regressions were performed of both percentage of foliage and the logarithm thereof onto the first several relevant PCs. The stepping method criterion was set to use the probability of F with entry of the variable at p = 0.009999 and removal at p = 0.010000. The data and regression were plotted in a scatterplot with the 95 % confidence interval of the group means. Additionally, the mean regression score of U. spelaeus was plotted as a vertical line. Analogously, a phylogenetically controlled regression analysis was performed to determine which of the PCs is most closely correlated to foliage content of the diet. Here, a backward elimination phylogenetic generalised least squares (pGLS) analysis was performed on the PC scores using the R package pGLS.
Phylogenetic flexible discriminant analyses (phylo FDA) were performed on the first four PCs both with equal prior probabilities and prior probabilities calculated from group size to determine dietary preference and most important food item for cave bears. This analysis flexibly takes phylogeny into account when appropriate by calculating the optimal Pagel’s lambda to determine the degree of phylogenetic correction that needs to be applied. To avoid pseudoreplication, species means and region means for brown bears were used. This analysis was run in R using the methodology of Schmitz and Motani (2011).
Results
Sexual dimorphism in extant bears
The first four PC axes were interpreted, because they collectively explain 77 % of the shape variance in the data set and the other axes explain less than 5 % each. The influence of sexual dimorphism on the data was tested by performing PCA directly on the Procrustes coordinates without any allometric correction (Supplementary Information 2) and comparing these results with a PCA based on the regression residuals through MANOVA. Of the first four PCs, sex has a significant influence on PC2 (F(1,38) = 5.273, p = 0.027) when no allometric correction is performed. The interaction term of species*sex is not significant on any of the PCs (df1 = 6, df2 = 38). After allometric correction, sex (df1 = 1, df2 = 38) and the interaction term species*sex (df1 = 6, df2 = 38) do not have a significant effect on any of the first four PCs. The tests show that sexual dimorphism is sufficiently filtered out by correcting for allometry and does not need to be taken into account in the following analyses.
Phylogenetic signal
There is a significant phylogenetic signal present in the regression residuals (K = 0.3794, p = 0.0146). The morphological variation was partitioned to assess the amount of variation determined by diet, by phylogeny and by both (Fig. 3). When correcting for phylogeny, for example using independent contrasts, almost a third of the variation that is explained by diet will be removed. Therefore, both phylogenetically corrected and uncorrected analyses will be presented and interpreted together.
Mandible shape in cave bears compared to extant bears
Figure 4 shows the shape changes associated with the first four PCs of shape space (see Supplementary Information 3 for form space PCAs). Within the spectrum of the genus Ursus, U. spelaeus is at the opposite end to U. maritimus on PC1. High scores on the first PC are indicative of a straight deep mandibular corpus with a low coronoid process, a masseteric fossa approximately level with the condyle and a narrow symphysis. U. spelaeus, however, has low PC1 scores, comparable with T. ornatus and A. melanoleuca, which are associated with a dorsoventrally concave mandibular corpus, high coronoid process, a dorsal position of the masseteric fossa and large distance between the angular process and the condyle. PC1 explains 36 % of the total variance.
PCAs on the regression residuals of all extant Ursidae and U. spelaeus after a regression analysis of the Procrustes coordinates onto log centroid size pooled per species (a, b) and on the regression residuals of the regression taking phylogeny into account (c, d). The colors indicate the most important food item in the diet; a red outline denotes that the specimen is a fossil and diet is unknown. a, c PCs 1 and 2. b, d PCs 3 and 4. Shapes at −0.1 and 0.1 of a and b on PCs 1 and 3 are shown outside the graph and on PC2 and PC4 inside the graph
Of the group of species with relatively low PC1 scores, A. melanoleuca has the highest PC2 values. The spectacled bear (T. ornatus) has intermediate PC2 scores and the cave bear (U. spelaeus) has the lowest PC2 scores (Fig. 4a). The scores of U. spelaeus are comparable to Melursus ursinus and the other members of the genus Ursus apart from U. maritimus. Relatively low PC2 scores, such as those displayed by U. spelaeus, represent a relatively small mandibular corpus height dorsoventrally, a low coronoid process and a high position of the masseteric fossa. PC2 explains 19 % of the total variance, which, together with PC1, is 55 % cumulatively.
U. spelaeus has PC3 scores similar to those of the other bear species, except for T. ornatus, which has extremely high PC3 scores (Fig. 4b). These aberrant scores of T. ornatus are caused by two main morphological features: the back of its tooth row starts at a relatively large distance from the condyle and its masseteric fossa has a relatively caudoventral position. PC3 explains 13 % of the total variance, which, together with the previous two PCs, is 68.2 % cumulatively.
U. spelaeus has PC4 scores that are most similar to those of A. melanoleuca but shows overlap with M. ursinus and U. arctos, as well (Fig. 4b). PC4 is mostly determined by shape changes associated with the position of the masseteric fossa and the shape of the symphysis. PC4 explains 9.1 % of the total variance.
In the PCA based on regression residuals of a regression that takes phylogeny into account, the first four PCs explain 33.5, 23.5, 14.2 and 5.5 % of the variance (Fig. 4c, d). This PCA looks rather different from the previous but also shows a clear separation between the various dietary preferences. In this PCA, the cave bear is even more clearly separated from the other members of the genus Ursus and approaches the panda in morphospace. This is particularly visible on PC2.
Mandibular morphology and diet in extant bears
A 2B-PLS of the regression residuals of the regression onto log centroid size pooled per species and the dietary variables listed in Table 1 was performed (Fig. 5). The overall strength of the association between the two blocks of the 2B-PLS is given by the RV coefficient (0.57). A permutation test against the null hypothesis of independence gives p = 0.0126, indicating that the two blocks are not independent. PLS scores of the 2B-PLS versus dietary variables are correlated with the phylogeny of Ursidae (p = 0.0008). A form space 2B-PLS is presented in Supplementary Information 3.
Figure 5 shows how species have adapted to varying diets. The lineages leading to U. spelaeus and A. melanoleuca are parallel in morphospace in a direction, which is determined by large amounts of foliage in their diets. There is some indication of convergent evolution in U. arctos and U. americanus. M. ursinus and Helarctos malayanus diverged relatively little, suggesting a rather long shared evolutionary history in the direction of increased amounts of invertebrates in their diets. The direction in morphospace of the lineage leading to U. maritimus corresponds to a decrease in foliage and a corresponding increase in vertebrates in its diet.
Mandibular morphology and diet in cave bears
U. spelaeus has relatively high PLS1 scores, comparable to those of T. ornatus (Fig. 5). PLS1 is primarily associated with the amounts of foliage and invertebrates in the diet. Therefore, U. spelaeus displays a mandibular morphology associated with much foliage in the diet.
U. spelaeus has much higher PLS2 scores than T. ornatus has and is more comparable to the other members of the genus Ursus. PLS2 is for the most part influenced by the percentage of vertebrate prey in the diet. The position of U. spelaeus in this PLS graph (Fig. 5a) is relatively neutral for this feature.
Figure 5 shows that U. spelaeus and A. melanoleuca both evolved in a direction in morphospace associated with the amount of foliage in the diet. The amount of foliage in the diet of cave bears was estimated by running a stepwise regression analysis of the percentage of foliage, and the logarithm thereof, unto the PC scores, which resulted in R 2 values of 0.505 and 0.786, respectively. In the regression with the logarithm of percentage of foliage, only PC1 was retained (Fig. 6). This regression is highly significant (F(1) = 33.144, p < 0.001). The average PC1 score of U. spelaeus (indicated by the vertical line) is much lower than that of all other members of the genus Ursus and similar to that of T. ornatus. Consequently, the foliage content of the diet of U. spelaeus is also predicted to be much higher than that of all extant members of the genus Ursus. Based on the regression analysis, the foliage content of the diet of U. spelaeus is reconstructed to 79 % and no less than 58 %. A phylogenetically corrected regression analysis corroborates the importance of foliage in the diet and estimates an even higher foliage proportion. The pGLS indicates that only PC1 is significantly correlated with foliage content in the diet. According to this analysis, U. spelaeus likely had a foliage intake that was 96 % of its total food intake.
In the phylo FDA, the optimal Pagel’s lambda was determined to be 0, indicating that no phylogenetic correction was applied. The phylo FDA on dietary preference yields four discriminant functions. Both when all groups have equal prior probabilities and when prior probabilities are calculated from group size, 81.8 % of the cross-validated grouped specimens are correctly classified, meaning both analyses perform equally well. The results based on equal prior probabilities indicate that the dietary preference of U. spelaeus was foliage only (p = 0.7305), whereas the results with prior probabilities based on sample size indicates soft mast and foliage as preferred food items (p = 0.6834). The phylo FDA on most important food item using equal probabilities indicates that 81.8 % of the cross-validated grouped specimens are correctly classified, whereas 72.7 % of the cross-validated grouped specimens are correctly classified in the analysis with the prior probabilities based on sample size. Both analyses show that cave bears had foliage as most important food item in the case of equal probabilities with p = 0.991 and in the other analysis with p = 0.997. All four phylo FDAs point to the importance of foliage in the cave bear diet, whereas soft mast might have been of secondary importance according to one of the analyses.
Discussion
Evolution of dietary adaptations
In extant Carnivora, craniodental features have been shown to be linked to masticatory function (Anyonge and Baker 2006; Arribas and Palmqvist 1999; Biknevicius et al. 1996; Binder and Van Valkenburgh 2000; Christiansen and Adolfssen 2005; Cox 2008; Emerson and Radinsky 1980; Martínez-Navarro and Palmqvist 1996; Meloro et al. 2008; Oldfield et al. 2012; Slater et al. 2007; Therrien 2005; Van Valkenburgh 2007; Werdelin 1989; Werdelin and Solounias 1991). Tooth (root) morphology (Evans et al. 2006a; Evans et al. 2006b; Kupczik and Stynder 2012; Stynder and Kupczik 2013) gives an indication of preferred diet, while toothwear indicates the physical properties of what has actually been masticated (Van Valkenburgh and Hertel 1993). Rounded canines and canines with large bending strength suggest struggling with prey (Christiansen 2007a, b, c). The eight extant bear species analysed in the present study have varied diets, from completely folivorous to completely carnivorous to mostly insectivorous. These variations in dietary habits are expected to be reflected in their masticatory apparatus.
Although size is an important diet predictor in Carnivora (Gittleman 1985; Meloro 2011; Meloro et al. 2015b), there are large size differences within bear taxa, both geographically (e.g., brown bears) and between males and females, which allometrically change the shape of the mandible unrelated to diet. The importance of using allometrically corrected data for dietary analyses of modern bear morphology and dietary reconstructions of fossil bears (Supplementary Information 2) is also illustrated by work done on hominoids (McNulty 2004). McNulty’s work has shown that allometry contributes substantially to hominoid cranial morphogenesis and may do so for other taxa as well. Additionally, in the case of hominoids, evolution in size follows phylogeny. Any differences in shape without allometric correction may therefore be erroneously interpreted as different adaptations or the result of phylogenetic inertia. Correcting for allometry ensures that non-allometric adaptations are no longer obscured by allometry and are easier to interpret correctly. In addition, as part of the morphological variance is influence by both phylogeny and diet, it is helpful to simultaneously interpet phylogenetically corrected and uncorrected analyses.
The analyses herein, using allometrically corrected data, show good separation between the different dietary categories in terms of mandible morphology, as has previously been shown for skull morphology (Figueirido et al. 2009; Mattson 1998; Sacco and Van Valkenburgh 2004; van Heteren et al. 2014; Van Valkenburgh 2007). Bears that eat mostly insects, as well as those that eat mostly vertebrates, have relatively straight mandibular corpi, whereas those that eat mostly foliage have rather dorsoventrally curved mandibular corpi (Fig. 4a). Foliage-eating bears also have much dorsoventrally deeper mandibular corpi and higher coronoid processes than vertebrate-eating bears have (Fig. 4a). These are all indications that the PCA morphospace is determined by diet. This is confirmed by the 2B-PLS analysis (Fig. 5), which, although the axes are reversed, shows a very similar general pattern.
The panda (A. melanoleuca) was the first to split off from the other species phylogenetically (Bininda-Emonds et al. 1999) and has developed the most specialised diet, consisting solely of bamboo. The morphology of the mandible of A. melanoleuca is indicated in the present study to be adapted to relatively high amounts of foliage and low amounts of invertebrates and vertebrates in its diet (Fig. 5). Bamboo is a very tough material (Senshu et al. 2007), and its consumption requires certain masticatory adaptations, including increased tooth root surface (Kupczik and Stynder 2012). In Fig. 4a, the mechanical advantage of the temporalis muscle increases from the left upper corner of the graph to the right lower corner. This implies that pandas have very high coronoid processes, making the moment arm of the temporal muscle longer, so the force of this muscle is more effectively applied. Indeed, a strong mechanical advantage has been reported before for A. melanoleuca (Figueirido et al. 2009; Sacco and Van Valkenburgh 2004), and this feature is also linked to high fibre herbivory in other animals (Baryshnikov 2007). The masseteric fossa is located relatively ventrally according to the present results (Fig. 4). This does not change the absolute length of the moment arm of the deep masseter, but it does change the position where the moment arm is longest. In this configuration, the deep masseter is most effective in a more closed mandibular position. The superficial masseter, represented by landmark 5, does have a longer moment arm in the present study (i.e., it is positioned relatively far from the condyle), making this part of the muscle more efficient. As the superficial masseter is more efficient at more closed gapes than is the deep masseter, this is clearly an adaptation to closed jaw mastication, such as grinding. The results of the present study indicate that the superficial and deep masseter and the temporal muscle all contribute to an effective grinding motion when the jaw is nearly closed (i.e., they have relatively long moment arms when the jaw is nearly closed (Fig. 4)), the masseter pulling rostrally and laterally, and the temporalis pulling caudally and medially. The muscles themselves are very powerful, too, according to previous studies, which can be seen from the large space available between the zygomatic arch and the supraorbital process for the masticatory muscles as well as the depth of the zygomatic arch, which provides a large attachment area for the masseter (Mattson 1998). The strong muscles, which reflect the toughness of bamboo (Senshu et al. 2007), lead to additional adaptations to resist torsion and bending in the mandible. The panda’s mandibular corpus is relatively curved, and the mandibular symphysis is rather deep in the present study (Fig. 4). Pandas are, thus, very well adapted to their diet. The initial evolutionary reasons why the panda began eating substantial amounts of bamboo around 7 Ma and become fully dependent on it by around 2 Ma is still a mystery (Jin et al. 2007; Jin et al. 2011). Genetic evidence suggests, however, that this change was coincident with changes in taste perception, making the eating of meat less rewarding for the panda (Jin et al. 2011).
The second species to split off from the evolutionary tree of bears was the spectacled bear (T. ornatus) (Bininda-Emonds et al. 1999). In the analyses presented above, T. ornatus consistently occupies positions in morphospace that may be unexpected based on what is known about its diet. This is due to a feature of the mandible of the spectacled bear that is not found in other extant ursids: the premasseteric fossa (Fig. 2c). The premasseteric fossa is not occupied by any soft tissue and could potentially function as a cheek pouch, although it has not been observed to be used in that way (Davis 1955). The function of this fossa remains unclear but must have been important, judging from the fact that it evolved convergently on at least three separate occasions within Ursidae (Hunt 1999) and that it may be related to an expansion of the musculus zygomaticomandibularis (Davis 1955), which has been linked to the supposed herbivorous diet of the spectacled bear (Trajano and Ferrarezzi 1995). The spectacled bear, however, has a non-specialised diet similar to that of the American black bear (Table 1), and its mandible does not show any specific masticatory adaptations related to the function of the temporal muscle or resistance against bending forces or torsion. The omnivorous diet of the spectacled bear consists of relatively little foliage (Table 1) and is not as tough as that of the panda. The presence of the premasseteric fossa influences the rest of the morphology of the mandible. A comparison of Fig. 2b and c shows that the masseteric fossa is pushed back caudally in T. ornatus due to the premasseteric fossa. This causes the masseter to be relatively inefficient when the jaw is widely opened and more efficient when the jaw is more closed. It is important to keep in mind though that this is not necessarily a direct adaptation of masseter functionality to diet, but might be an indirect adaptation due to the premasseteric fossa, of which the function is still unknown.
The genus Ursus is the only genus of bears with a well-documented fossil record (Herrero 1972), and it is the most generalised genus of the bear family, apart from its most recent offshoot, the polar bear. In the present study, it is possible to reconstruct the diet of U. minimus. U. minimus was about the size of H. malayanus but in general resembled black bears morphologically (Herrero 1972). Based on the present work, some predictions about its diet can be made. U. minimus, which was on the lineage leading to both the last common ancestor of the extant members of the genus Ursus and the lineage leading to the sloth and Malayan sun bear (Fig. 1), is predicted possibly to have had an elevated vertebrate intake (Fig. 5). U. minimus was a small, primitive ursine bear that generally increased in size over evolutionary time (McLellan and Reiner 1994). U. minimus has previously been proposed to have been omnivorous with a preference for invertebrates based on two-dimensional geometric morphometrics of the mandibular outline in lateral view (Meloro 2007). This conclusion was, however, based only on the position in PCA morphospace of U. minimus but not the direction through a diet-specific morphospace of the evolutionary lineage. In Fig. 5, the direction of the lineage of U. minimus in 2B-PLS morphospace indicates a decrease in foliage, with a possible increase in vertebrates, relative to its ancestor.
The two insectivorous species, the Malayan sun bear (H. malayanus) and the sloth bear (M. ursinus), split off from the bear family tree together (Bininda-Emonds et al. 1999) and developed adaptations to eating insects and fruits. H. malayanus and, particularly, M. ursinus hardly have any fibrous food items in their diet. These species need very little mechanical advantage, seen in the very low coronoid process (Fig. 4). The results of the present study correctly indicate that the morphology of both H. malayanus and M. ursinus indicates relatively low amounts of foliage and high amounts of invertebrates in their diets (Fig. 5). Their diet, and probably that of their ancestors, is not very tough. The morphometric analyses in the present study suggest that their mandibles do not show any specific adaptations of the masseter and temporalis muscles. Both species, and presumably their ancestors, have a particularly long tongue to suck and lick up insects (Augeri 1995; Fredriksson et al. 2006; Kurt 1990; Meijaard 1998; Yoganand et al. 2012). Such a tongue requires a lot of space in the mouth cavity, and the present analyses suggest that this is provided by a relatively deep mandibular corpus and associated mandibular symphysis (Fig. 4). Later in evolutionary history, the two species split from each other adapting to their own niches. The Malayan sun bear eats slightly fewer insects and less soft mast than does the sloth bear but eats approximately as many vertebrates as the brown bear. This makes Malayan sun bears more omnivorous than sloth bears and restricts potential adaptations to the insectivorous part of its diet. The mastication of vertebrates requires more force than does the mastication of most fruits and insects, which are less fracture-resistant (Popowics 2003). In the present analyses, the coronoid process of the Malayan sun bear is higher, and the masseteric fossa is located more rostrally than are those of the sloth bear (Fig. 4), lengthening the moment arms of both the masseter and the temporalis, making both these muscles more effective. Both species are, thus, very well adapted to their respective diets. The fossil record for the lineage leading to the Malayan sun bear and the sloth bear is scant, but the pronounced morphological divergence of the Malayan sun bear and sloth bear relative to the other ursine bears is likely a recent adaptation to the exploitation of an insectivorous niche by these South Asian bears (McLellan and Reiner 1994). The split between the two bears roughly coincides with the mid-Pleistocene transition from obliquity to eccentricity-led climatic cycles (Clark et al. 2006), and it is conceivable that this transition is somehow related to their speciation and dietary adaptations.
The oldest split within the genus Ursus is that of the American black bear (U. americanus) and U. etruscus, the last common ancestor of Asiatic black bear and the lineage leading to modern brown bear and polar bear. The results of the present analyses suggest that the American black bear does not show any particular adaptations in the temporal or the masseter muscle functionality and its diet is unspecialised (Fig. 4, Table 1). Early forms of U. etruscus were about the size of the Asiatic black bear, but over time they increased in size up to the size of brown bears (Herrero 1972). U. etruscus was probably a forest-adapted species (Herrero 1972). The U. etruscus lineage is predicted, based on the present results, to have been parallel to that of the American black bear (Fig. 5), which may be associated with a decrease in the amount of invertebrates and an increase in meat in the diet. This makes sense in terms of its evolutionary size increase, as it is difficult to sustain a large body size on invertebrates. Based on dental morphology, U. etruscus has, indeed, been thought to have been an unspecialised omnivore, despite its high levels of δ15N, which have been attributed to either the consumption of fish or the reuse of urea during hibernation (Palmqvist et al. 2003; Palmqvist et al. 2008a; Palmqvist et al. 2008b). Based on two-dimensional geometric morphometrics, however, it has been suggested that U. etruscus was omnivorous with a preference for meat (Meloro 2007). In the present work, the direction of its lineage in morphospace also implies that it consumed more vertebrates than its ancestor did, supporting the conclusions of Meloro (2007).
The next species to split off within the genus Ursus was the Asiatic black bear (U. thibetanus), although it can morphologically be viewed as a slightly modified surviving U. etruscus (Herrero 1972). The Asiatic black bear is omnivorous but eats mostly hard mast. The position of U. thibetanus in morphospace is difficult to explain. With such a large portion of hard mast in their diet, one might expect the necessity for more mechanical advantage, for example to crack open nuts, but Fig. 4 shows that this bear has very little mechanical advantage concerning the masseter. This may be compensated, however, by a relatively large cross-section of the masseter muscle (Christiansen 2007c). The results of the present analyses show that this required only slight masticatory adaptations of the mandible (Fig. 4). The present analyses show that U. thibetanus has smaller foliage component in its diet as compared to the invertebrate component than U. arctos and U. americanus (Fig. 5).
Brown bears (U. arctos) and polar bears (U. maritimus) are the last two species of the genus Ursus to diverge. Whereas brown bears retained a relatively diverse diet, polar bears specialised in eating meat. The results of the present analyses show that the morphology and muscle function of brown bears is very similar to that of the other omnivorous bears and does not show any specific adaptations. The present analyses suggest that polar bears, on the other hand, have developed adaptations to eating meat in contrast to the other members of the genus Ursus for whom soft mast and other plant matter are consistently an important determinant of morphology, as indicated by the 2B-PLS analyses (Fig. 5). In PCA morphospace, this is also visible in the present study, as the polar bear has a straighter mandibular corpus than the panda has with a lower coronoid process (Fig. 4). In terms of biomechanics, this means that in the present analyses, the temporal muscle of the polar bear has a relatively short moment arm, as opposed to the long moment arm of the panda (Fig. 4). It may seem counter-intuitive that U. maritimus, being carnivorous, should need so little mechanical advantage for the temporal muscle. Bears, however, mostly eat prey that are relatively smaller than those of canids or felids (Sacco and Van Valkenburgh 2004). Felids (and canids even more so) use their jaws as an important tool for subduing prey, although is not their only weapon (e.g., claws). Bears, being relatively larger, do not need the same mechanical advantage of their temporal muscle as canids and felids do, because they can subdue their prey with their strong paws and large body weight and are less dependent on their jaws to hold the prey. U. maritimus is no exception; it eats mostly seal pups (Stirling and Archibald 1977), which are not only small, but also vulnerable. This is consistent with a previous study (Slater et al. 2010) that showed that the skull of U. maritimus is relatively weak. Additionally, the polar bear has a rather short grinding basin, as its meat-based diet does not require grinding. The masseter of polar bears has a long moment arm and its attachment is located rather rostrally (Fig. 4), making the masseter very efficient when the jaw is at a wider gape angle for grabbing prey. The split between brown and polar bears, again, coincides approximately with the mid-Pleistocene transition (Clark et al. 2006). This climatic transition resulted in more extensive ice sheets, due to longer formation periods. This likely forced some of the brown bears to adapt to colder, arctic conditions, resulting in what we now know as the polar bear.
Overall, all the extant bear species show mandibular shape variations that are compatible with the hypothesis that their morphology is influenced by their respective diets. Even the polar bear, whose lineage only diverged from that of the brown bear relatively recently (i.e., 1 Ma (Bininda-Emonds et al. 1999)), displays clear masticatory adaptations to its specialist meat-based diet. The reconstructions of the diets of U. minimus and U. etruscus, particularly the latter, based on the present study, are consistent with analyses of teeth and two-dimensional geometric morphometrics from the literature. This indicates that overlaying a phylogeny onto morphospace can provide valuable information on taxa that are rarely or incompletely preserved in the fossil record. Additionally, this offers the possibility of interpreting the diet of extinct bears, such as the cave bear, according to the position in morphospace of their fossils.
Cave bear diet
The present study assesses the mandibular adaptations of fossil and extant bears, with the aim of identifying the likely diet of U. spelaeus, which inhabited Europe during the Pleistocene. Previous work on U. spelaeus based on morphology, stable isotopes and tooth microwear analysis has not led to an agreement on their diet or behaviour (Athen 2006, 2007; Bocherens et al. 1994, 1997; Figueirido et al. 2009; Hilderbrand et al. 1996; Osborn 2010; Peigné et al. 2009; Pinto Llona 2006; Quilès et al. 2006; Rabal-Garcés et al. 2012; Richards et al. 2008). The present study thus contributes to the current debate on the diet of cave bears by analysing the relationship between form and function of the masticatory apparatus of bears.
The results of the present analyses show that, in terms of masticatory functional morphology, U. spelaeus occupies a position in morphospace close to extant brown bears, but its mandible is slightly more curved approaching the configuration of the folivorous panda (Fig. 4), possibly indicating an elevated amount of foliage in its diet relative to modern brown bears. This is supported by the fact that the position in morphospace of cave bears and polar bears are on opposite sides relative to other members of the genus Ursus (Fig. 4; van Heteren et al. 2009), suggesting that as polar bears eat more meat than do brown bears, cave bears are likely to have eaten less meat. The moment arm of the superficial masseter of U. spelaeus is much longer and the moment arm of the deep masseter slightly longer than that of the extant brown bear, approaching the configuration of the panda (Fig. 4). The masseter, and particularly the superficial masseter, is relatively effective when the jaw is nearly closed (Ewer 1973). Increased effectiveness of muscle force at this gape angle may be an adaptation to grinding plant matter. The lineage leading to the cave bear runs parallel to the folivorous panda in 2B-PLS morphospace (Fig. 5). Thus, U. spelaeus and A. melanoleuca appear to have displayed parallel evolution and the different morphologies of these species can largely be attributed to different ancestries. This indicates that cave bears evolved mandibular adaptations suited for a diet with similar properties to that of the panda.
The moment arm of the temporalis muscle of cave bears is longer than that of modern brown bears and approaches that of the panda. The temporalis muscle of cave bears is inferred to have been most efficient at a jaw position that is more closed than at the most efficient position for either the modern brown bear or the panda (Fig. 4); this is unique and not seen in any extant bear. As there is no modern analogue, it is difficult to infer to what this may be an adaptation, but it is conceivable that this increased efficiency of the temporal muscle when the jaw is nearly closed aids in delivering a more powerful grinding motion compared to U. arctos. This may be an adaptation to elevated levels of herbivory, consistent with the interpretation of the masseter morphology. This may have evolved in response to the Pleistocene climate, which became increasingly varied and cold over time (Lisiecki and Raymo 2007). In any case, Fig. 4 shows that U. spelaeus had less mechanical advantage of the temporalis muscle than A. melanoleuca had, but more than the other extant bears have, suggesting a relatively high fibre diet. These results correspond with recent stable isotope analyses of cave bears (Bocherens et al. 2011). According to the phylo FDA analysis, U. spelaeus would have had foliage as its most important food item with possibly soft mast as second most important food item. Futhermore, the regression analyses suggest that the foliage content of the cave bear diet was likely between 79 and 96 %.
Mattson (1998) performed comparable analyses to those presented here but using linear measurements on the skull and mandible of a subset of modern bear species and two fossil bear species, including the cave bear. He showed that there was good agreement between skull and mandible morphology and the composition of the faeces, which he used as a proxy for diet. Based on his results, he predicted that cave bear faeces should consist almost wholly of foliage and/roots, and he interpreted this as an indication of a mostly herbivorous diet for the cave bear, consistent with the results of our study. Isotope analyses of cave bear teeth have provided variable results, sometimes pointing to the cave bear as being herbivorous (Bocherens et al. 1994) and sometimes to a substantial amount of meat in its diet (Hilderbrand et al. 1996). However, this latter effect may be due to metabolic peculiarities associated with hibernation (Nelson et al. 1983; Palmqvist et al. 2003; Palmqvist et al. 2008a; Palmqvist et al. 2008b). Previous geometric morphometric analyses of the skull (van Heteren et al. 2014) also indicated that the cave bear had a diet that predominantly consisted of foliage and/or roots and tubers, which possibly involved mastication of grit as well (Stiner et al. 1998).
Conclusion
The results of the analyses presented here, using 3D geometric morphometrics with allometric and phylogenetic correction, suggest that bear mandibular morphology reflects diet with a clear separation between the species, which allows for easy classification of the diet of cave bears. As hypothesised, the position of the cave bear in morphospace reflects both its phylogeny and its diet. The influence of these two factors can be visualised by plotting the phylogeny of the represented taxa onto diet-related morphospace. The evolutionary directions in morphospace then represent dietary adaptations irrespective of the species’ phylogenetic starting point.
Using various approaches, the present study suggests that cave bears were primarily folivorous, which is in support of the prevailing hypothesis that the cave bears were herbivorous (Bocherens et al. 1997; Bocherens et al. 2014; Bocherens et al. 1994; Bocherens et al. 2011; Christiansen 2007c; Rabeder et al. 2010; Stiner et al. 1998). There is no support for the interpretation of adaptations to scavenging or hunting behaviour in cave bears. This implies that the cave bear’s extinction is not caused by a lack of food supply after the end-Pleistocene large herbivore extinction, but rather that the cave bear was one of the large herbivores.
References
Adams, D. C. (2014). A generalized K statistic for estimating phylogenetic signal from shape and other high-dimensional multivariate data. Systematic Biology, 63(5), 685–697.
Anyonge, W., & Baker, A. (2006). Craniofacial morphology and feeding behavior in Canis dirus, the extinct Pleistocene dire wolf. Journal of Zoology, 269(3), 309–316.
Arribas, A., & Palmqvist, P. (1999). On the ecological connection between sabre-tooths and hominids: faunal dispersal events in the Lower Pleistocene and a review of the evidence for the first human arrival in Europe. Journal of Archaeological Science, 26(5), 571–585.
Athen, K. (2006). Biostatistical investigation of long bones and metapodial bones of Ursus spelaeus and Ursus deningeri. Scientific Annals, School of Geology Aristotle University of Thessaloniki, 98, 159–162.
Athen, K. (2007). Biometrische Untersuchungen des Stylopodiums, Zygopodiums und Metapodiums pleistozäner Ursiden im Hinblick auf die Evolution des Höhlenbären und die Klassifizierung des Fundmaterials Einhornhöhle/Harz. Ph. D. thesis, Eberhard-Karls-Universität, Tübingen.
Augeri, D. M. (1995). On the biogeographic ecology of the Malayan sun bear. Cambridge: University of Cambridge.
Azzaroli, A. (1983). Quaternary mammals and the “end-Villafranchian” dispersal event—a turning point in the history of Eurasia. Palaeogeography, Palaeoclimatology, Palaeoecology, 44(1–2), 117–139. doi:10.1016/0031-0182(83)90008-1.
Baca, M., Stankovic, A., Stefaniak, K., Marciszak, A., Hofreiter, M., Nadachowski, A., et al. (2012). Genetic analysis of cave bear specimens from Niedźwiedzia Cave, Sudetes, Poland. Palaeontologia Electronica, 15(2), 21A.
Bargali, H. S., Akhtar, N., & Chauhan, N. P. S. (2009). Feeding ecology of sloth bears in a disturbed area in central India. Ursus, 15(2), 212–217. doi:10.2192/1537-6176(2004)015<0212:feosbi>2.0.co;2.
Baryshnikov, G. (2007). Ursidae (Fauna of Russia and neighbouring countries, new ser. 147 ). Saint Petersburg: Nauka. (in Russian)
Biknevicius, A. R., Van Valkenburgh, B., & Walker, J. (1996). Incisor size and shape: implications for feeding behaviors in saber-toothed “cats”. Journal of Vertebrate Paleontology, 16(3), 510–521.
Binder, W. J., & Van Valkenburgh, B. (2000). Development of bite strength and feeding behaviour in juvenile spotted hyenas (Crocuta crocuta). Journal of Zoology, 252, 273–283.
Bininda-Emonds, O. R. P., Gittleman, J. L., & Purvis, A. (1999). Building large trees by combining phylogenetic information: a complete phylogeny of the extant Carnivora (Mammalia). Biological Reviews, 74(2), 143–175.
Bocherens, H., Fizet, M., & Mariotti, A. (1994). Diet, physiology and ecology of fossil mammals as inferred from stable carbon and nitrogen isotope biogeochemistry: implications for Pleistocene bears. Palaeogeography, Palaeoclimatology, Palaeoecology, 107(3–4), 213–225.
Bocherens, H., Billiou, D., Patou-Mathis, M., Bonjean, D., Otte, M., & Mariotti, A. (1997). Paleobiological implications of the isotopic signatures (13C,15N) of fossil mammal collagen in Scladina cave (Sclayn, Belgium). Quaternary Research, 48(3), 370–380.
Bocherens, H., Stiller, M., Hobson, K. A., Pacher, M., Rabeder, G., Burns, J. A., et al. (2011). Niche partitioning between two sympatric genetically distinct cave bears (Ursus spelaeus and Ursus ingressus) and brown bear (Ursus arctos) from Austria: isotopic evidence from fossil bones. Quaternary International, 245(2), 238–248.
Bocherens, H., Bridault, A., Drucker, D. G., Hofreiter, M., Münzel, S. C., Stiller, M., et al. (2014). The last of its kind? Radiocarbon, ancient DNA and stable isotope evidence from a late cave bear (Ursus spelaeus ROSENMÜLLER, 1794) from Rochedane (France). Quaternary International, 339–340, 179–188.
Bojarska, K., & Selva, N. (2012). Spatial patterns in brown bear Ursus arctos diet: the role of geographical and environmental factors. Mammal Review, 42(2), 120–143. doi:10.1111/j.1365-2907.2011.00192.x.
Bon, C., Caudy, N., de Dieuleveult, M., Fosse, P., Philippe, M., Maksud, F., et al. (2008). Deciphering the complete mitochondrial genome and phylogeny of the extinct cave bear in the Paleolithic painted cave of Chauvet. Proceedings of the National Academy of Sciences of the United States of America, 105(45), 17447–17452. doi:10.1073/pnas.0806143105.
Bookstein, F. L. (1991). Morphometric tools for landmark data geometry and biology. Cambridge: Cambridge University Press.
Bruner, E., & Costantini, D. (2009). Head morphology and degree of variation in Lacerta bilineata, Podarcis muralis and Podarcis sicula. International Journal of Morphology, 27(3), 667–676.
Cahill, J. A., Green, R. E., Fulton, T. L., Stiller, M., Jay, F., Ovsyanikov, N., et al. (2013). Genomic evidence for island population conversion resolves conflicting theories of polar bear evolution. PLoS Genetics, 9(3), e1003345.
Christiansen, P. (2007a). Canine morphology in the larger Felidae: implications for feeding ecology. Biological Journal of the Linnean Society, 91(4), 573–592.
Christiansen, P. (2007b). Comparative bite forces and canine bending strength in feline and sabretooth felids: implications for predatory ecology. Zoological Journal of the Linnean Society, 151, 423–437.
Christiansen, P. (2007c). Evolutionary implications of bite mechanics and feeding ecology in bears. Journal of Zoology, 272(4), 423–443.
Christiansen, P. (2008). Feeding ecology and morphology of the upper canines in bears (Carnivora: Ursidae). Journal of Morphology, 269(7), 896–908.
Christiansen, P., & Adolfssen, J. S. (2005). Bite forces, canine strength and skull allometry in carnivores (Mammalia, Carnivora). Journal of Zoology, 266, 133–151.
Clark, P. U., Archer, D., Pollard, D., Blum, J. D., Rial, J. A., Brovkin, V., et al. (2006). The middle Pleistocene transition: characteristics, mechanisms, and implications for long-term changes in atmospheric pCO2. Quaternary Science Reviews, 25(23–24), 3150–3184.
Cox, P. G. (2008). A quantitative analysis of the Eutherian orbit: correlations with masticatory apparatus. Biological Reviews, 83(1), 35–69. doi:10.1111/j.1469-185X.2007.00031.x.
Davis, D. D. (1955). Masticatory apparatus in the spectacled bear. Fieldiana: Zoology, 37, 25–46.
Desdevises, Y., Legendre, P., Azouzi, L., & Morand, S. (2003). Quantifying phylogenetically structured environmental variation. Evolution, 57(11), 2647–2652.
Emerson, S. B., & Radinsky, L. (1980). Functional-analysis of sabertooth cranial morphology. Paleobiology, 6(3), 295–312.
Enloe, J. G., David, F., & Baryshnikov, G. (2000). Hyenas and hunters: zooarchaeological investigations at Prolom II Cave, Crimea. International Journal of Osteoarchaeology, 10(5), 310–324.
Evans, A. R., Martin, T., Fortelius, M., & Jernvall, J. (2006a). Reconstructing dental occlusion in 3D: from carnivorans to Asfaltomylos. Journal of Vertebrate Paleontology, 26(3), 59A–59A.
Evans, A. R., Wilson, G. P., Fortelius, M., & Jernvall, J. (2006b). High-level similarity of dentitions in carnivorans and rodents. Nature, 445, 78–81.
Ewer, R. G. (1973). The carnivores. Ithaca: Cornell University Press.
Figueirido, B., Palmqvist, P., & Pérez-Claros, J. A. (2009). Ecomorphological correlates of craniodental variation in bears and paleobiological implications for extinct taxa: an approach based on geometric morphometrics. Journal of Zoology, 277(1), 70–80.
Figueirido, B., Serrano-Alarcón, F. J., & Palmqvist, P. (2012). Geometric morphometrics shows differences and similarities in skull shape between the red and giant pandas. Journal of Zoology, 286(4), 293–302. doi:10.1111/j.1469-7998.2011.00879.x.
Flynn, J. J., & Nedbal, M. A. (1998). Phylogeny of the Carnivora (Mammalia): congruence vs incompatibility among multiple data sets. Molecular Phylogenetics and Evolution, 9(3), 414–426. doi:10.1006/mpev.1998.0504.
Fredriksson, G. M., Wich, S. A., & Trisno. (2006). Frugivory in sun bears (Helarctos malayanus) is linked to El Niño-related fluctuations in fruiting phenology, East Kalimantan, Indonesia. Biological Journal of the Linnean Society, 89(3), 489–508.
Fulton, T. L., & Strobeck, C. (2006). Molecular phylogeny of the Arctoidea (Carnivora): effect of missing data on supertree and supermatrix analyses of multiple gene data sets. Molecular Phylogenetics and Evolution, 41(1), 165–181. doi:10.1016/j.ympev.2006.05.025.
Ginsburg, L., & Morales, J. (1998). Les Hemicyoninae (Ursidae, Carnivora, Mammalia) et les formes apparentées du Miocène inférieur et moyen d’Europe occidentale. Annales de Paleontologie, 84(1), 71–123. doi:10.1016/s0753-3969(98)80003-7.
Gittleman, J. L. (1985). Carnivore body size—ecological and taxonomic correlates. Oecologia, 67(4), 540–554.
Grandal-d’Anglade, A., & Vidal Romaní, J. R. (1997). A population study on the cave bear (Ursus spelaeus Ros.-Hein.) from Cova Eirós (Triacastela, Galicia, Spain). Geobios, 30(5), 723–731.
Hänni, C., Laudet, V., Stehelin, D., & Taberlet, P. (1994). Tracking the origins of the cave bear (Ursus spelaeus) by mitochondrial DNA sequencing. Proceedings of the National Academy of Sciences of the United States of America, 91(25), 12336–12340.
Herrero, S. (1972). Aspects of evolution and adaptation in American black bears (Ursus americanus Pallus) and brown and grizzly bears (Ursus arctos Linn.) of North America. Bears - Their Biology and Management, 23, 221–231.
Hilderbrand, G. V., Farley, S. D., Robbins, C. T., Hanley, T. A., Titus, K., & Servheen, C. (1996). Use of stable isotopes to determine diets of living and extinct bears. Canadian Journal of Zoology, 74, 2080–2088.
Hofreiter, M., Capelli, C., Krings, M., Waits, L., Conard, N., Münzel, S., et al. (2002). Ancient DNA analyses reveal high mitochondrial DNA sequence diversity and parallel morphological evolution of Late Pleistocene cave bears. Molecular Biology and Evolution, 19(8), 1244–1250.
Hunt, R. M. (1999). Ursidae. In C. M. Janis, K. M. Scott, & L. L. Jacobs (Eds.), Evolution of tertiary mammals of North America (pp. 174–195). New York: Cambridge University Press.
Hunt, R. M. (2004). A paleontologist’s perspective on the origin and relationships of the giant panda. In D. G. Lindburg & K. Baragona (Eds.), Giant pandas: biology and conservation (p. 308). Berkely: University of California Press.
Ives, A. R., Midford, P. E., & Garland, T., Jr. (2007). Within-species measurement error in phylogenetic comparative methods. Systematic Biology, 56, 252–270.
Jin, C., Ciochon, R. L., Dong, W., Hunt, R. M., Liu, J., Jaeger, M., et al. (2007). The first skull of the earliest giant panda. Proceedings of the National Academy of Sciences of the United States of America, 104(26), 10932–10937. doi:10.1073/pnas.0704198104.
Jin, K., Xue, C., Wu, X., Qian, J., Zhu, Y., Yang, Z., et al. (2011). Why does the giant panda eat bamboo? A comparative analysis of appetite-reward-related genes among mammals. PLoS ONE, 6(7), e22602. doi:10.1371/journal.pone.0022602.
Joshi, A. R., Garshelis, D. L., & Smith, J. L. D. (1997). Seasonal and habitat-related diets of sloth bears in Nepal. Journal of Mammalogy, 78(2), 584–597.
Klingenberg, C. P. (2010a). MorphoJ: an integrated software package for geometric morphometrics. Molecular Ecology Resources, 353–357, doi:10.1111/j.1755-0998.2010.02924.x.
Klingenberg, C. P. (2010b). New Procrustes Fit. http://www.flywings.org.uk/MorphoJ_guide/frameset.htm?index.htm. Accessed September 16th 2010.
Klingenberg, C. P. (2010c). Regression. http://www.flywings.org.uk/MorphoJ_guide/frameset.htm?index.htm. Accessed June 1st 2010.
Kosintsev, P. (2007). Late Pleistocene large mammal faunas from the Urals. Quaternary International, 160, 112–120. doi:10.1016/j.quaint.2006.09.012.
Krause, J., Unger, T., Nocon, A., Malaspinas, A.-S., Koloktronis, S.-O., Stiller, M., et al. (2008). Mitochondrial genomes reveal an explosive radiation of extinct and extant bears near the Miocene-Pliocene boundary. Evolutionary Biology, 8(220), 1–12.
Kupczik, K., & Stynder, D. D. (2012). Tooth root morphology as an indicator for dietary specialisation in carnivores (Mammalia: Carnivora). Biological Journal of the Linnean Society, 105(2), 456–471.
Kurt, F. (1990). Malayan Sun Bear. In S. P. Parker (Ed.), Grzimek’s encyclopedia of mammals (Vol. 3, p. 504). New York: McGraw-Hill Publishing Company.
Laurie, A., & Seidensticker, J. (1977). Behavioural ecology of the Sloth bear (Melursus ursinus). Journal of Zoology, 182(2), 187–204.
Lisiecki, L. E., & Raymo, M. E. (2007). Plio-Pleistocene climate evolution: trends and transitions in glacial cycle dynamics. Quaternary Science Reviews, 26(1–2), 56–69.
Martin, L. D. (1989). Fossil history of the terrestrial Carnivora. In J. L. Gittleman (Ed.), Carnivore behaviour, ecology and evolution (pp. 536–568). London: Chapman and Hall.
Martínez-Navarro, B., & Palmqvist, P. (1996). Presence of the African saber-toothed felid Megantereon whitei (Broom, 1937) (Mammalia, Carnivora, Machairodontinae) in Apollonia-1 (Mygdonia Basin, Macedonia, Greece). Journal of Archaeological Science, 23(6), 869–872.
Mattson, D. J. (1998). Diet and morphology of extant and recently extinct northern bears. Ursus, 10, 479–496.
McLellan, B., & Reiner, D. C. (1994). A review of bear evolution. Bears - Their Biology and Management, 85–96.
McNulty, K. P. (2004). A geometric morphometric assessment of hominoid crania: conservative African apes and their liberal implications. Annals of Anatomy - Anatomischer Anzeiger, 186(5–6), 429–433.
Meijaard, E. (1998). Ursus (Helarctos) malayanus, the neglected Malayan sun bear. Nederlandse Commissie voor Internationale Natuurbescherming. Mededelingen, 34, 1-62.
Meloro, C. (2007). Plio-Pleistocene large carnivores from the Italian pensinsula: functional morphology and macroecology. Ph. D. thesis, Università degli Studi di Napoli “Federico II”, Napoli.
Meloro, C. (2011). Feeding habits of Plio-Pleistocene large carnivores as revealed by the mandibular geometry. Journal of Vertebrate Paleontology, 31(2), 428–446.
Meloro, C., Raia, P., Piras, P., Barbera, C., & O’Higgins, P. (2008). The shape of the mandibular corpus in large fissiped carnivores: allometry, function and phylogeny. Zoological Journal of the Linnean Society, 154, 832–845.
Meloro, C., Clauss, M., & Raia, P. (2015a). Ecomorphology of Carnivora challenges convergent evolution. Organisms Diversity & Evolution, 1–10, doi:10.1007/s13127-015-0227-5.
Meloro, C., Hudson, A., & Rook, L. (2015b). Feeding habits of extant and fossil canids as determined by their skull geometry. Journal of Zoology, 295(3), 178–188.
Mutsvangwa, T. E. M., Meintjes, E. M., Viljoen, D. L., & Douglas, T. S. (2010). Morphometric analysis and classification of the facial phenotype associated with fetal alcohol syndrome in 5- and 12-year-old children. American Journal of Medical Genetics Part A, 152A, 32–41.
Nawaz, M. A. (2008). Ecology, Genetics and Conservation of Himalayan Brown Bears. Ph. D. thesis, Norwegian University of Life Sciences, Ås.
Nelson, R. A., Folk, G. E., Pfeiffer, E. W., Craighead, J. J., Jonkel, C. J., & Steiger, D. L. (1983). Behavior, biochemistry, and hibernation in black, grizzly, and polar bears. International Conference on Bear Research and Management, 5, 284–290.
Oldfield, C. C., McHenry, C. R., Clausen, P. D., Chamoli, U., Parr, W. C. H., Stynder, D. D., et al. (2012). Finite element analysis of ursid cranial mechanics and the prediction of feeding behaviour in the extinct giant Agriotherium. Journal of Zoology, 286(2), 163–170.
Osborn, H. F. (2010). The age of mammals in Europe, Asia and North America. New York: MacMillan Co.
Pacher, M., & Stuart, A. J. (2008). Extinction chronology and palaeobiology of the cave bear (Ursus spelaeus). Boreas, 38, 189–206.
Palmqvist, P., Gröcke, D. R., Arribas, A., & Fariña, R. A. (2003). Paleoecological reconstruction of a Lower Pleistocene large mammal community using biogeochemical (δ13C, δ15N, δ18O, Sr: Zn) and ecomorphological approaches. Paleobiology, 29(2), 205–229.
Palmqvist, P., Pérez-Claros, J. A., Janis, C. M., Figueirido, B., Torregrosa, V., & Gröcke, D. R. (2008a). Biogeochemical and ecomorphological inferences on prey selection and resource partitioning among mammalian carnivores in an Early Pleistocene community. Palaios, 23(11), 724–737. doi:10.2110/palo.2007.p07-073r.
Palmqvist, P., Pérez-Claros, J. A., Janis, C. M., & Gröcke, D. R. (2008b). Tracing the ecophysiology of ungulates and predator–prey relationships in an early Pleistocene large mammal community. Palaeogeography, Palaeoclimatology, Palaeoecology, 266(1–2), 95–111.
Peigné, S., Goillot, C., Germonpré, M., Blondel, C., Bignon, O., & Merceron, G. (2009). Predormancy omnivory in European cave bears evidenced by a dental microwear analysis of Ursus spelaeus from Goyet, Belgium. Proceedings of the National Academy of Sciences, 106(36), 15390–15393. doi:10.1073/pnas.0907373106.
Pierce, G. J., Hastie, L. C., Guerra, A., Thorpe, R. S., Howard, F. G., & Boyle, P. R. (1994). Morphometric variation in Loligo forbesi and Loligo vulgaris: regional, seasonal, sex, maturity and worker differences. Fisheries Research, 21, 127–148.
Pilgrim, G. E. (1932). The fossil carnivora of India. Palaeontologia Indica, 18, 1–232.
Pinto Llona, A. C. (2006). Comparative dental microwear analysis of cave bears Ursus spelaeus Rosenmüller, 1794 and brown bear Ursus arctos Linnaeus, 1758. Scientific Annals, School of Geology Aristotle University of Thessaloniki, 98, 103–108.
Popowics, T. E. (2003). Postcanine dental form in the mustelidae and viverridae (Carnivora: Mammalia). Journal of Morphology, 256(3), 322–341. doi:10.1002/jmor.10091.
Prevosti, F. J., Turazzini, G. F., Ercoli, M. D., & Hingst-Zaher, E. (2012). Mandible shape in marsupial and placental carnivorous mammals: a morphological comparative study using geometric morphometrics. Zoological Journal of the Linnean Society, 164(4), 836–855. doi:10.1111/j.1096-3642.2011.00785.x.
Quilès, J. (2002). Les Ursidae du Pléistocène moyen et supérieur en Midi méditerranéen: Apports paléontologiques et archéozoologique. Ph. D. thesis, Muséum National d’Histoire Naturelle, Paris.
Quilès, J., Petrea, C., Moldovan, O., Zilhao, J., Rodrigo, R., Rougier, H., et al. (2006). Cave bears (Ursus spelaeus) from the Peştera cu Oase (Banat, Romania): paleobiology and taphonomy. Comptes Rendus Palevol, 5(8), 927–934. doi:10.1016/j.crpv.2006.09.005.
Rabal-Garcés, R., Cuenca-Bescos, G., Ignacio Canudo, J., & De Torres, T. (2012). Was the European cave bear an occasional scavenger? Lethaia, 45(1), 96–108. doi:10.1111/j.1502-3931.2011.00260.x.
Rabeder, G., & Hofreiter, M. (2004). Der neue Stammbaum der alpinen Höhlenbären. Die Höhle, 55(1–4), 58–77.
Rabeder, G., Hofreiter, M., Nagel, D., & Withalm, G. (2004). New taxa of Alpine cave bears (Ursidae, Carnivora). Cahiers scientifiques Muséum Lyon, 2, 49–67.
Rabeder, G., Debeljak, I., Hofreiter, M., & Withalm, G. (2008). Morphological responses of cave bears (Ursus spelaeus group) to high-alpine habitats. Die Höhle, 59(1–4), 59–72.
Rabeder, G., Pacher, M., & Withalm, G. (2010). Early Pleistocene bear remains from Deutsch-Altenburg (Vol. 17). Wien: Verlag der Österreichischen Akademie der Wissenschaften.
Raia, P. (2004). Morphological correlates of tough food consumption in large land carnivores. Italian Journal of Zoology, 71(1), 45–50.
Revell, L. J. (2012). Phytools: an R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution, 3, 217–223.
Richards, M. P., Pacher, M., Stiller, M., Quilès, J., Hofreiter, M., Constantin, S., et al. (2008). Isotopic evidence for omnivory among European cave bears: Late Pleistocene Ursus spelaeus from the Peştera cu Oase, Romania. Proceedings of the National Academy of Sciences of the United States of America, 105(2), 600–604. doi:10.1073/pnas.0711063105.
Robu, M., Fortin, J. K., Richards, M. P., Schwartz, C. C., Wynn, J. G., Robbins, C. T., et al. (2013). Isotopic evidence for dietary flexibility among European Late Pleistocene cave bears (Ursus spelaeus). Canadian Journal of Zoology, 91(4), 227–234. doi:10.1139/cjz-2012-0222.
Rohlf, F. J., & Corti, M. (2000). Use of two-block partial least squares to study covariation in shape. Systematic Biology, 49(4), 740–753.
Rossi, M., & Santi, G. (2001). Archaic and recent Ursus spelaeus forms from Lombardy and Venetia Region (North Italy). Cadernos do Laboratorio Xeolóxico de Laxe, 26, 317–323.
Sacco, T., & Van Valkenburgh, B. (2004). Ecomorphological indicators of feeding behaviour in the bears (Carnivora: Ursidae). Journal of Zoology, 263, 41–54.
Schmitz, L., & Motani, R. (2011). Nocturnality in dinosaurs inferred from scleral ring and orbit morphology. Science, 332(6030), 705–708.
Seetah, T. K., Cardini, A., & Miracle, P. T. (2012). Can morphospace shed light on cave bear spatial-temporal variation? Population dynamics of Ursus spelaeus from Romualdova pećina and Vindija, (Croatia). Journal of Archaeological Science, 39(2), 500–510.
Senshu, T., Ohya, A., Ide, K., Mikogai, J., Morita, M., Nakao, T., et al. (2007). Studies on the digestion in the Giant Panda, Ailuropoda melanoleuca, fed feedstuffs including bamboo. Mammal Study, 32(4), 139–149. doi:10.3106/1348-6160(2007)32[139:sotdit]2.0.co;2.
Slater, G. J., Dumont, E., & Van Valkenburgh, B. (2007). Implications of craniofacial form for performance of the canid skull during prey killing and feeding. Journal of Vertebrate Paleontology, 27(3), 148A–148A.
Slater, G. J., Figueirido, B., Louis, L., Yang, P., & Van Valkenburgh, B. (2010). Biomechanical consequences of rapid evolution in the polar bear lineage. PLoS ONE, 5(11), e13870.
SPSS Inc. (2009). PASW Statistics 17.0. (17.0.3 ed.). Chigago: SPSS Inc.
Stiller, M., Molak, M., Prost, S., Rabeder, G., Baryshnikov, G., Rosendahl, W., et al. (2014). Mitochondrial DNA diversity and evolution of the Pleistocene cave bear complex. Quaternary International, 339–340, 224–231.
Stiner, M. C., Achyuthan, H., Arsebuk, G., Howell, F. C., Josephson, S. C., Juell, K. E., et al. (1998). Reconstructing cave bear paleoecology from skeletons: a cross-disciplinary study of Middle Pleistocene bears from Yarimburgaz cave, Turkey. Paleobiology, 24(1), 74–98.
Stirling, I., & Archibald, W. R. (1977). Aspects of predation of seals by polar bears. Journal of the Fisheries Research Board of Canada, 34(8), 1126–1129. doi:10.1139/f77-169.
Stynder, D. D., & Kupczik, K. (2013). Tooth root morphology in the early Pliocene African bear Agriotherium africanum (Mammalia, Carnivora, Ursidae) and its implications for feeding ecology. Journal of Mammalian Evolution, 20(3), 227–237.
Tagle, D. A., Miyamoto, M. M., Goodman, M., Hofmann, O., Braunitzer, G., Göltenboth, R., et al. (1986). Hemoglobin of pandas: phylogenetic relationships of carnivores as ascertained with protein sequence data. Naturwissenschaften, 73(8), 512–514.
Talbot, S. L., & Shields, G. F. (1996). A phylogeny of the bears (Ursidae) inferred from complete sequences of three mitochondrial genes. Molecular Phylogenetics and Evolution, 5(3), 567–575. doi:10.1006/mpev.1996.0051.
Therrien, F. (2005). Mandibular force profiles of extant carnivorans and implications for the feeding behaviour of extinct predators. Journal of Zoology, 267, 249–270.
Trajano, E., & Ferrarezzi, H. (1995). A fossil bear from northeastern Brazil, with a phylogenetic analysis of the South American extinct Tremarctinae (Ursidae). Journal of Vertebrate Paleontology, 14(4), 552–561. doi:10.1080/02724634.1995.10011577.
van Heteren, A. H., MacLarnon, A., Rae, T. C., & Soligo, C. (2009). Cave bears and their closest living relatives: a 3D geometric morphometrical approach to the functional morphology of the cave bear Ursus spelaeus. Slovenský Kras Acta Carsologica Slovaca, 47(supplement 1), 33–46.
van Heteren, A. H., MacLarnon, A. M., Soligo, C., & Rae, T. C. (2012). 3D geometric morphometrical analyses of intraspecific variation in the mandible of Ursus spelaeus from the Alpine region. Braunschweiger Naturkundliche Schriften, 11, 111–128.
van Heteren, A. H., MacLarnon, A. M., Soligo, C., & Rae, T. C. (2014). Functional morphology of the cave bear (Ursus spelaeus) cranium: a three-dimensional geometric morphometric analysis. Quaternary International, 339–340, 209–216.
Van Valkenburgh, B. (2007). Deja vu: the evolution of feeding morphologies in the Carnivora. Integrative and Comparative Biology, 47(1), 147–163.
Van Valkenburgh, B., & Hertel, F. (1993). Tough times at La-Brea—tooth breakage in large carnivores of the Late Pleistocene. Science, 261(5120), 456–459.
Werdelin, L. (1989). Constraint and adaptation in the bone-cracking canid Osteoborus (Mammalia, Canidae). Paleobiology, 15(4), 387–401.
Werdelin, L., & Solounias, N. (1991). The Hyaenidae: taxonomy, systematics and evolution. Fossils and Strata, 30, 1–104.
Wong, S. T. (2002). The ecology of Malayan sun bears (Helarctos malayanus) in the lowland tropical rainforest of Sabah, Malaysian Borneo. M. Sc. thesis, University of Montana, Montana.
Wozencraft, W. C. (1989). Phylogeny of the recent carnivora. In J. L. Gittleman (Ed.), Carnivore behaviour, ecology and evolution (pp. 495–535). London: Chapman and Hall.
Yoganand, K., Rice, C. G., & Johnsing, A. J. T. (2012). Sloth bear. In A. J. T. Johnsing & N. Manjrekar (Eds.), Mammals of South Asia (Vol. 1, pp. 328–456). Telangana: Orient Blackswan.
Zhang, Y.-P., & Shi, L.-M. (1991). Riddle of the giant panda. Nature, 352(6336), 573–573. doi:10.1038/352573a0.
Acknowledgments
Specimens were studied in the following museums: Haus der Natur, Salzburg, Austria; Musée de Préhistoire de Tautavel; Museum für Naturkunde, Leibnitz-Institut für Evolutions- und Biodiversitätsforschung and der Humboldt Universtität, Berlin, Germany; Landesmuseum Joanneum, Graz, Austria; Museum of Zoology and Natural History “La Specola”, Firenze, Italy; Natural History Museum, London, United Kingdom; Natural History Museum, University of Oslo, Oslo, Norway; Naturhistorisches Museum Wien, Vienna, Austria; Royal Belgian Institute of Natural Sciences, Brussels, Belgium; Staatliches Museum für Naturkunde Stuttgart, Museum am Löwentor, Germany; Universität Tübingen, Tübingen, Germany; Universität Wien, Vienna, Austria; Université de Liège, Liège, Belgium. The authors would like to thank the curators at these institutions for granting permission to study the fossils. The authors are also grateful to G. Rabeder, E. Heiss and two anonymous reviewers for helpful comments on earlier versions of this manuscript, as well as C. Meloro and J. Smaers for advice on statistics. This research received support from the SYNTHESYS Project http://www.synthesys.info/ which is financed by European Community Research Infrastructure Action under the FP6 ‘Structuring the European Research Area’ Programme. This research was partly funded by internal grant 37913 and a 3-year bursary from the University of Roehampton.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Information 1
List of specimens used in the analyses. (PDF 285 kb)
Supplementary Information 2
Statistics pertaining to the allometric correction. (PDF 337 kb)
Supplementary Information 3
Form spaces. (PDF 351 kb)
Rights and permissions
About this article
Cite this article
van Heteren, A.H., MacLarnon, A., Soligo, C. et al. Functional morphology of the cave bear (Ursus spelaeus) mandible: a 3D geometric morphometric analysis. Org Divers Evol 16, 299–314 (2016). https://doi.org/10.1007/s13127-015-0238-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-015-0238-2