Abstract
Sericini chafer beetles are inconspicuous and morphologically rather uniform. Their morphological homogeneity has resulted in taxonomic difficulties particularly with the generic classification but also with the recognition of the species in general. Here, we present a phylogenetic analysis of Sericini using maximum likelihood and Bayesian tree inference based on partial 28S ribosomal DNA (rDNA), cytochrome oxidase I (cox 1), and 16S rDNA (rrnL) for 26 genera and 173 species of Sericini, with major focus on Chinese taxa. Chinese taxa were resolved within two major clades of the subtribe Sericina, while basal lineages of Sericini as well as Trochalina did not include species from China. Our results confirm previous findings in which species relationships were not consistent with the current genus-level classification, as well as in a previously hypothesized close phylogenetic link between southwestern Chinese and Himalayan species. Large genera such as Neoserica, Serica, and Maladera all resulted to be polyphyletic, being split in several distantly related branches. But also, less intensely sampled groups like Lasioserica and Microserica were non-monophyletic. The major implication from this analysis is that the existing taxonomic system needs to be significantly revised by integrating morphology carefully in the context of a robust backbone phylogeny to allow delineation of monophyletic but unambiguously diagnosable genera.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The family Scarabaeidae is one of the more intensively studied groups of beetles with respect to their biology, taxonomy, and phylogeny (Scholtz 1990). However, Sericini chafers, with nearly 3500 described species in about 200 genera (Ahrens 2006a), represent a striking exception being poorly known in terms of phylogeny, taxonomy, and larval morphology. Sericini are inconspicuous and rather uniform in external morphology. They feed on leaves of angiosperm plants as adults and on roots as larvae. Sericini occur predominantly in the Old World, while only seven genera (two of them introduced) are known in the New World (Ahrens 2006a; Evans and Smith 2009). They are entirely absent from the Australian region. Their homogeneity and the resulting taxonomic difficulties have limited studies of their distribution, ecology, and morphology (e.g. Scholtz and Chown 1995; Ahrens 2006a). This lack of knowledge, the high amount of homoplasy of diagnostic features (Ahrens and Vogler 2008), and their uniform morphology hamper also the lower-level systematics of the group, particularly the genus group assignment of the vast diversity of species (Ahrens 2004). In many regions of the world, some of the species are serious crop pests (Nair 1986), but their poor taxonomy hampers successful control (e.g., Pal and Misra 1973; Ahrens 2000; Ahrens et al. 2007).
Based on morphological characters of Sericini, Ahrens (Ahrens 2006b, c, d; 2007a) hypothesized a close link of the mountainous fauna of middle elevations between the Himalaya and southern China, which is also reflected by many biogeographic hypotheses and recent studies (Mani 1974; de Lattin 1967; Dobremez 1976; Martens 1993). However, most of these relations were hypothesized only for selected species groups without the perspective of the phylogeny of the entire group. The phylogeny of Sericini was so far studied only in a few papers based on morphology (Ahrens 2006a) and DNA data (Ahrens and Vogler 2008). However, both these works had a rather limited sampling, particularly with regard to Chinese species.
Therefore, we investigated here the phylogenetic relationships of the very diverse Sericini, which are represented in China by several hundreds of species. We expanded the existing dataset (Ahrens and Vogler 2008) by more than 80 species collected in China using partial sequences from two mitochondrial genes, cytochrome oxidase subunit I (cox1) and the large subunit ribosomal DNA (rDNA) (rrnL), and one nuclear gene, the large subunit rDNA (28S).
Material and methods
The data set includes 188 terminals of which 81 species of Sericini are newly sequenced from 45 sites in China (Fig. 1); all others were taken from the dataset in Ahrens and Vogler (2008) (Supplement Table 1, see here also for full taxon names including author and year). The latter data set includes species from all over the world, representing a large number of genera of major lineages not occurring in China. Three outgroup taxa were included as follows: Melolontha melolontha (Linnaeus, 1758), Liparetrus sp., and Maechidius sp.
DNA was extracted from thoracic leg muscle tissue using Qiagen DNeasy columns. Subsequently, beetles were dry mounted. Vouchers were deposited in the Zoological Research Museum A. Koenig Bonn (ZFMK) and in the Institute of Zoology, Chinese Academy of Sciences (IZAS). Two mitochondrial and one nuclear gene regions were amplified and sequenced. These included the 3′ end of cytochrome oxidase subunit 1 (cox 1) and 16S ribosomal DNA (rrnL). PCR and sequencing was performed using primers stevPat and stevJerry for cox1 (Timmermans et al. 2010) and 16Sar and 16sB2 for rrnL (Simon et al. 1994). A fragment of nuclear 28S rDNA containing the variable domains D3-D6 was amplified using primers FF and DD (Monaghan et al. 2007). Double-stranded sequencing was carried out by a sequencing facility (Macrogen, Seoul, South Korea) on ABI 3730XL sequencers. Sequences were edited and aligned in Geneious version 6.1.6 (Biomatters; available from http://www.geneious.com/); for the latter, we used the implemented MAFFT algorithm with the default settings (Standley 2013). Terminals with more than one marker missing were not included in the tree search. Optimal partition schemes and substitution models were simultaneously identified with a greedy search using the Bayesian information criterion implemented in PartitionFinder (Lanfear et al. 2012), with branch lengths linked across partitions.
Subsequently, maximum likelihood (ML; Felsenstein 1973) searches were performed on the concatenated data matrices in PhyML 3.0 (Guindon and Gascuel 2003) on the South of France bioinformatics platform (http://atgc.lirmm.fr/phyml/) using a GTR + Γ + I model with all parameters estimated from the data and four substitution rate categories. We used the aLRT statistics as a measure of branch support (Anisimova and Gascuel 2006; Guindon et al. 2010). Additionally, a Bayesian analysis was conducted using parallel MrBayes 3.2 (Ronquist et al. 2012) on the ZFMK cluster. Bayesian MCMC analyses (Yang and Rannala 1997) were performed with partitioned data (as determined by PartitionFinder) separating the combined matrix into five partitions: rrnL, 28S, and three partitions for each codon position of cox1. Tree searches were conducted for 70 × 106 generations, using a random starting tree and two runs of three heated and one cold Markov chains (heating of 0.1). Chains were sampled every 1000 generations, and 25 % of the generations were discarded as burn-in based on the average standard deviation of split frequencies as well as by plotting -lnL against generation time. Tracer 1.6 (Rambaut et al. 2013) was used to determine stationarity and convergence of runs. The trees were rooted with M. melolontha. Newly generated sequences were submitted to GenBank (Supplement Table 1); phylogenetic data (trees and matrix) are deposited in TreeBASE (xxxx). Biogeographic patterns of selected clades of Sericina were examined by projecting phylogenetic relationships into geographic space with a phylomorphospace plot (Sidlauskas 2008) using the R-package phytools 0.4-31 (Revell 2012) and R-package maps (Brownrigg 2014).
Results
Sequences (n = 188) for three loci (cox1, rrnL, 28S) were aligned and concatenated to a matrix of 1983 nucleotide positions. The length of aligned sequences was 522, 650, and 811 bp for rrnL, 28S, and cox1, respectively. Length variation was minimal for 28S and low for rrnL, resulting in 5 and 17 indels, respectively. Major topologies among Sericini obtained from the ML and Bayesian tree searches (Fig. 2) were widely consistent among each other. This includes the hypothesis in both studies that Ablaberini is the sister group of Sericini and the Neotropical Astaena species are sister to the Old World Sericini taxa.
Majority-rule consensus-tree from Bayesian analysis (left) and the maximum likelihood tree (right). Posterior probabilities aLRT values >50, respectively, are given next to the nodes. Terminals of Maladera, Neoserica, Serica, and Microserica are marked with blue, green, red, and orange, respectively, their type species with a T. The two major clades of subtribe Sericina are indicated with 1 and 2; 3 and 4 indicate clades with strong Himalayan-Chinese disjunction (see Fig. 3). Chinese and Himalayan terminals are highlighted along the Bayesian tree with an orange or blue dot, respectively
The position of Omaloplia + Hellaserica in the different trees was ambiguous: while it diverged at the most basal branching of the Old World clade in the Bayesian tree, it clustered within the Malagasy clade of Hyposerica and Comaserica in the ML tree. Conflicting results were also found for the Triodontella-clade, containing Triodontella, Hymenoplia, and Paratriodonta. This group was paraphyletic in the ML search of this dataset, but monophyletic under Bayesian inference. In both trees, the subtribe Trochalina (Machatschke 1959) was resolved as sister of the subtribe Sericina. While the former is entirely distributed in the Afrotropics, Chinese taxa, like all other Asian Sericini, are exclusively placed within the subtribe Sericina. The subtribe Sericina is subdivided into two major clades (Fig. 2), both of them include Afrotropical and Asian (and Chinese) taxa.
In the Bayesian and ML trees, Chinese species were not monophyletic nor were the major Chinese genera such as Neoserica, Maladera, and Serica (s.l.) (Fig. 2). Their type species (T, see Fig. 2) indicate the more strict concept (sensu strictu) of those genera. Species so far classified as Serica sensu lato (s.l.) (Ahrens 2004) were found at several distal branches of the tree: one branch comprised the North American species of Serica (Serica intermixta, Serica loxia, Serica mystica, Serica sp. 671336) nested with Nipponoserica and Maladera LW1128. Serica solivaga nested with Maladera. Maladera and Neoserica were associated with some more basal branches among the Asian Sericini, being separated into numerous smaller lineages. Many species in these groups from China are newly discovered species, and the formal descriptions complying with the Code of Zoological Nomenclature are not published yet.
Maladera was divided into six larger or smaller unrelated lineages, nesting together with Neoserica (s.l.), Serica (s.l.), Lasioserica, although sometimes with low support only. The most interesting finding was that the subgenus Omaladera (represented by Maladera lignicolor, Maladera simlana, Maladera joachimi, Maladera himalayica) nested within the subgenus Cephaloserica (Maladera veriticalis, Maladera affinis, Maladera cardoni, Maladera iridescens).
The situation among Neoserica (in the wider sense [sensu lato, s.l.]) species is similarly complicated. It is divided into 19 separate lineages which are not discussed in more detail here (see Fig. 2). Lasioserica was separated into three lineages while Microserica is divided under the current sampling into four separate groups: Some Himalayan Microserica species nesting around the endemic Himalayan genus Oxyserica (Microserica gandakiensis, Microserica interrogator, and Microserica pruinosa). Others (Microserica fukiensis and Microserica sp. LW1321) seem to be associated in the Bayesian tree with the Gastroserica-Neoserica clade. One lineage of Microserica (Microserica sp. LW 1264) is grouped with the first major basal clade of Sericina (Fig. 2).
Other smaller or less numerously sampled genera resulted to be monophyletic with high branch support (posterior probability/aLRT): Nepaloserica (0.95/0.91), Gynaecoserica (1/0.97), Anomalophylla (1/1), Nipponoserica (0.88/0.84), and Gastroserica (1/0.99). Tetraserica species were not monophyletic as they were associated still with some other Neoserica species whose genus assignment could not be stated confidently because these specimens were females.
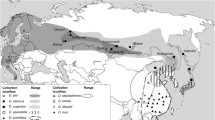
In a few cases, Chinese species are strictly associated to each other, e.g., for the Neoserica (sensu stricto) + Gastroserica sister group relationship, found however only with the ML tree, representing a clade comprising in total of ca. 75 known species restricted to China and northern Indochina (Liu et al. 2014a, 2014b). While a few other Chinese taxa are associated in the tree with species from India or other parts of the Oriental region, the vast majority of Chinese species relate with clades with species from the Himalayas (Figs. 2 and 3; e.g., subclades 3 and 4) and confirm, given the current sampling limits, previous hypotheses derived from morphology-based phylogenies of selected lineages (Ahrens 2006b, c, d, 2007a, c, d, 2012). This is particularly true not only for the genus Serica (Ahrens 2007a), but also for the entire clade comprising the genera Serica (s.str.), Pachyserica, Gynaecoserica, Nepaloserica, Chrysoserica, and the Neoserica abnormis group (Fig. 3c,d). These genera or groups are very species rich in the Indo-Himalayan region, especially in the middle elevations from 1000 to 3000 m (Ahrens 1999, 2004, 2005, 2006b, 2012; Ahrens and Fabrizi 2009; Ahrens et al. 2014). Serica (s.str.), represented by 14 species (of the 115 known taxa; Ahrens 2007b; Ahrens and Fabrizi 2009, 2011) in this analysis, resulted monophyletic, having the Serica nigroguttata group (Ahrens 2007c) (represented here by Serica tryznai, Serica jindrai, Serica maculosa, Serica wrasei, and Serica inaequalis) as sister.
Projections of phylogenetic relationships of selected Sericina clades into geographical space, illustrating the spatial within clade divergence between Himalayan and Chinese lowland species. a Sericina subclade 2 (Fig. 2), the North American clade is not shown, b detail of Chinese and Himalayan taxa, c Sericina subclade 3, and d Sericina subclade 4 including Serica s. str. Internal nodes are merely projections of the phylogeny and have no biological meaning
Discussion
The results with a more extensive sampling among Asian and particularly Chinese taxa largely confirmed previous findings (Ahrens and Vogler 2008) towards the phylogeny of Sericini. While subtribes Trochalina and Sericina were recovered sister clades, all lineages branching basally to Trochalina + Sericina, including Omaloplia, Astaena, Hymenoplia, Paratriodonta, Triodontella, and the malagassy Hyposerica and Comaserica, remain outside of any of the so far recognized subtribes (Machatschke 1959). However, to establish for those genera subtribal family-group names, a more complete sampling of basal Sericini lineages and comprehensive backing from morphology would be desirable.
Already, Ahrens and Vogler (2008) questioned the traditional definitions of major genera of Sericini based on the principal works of Brenske (1897, 1899) and subsequent authors (see Machatschke 1959; Ahrens 2004, 2007b), which mostly referred to the number of antennomeres in the male antennal club as major diagnostic features. Many other characteristic features traditionally used to distinguish major lineages (i.e., genera) of Sericini, such as the serrated lines beside dorsal margin of metatibia or adjacent to the anterior margin of metafemur, the reduced anterior angles of pronotum, the absence of the membranous rim of apex of elytra, and the tri- or bidentate protibia, are affected by a high degree of homoplasy (e.g., Ahrens 2006d). In fact, the trees inferred from the current data set underline that a large proportion of the diversity of species is affected by the difficulties of genus taxonomy within Sericini, although sampling is still far from being complete likewise in the study of Ahrens and Vogler (2008) and previous morphology-based phylogenies of selected lineages (Ahrens 2006b, c, d, 2007a, c, d, 2012).
The state of the art for the taxonomy of the most diverse genera is summarized as follows:
-
1.
The monophyly of Neoserica
The genus Neoserica Brenske, 1897, with ca. 200 taxa, is one of the most species-rich groups of Sericini. Since the redefinition of the genus (Pope 1960; Ahrens 2003), many species conventionally grouped under Neoserica and being not directly related to the type species Neoserica ursina (Brenske, 1894) (i.e., Neoserica (s.str.) group; Ahrens 2003), were grouped preliminarily as Neoserica (s.l.) (e.g., Ahrens 2004), a collective group that was neither related to Neoserica (s.str.) (Ahrens 2003) nor monophyletic (Ahrens and Vogler 2008). This was reconfirmed by our results here, which for the first time included true Neoserica (s.str.) including its type species, N. ursina, in a DNA-based phylogeny. The species of Neoserica sensu lato all await taxonomic revision and a re-classification based on a robust analysis of their relationships that incorporates morphology as well.
-
2.
The monophyly of Serica
The genus Serica MacLeay, 1819, is one of the most species-rich groups of Sericini to which more than 450 nominal species have been associated in the past (some now assigned to other genera). Parts of Serica were revised by Ahrens (1999, 2004, 2005, 2007b) and the phylogeny of Serica (s. str.) was explored (Ahrens 2007a). Results here imply, based on the widest so far available sampling, the polyphyly of Serica in its wider sense (s.l.) but monophyly of the correctly (strictly) redefined genus (Ahrens 2007a, b) of which the type species Serica brunnea was included in this analysis.
-
3.
The monophyly of Maladera
The genus Maladera Mulsant and Rey, 1871, is also one of most species-rich groups of Sericini. Many species were originally described as Autoserica auctorum (nec Brenske 1897; Ahrens 2004), which was synonymised by Pope (1960) with Maladera. So far, ca. 250 species are formally assigned to Maladera. The ‘Maladera complex’ was revised for some geographical regions (Petrovitz 1969; Nomura 1974; Ahrens 2004) and the phylogeny was explored for some of its species groups (Ahrens 2006c, e). The current tree hypothesis confirms the polyphyly of Maladera already found by Ahrens and Vogler (2008) indicating the further need of efforts on the re-classification of these species.
Conclusions
The results of this study provide insight into the difficulties of the current classification of Sericini genera, which suffers from high homoplasy in many of the diagnostic traits used in the past to establish and diagnose generic groups. As in previous studies, polyphyly of larger and more species-rich groups was shown, justifying long-standing efforts to improve the systematic knowledge on this tribe (e.g., Ahrens 2004; Ahrens and Vogler 2008). Therefore, the major conclusion here is that most genus assignments for these larger groups, like Maladera, Neoserica, and Serica, as well as from smaller groups such as Microserica and Lasioserica, have to be regarded as preliminary. Local taxonomic treatments that may lead to the recognition of locally diagnosable (and thus apparently endemic) genera, as done for the faunas of for Taiwan and Japan (e.g., Nomura 1974), seems under these circumstances little helpful. The generic classification needs exhaustive and thorough revision at a worldwide scale to ensure that genera represent monophyletic units. However, current collective groups (as defined by Mayr 1969) are still to be retained for practical/technical reasons, to allow biodiversity research, taxonomic revisions, and morphological investigation at species level to continue. Nevertheless, given the prospect of possible changes in genus assignment, taxonomic authors should be careful to give new species names only once for the entire tribe (in particular for problematic groups as Sericina) in order to avoid subsequently necessary replacement names due to secondary homonomy.
The process of such a “re-classification” demands a near-comprehensive phylogenetic hypothesis of the group (considering all major genus-level lineages), which, in connection with a broad morphological data set for these taxa, will allow the identification of suitable diagnostic features for the resulting monophyletic taxa. This also requires, of course, robust topologies. Several nodes on the current hypothesis still show low branch support, which might be due to a rapid diversification, problems with the alignment of the length-variable markers, or insufficient character congruence of the markers. In future research, more data (i.e., markers) need to be included to resolve an increasingly complex tree. Given the huge diversity of Sericini, both tasks will be highly challenging, and will need care, time, and sufficient funding.
References
Ahrens, D. (1999). Revision der Gattung Serica (s. str.) MacLeay des Himalaya-Gebiets (Coleoptera, Melolonthidae). Fragmenta Entomologica, 31, 205–332.
Ahrens, D. (2000). The fauna of the Arabian Peninsular: Sericinae (Coleoptera, Scarabaeoidea). Fauna of Arabia, 18, 177–210.
Ahrens, D. (2003). Zur Identität der Gattung Neoserica Brenske, 1894, nebst Beschreibung neuer Arten (Coleoptera, Melolonthidae, Sericini). Koleopterologische Rundschau, 73, 169–226.
Ahrens, D. (2004). Monographie der Sericini des Himalaya (Coleoptera, Scarabaeidae). Dissertation.de - Verlag im Internet GmbH, Berlin, 534pp.
Ahrens, D. (2005). A taxonomic review on the Serica (s. str.) MacLeay, 1819 species of Asiatic mainland (Coleoptera, Scarabaeidae, Sericini). Nova Supplemeta Entomologica, 18, 1–163.
Ahrens, D. (2006a). The phylogeny of Sericini and their position within the Scarabaeidae based on morphological characters (Coleoptera: Scarabaeidae). Systematic Entomology, 31, 113–144.
Ahrens, D. (2006b). Revision und phylogenetische Analyse der Gattung Pachyserica Brenske, 1897 (Coleoptera, Melolonthidae, Sericini). Revue Suisse de Zoologie, 113, 487–557.
Ahrens, D. (2006c). Cladistic analysis of Maladera (Omaladera): implications on taxonomy, evolution and biogeography of the Himalayan species (Coleoptera: Scarabaeidae: Sericini). Organisms, Diversity and Evolution, 6, 1–16.
Ahrens, D. (2006d). The phylogeny of the genus Lasioserica inferred from adult morphology—implications on the evolution of montane fauna of the South Asian orogenic belt (Coleoptera: Scarabaeidae: Sericini). Journal of Zoological Systematics and Evolutionary Research, 44, 34–53.
Ahrens, D. (2006e). Evolution of Asian ‘lowland’ taxa and Alpine-Himalayan tertiary orogenic belt—insight from a preliminary cladistic analysis of Maladera (Cycloserica) (Coleoptera: Scarabaeidae: Sericini). Zoologischer Anzeiger, 244, 193–203.
Ahrens, D. (2007a). Beetle evolution in the Asian highlands: insight from a phylogeny of the scarabaeid subgenus Serica (Coleoptera, Scarabaeidae). Systematic Entomology, 32, 450–476.
Ahrens, D. (2007b). Taxonomic changes and an updated catalogue of Palearctic Sericini (Coleoptera: Scarabaeidae: Melolonthinae). Zootaxa, 1504, 1–51.
Ahrens, D. (2007c). Revision der Serica nigroguttata BRENSKE, 1897—Gruppe (Coleoptera, Scarabaeidae, Sericini). Bulletin de l’Institut Royal des Sciences Naturelles de Belgique, 77, 5–37.
Ahrens, D. (2007d). Cladistic analysis of Sericania (Coleoptera, Scarabaeidae: Sericini)—implications on the evolution of the xerophilous fauna of the Himalaya. European Journal of Entomology, 104, 517–530.
Ahrens, D. (2012). New species and a morphology-based phylogeny of the genus Nepaloserica (Coleoptera, Scarabaeidae). In M. Hartmann & M. Weipert (Eds.), Biodiversität und Naturausstattung im Himalaya III (pp. 305–316). Erfurt: Verein der Freunde und Förderer des Naturkundemuseums Erfurt e.V.
Ahrens, D., & Fabrizi, S. (2009). New species of Sericini from the Eastern Himalaya and Tibet (Coleoptera, Scarabaeidae): 249-284. In M. Hartmann & M. Weipert (Eds.), Biodiversität und Naturausstattung im Himalaya III. Erfurt: Verein der Freunde und Förderer des Naturkundemuseums Erfurt e.V.
Ahrens, D., & Fabrizi, S. (2011). New species of Sericini from the Himalaya and adjacent mountains (Coleoptera: Scarabaeidae). Bonn Zoological Bulletin, 60, 139–164.
Ahrens, D., & Vogler, A. P. (2008). Towards the phylogeny of chafers (Sericini): analysis of alignment-variable sequences and the evolution of segment numbers in the antennal club. Molecular Phylogenetics and Evolution, 47, 783–798.
Ahrens, D., Zorn, C., GC, Y., Keller, S., & Nagel, P. (2007). Illustrated key of phytophagous scarabs of Nepal. A guide to white grubs and chafers of the lower central regions (Coleoptera, Scarabaeidae). Opuscula Biogeographica Basiliensis, 5, 1–44.
Ahrens, D., Liu, W. G., Fabrizi, S., Bai, M., & Yang, X. K. (2014). A taxonomic review of the Neoserica (sensu lato) abnormis group (Coleoptera: Scarabaeidae: Sericini). Zookeys, 439, 28–82.
Anisimova, M., & Gascuel, O. (2006). Approximate likelihood-ratio test for branches: a fast, accurate, and powerful alternative. Systematic Biology, 55, 539–552.
Brenske, E. (1897). Die Serica—Arten der Erde. I. Berliner Entomologische Zeitschrift, 42, 345–438.
Brenske, E. (1899). Die Serica—Arten der Erde. III. Berliner Entomologische Zeitschrift, 44, 161–272.
Brownrigg, R (2014). Maps: Draw Geographical Maps. R package version 2.3-9. Enhancements by Thomas P Minka. Original S code by Richard A. Becker and Allan R. Wilks. http://CRAN.R-project.org/package=maps
de Lattin, G. (1967). Grundriss der Zoogeographie. Jena: Gustav Fischer Verlag. 602pp.
Dobremez, J.-F. (1976). Le Nepal. Ecologie et biogeographie. (Cahais Nepalais). Paris: Editions du Centre national de la recherche scientifique, 356pp.
Evans, A. V. & Smith, A. B. T. (2009). An electronic checklist of the New World chafers (Coleoptera: Scarabaeidae: Melolonthinae). Version 3. (http://museum.unl.edu/research/entomology/SSSA/nwmelos.htm). Accessed 14 Nov 2014.
Felsenstein, J. (1973). Maximum likelihood and minimum-step methods for estimating evolutionary trees from data on discrete characters. Systematic Zoology, 22, 240–249.
Guindon, S., & Gascuel, O. (2003). A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology, 52(5), 696–704.
Guindon, S., Dufayard, J. F., Lefort, V., Anisimova, M., Hordijk, W., & Gascuel, O. (2010). New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic Biology, 59, 307–321.
Lanfear, R., Calcott, B., Ho, S. Y. W., & Guindon, S. (2012). PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Molecular Biology and Evolution, 29, 1695–1701.
Liu, W. G., Bai, M., Yang, X. K., & Ahrens, D. (2014a). An update to the taxonomy of the genus Gastroserica Brenske (Coleoptera, Scarabaeidae, Sericini). Zookeys, 426, 87–110.
Liu, W. G., Bai, M., Yang, X. K., & Ahrens, D. (2014b). New species and records of the Neoserica (sensu stricto) group (Coleoptera, Scarabaeidae, Sericini). Journal of Natural History. doi:10.1080/00222933.2014.974707.
Machatschke, J. W. (1959). Phylogenetische Untersuchungen über die Sericini (sensu Dalla Torre 1912) (Coleoptera: Lamellicornia: Melolonthidae). Beiträge zur Entomologie, 9, 730–746.
Mani, M. S. (1974). Biogeographical Evolution in India. In: M. S. Mani (Ed.) Ecology and Biogeography in India (pp. 698-724). Netherlands: Springer.
Martens, J. (1993). Bodenlebende Arthropoden im zentralen Himalaya: Bestandsaufnahme, Wege zur Vielfalt und ökologische Nischen. In U. Schweinfurth (Ed.), Neue Forschungen im Himalaya (pp. 231–250). Stuttgart: F. Steiner Verlag.
Mayr, E. (1969). Principles of systematic zoology. New York: McGraw-Hill Book Company. xi + 428 pp.
Monaghan, M. T., Inward, D. G., Hunt, T., & Vogler, A. P. (2007). A molecular phylogenetic analysis of the Scarabaeinae (dung beetles). Molecular Phylogenetics and Evolution, 45, 674–692.
Nair, M. R. G. K. (1986). Insects and mites of crops in India (2nd ed.). New Dehli: Indian Council of Agricultural Research. 408pp.
Nomura, S. (1974). On the Sericini of Taiwan. Tôhô-Gakuhô, 24, 81–115.
Pal, S. K., & Misra, S. D. (1973). Studies on the biology and bionomics of Aserica sp. (Melolonthidae: Coleoptera) in the arid region of Rajasthan. Indian Journal of Agricultural Research, 7, 169–172.
Petrovitz, R. (1969). Ergebnisse zoologischer Sammelreisen in der Türkei: Lamellicornia, Coleoptera. Die mediterranen Arten der Gattung Maladera Mulsant. Annalen des Naturhistorischen Museums Wien, 73, 383–400.
Pope, R. D. (1960). Aserica, Autoserica, Neoserica or Maladera? (Col., Melolonthidae). Annals and Magazine of Natural History, 13(3), 545–550.
Rambaut, A., Suchard, M. A., Xie, W., Drummond, A. J. (2013). Tracer v1.6. Available: http://tree.bio.ed.ac.uk/software/tracer. Accessed 14 April 2014.
Revell, L. J. (2012). Phytools: an R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution, 3, 217–223. doi:10.1111/j.2041-210X.2011.00169.x.
Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M. A., & Huelsenbeck, J. P. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61, 539–542.
Scholtz, C. H. (1990). Phylogenetic trends in the Scarabaeoidea. Journal of Natural History, 24, 1027–1060.
Scholtz, C. H., & Chown, S. L. (1995). The evolution of habitat use and diet in the Scarabaeoidea: a phylogenetic approach. In J. Pakaluk & S. A. Slipinski (Eds.), Biology, phylogeny, and classification of Coleoptera: papers celebrating the 80th birthday of Roy A (pp. 355–374). Warszawa: Crowson. Muzeum i Instytut Zoologii PAN.
Sidlauskas, B. (2008). Continuous and arrested morphological diversification in sister clades of characiform fishes: a phylomorphospace approach. Evolution, 62, 3135–3156.
Simon, C., Frati, F., Beckenbach, A., et al. (1994). Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Annals of the Entomological Society of America, 87, 651–701.
Standley, K. (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. (outlines version 7). Molecular Biology and Evolution, 30, 772–780.
Timmermans, M. J. T. N., Dodsworth, S., Culverwell, C. L., L. Bocak, L., Ahrens, D., Littlewood, D. T. J., Pons, J., & Vogler, A. P. (2010). Why barcode? High-throughput multiplex sequencing of mitochondrial genomes for molecular systematics. Nucleic Acids Research, 2010, 1–14.
Yang, Z., & Rannala, B. (1997). Bayesian phylogenetic inference using DNA sequences: a Markov chain Monte carlo method. Molecular Biology and Evolution, 14, 717–724.
Acknowledgments
This research was supported by the National Basic Research Program of China (973 Program) (no. 2011CB302102), the National Natural Science Foundation of China (nos. 31010103913, 31172143), and the Knowledge Innovation Program of Chinese Academy of Sciences (no. KSCX3-IOZ-1004) and by a Fellowship (to M.B.) from Alexander von Humboldt Foundation. We are grateful to Li Yan, Wang Zhiliang, Zhan Qingbin, and Meng Lingzeng for their help to collect specimens in ethanol and to the two anonymous referees for the helpful comments and improvements. Funding for DNA labwork of W.-G. L. was provided by ZFMK institutional funding.
Conflicts of interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
List of specimens sequenced for the study, including species names, voucher number, sampling locality, and GenBank accession numbers (PDF 166 kb)
Rights and permissions
About this article
Cite this article
Liu, WG., Eberle, J., Bai, M. et al. A phylogeny of Sericini with particular reference to Chinese species using mitochondrial and ribosomal DNA (Coleoptera: Scarabaeidae). Org Divers Evol 15, 343–350 (2015). https://doi.org/10.1007/s13127-015-0204-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-015-0204-z