Abstract
Hepatocellular carcinoma (HCC) is the most common form of primary liver cancer in the world with high mortality due to its high potential of metastasis. Epithelial-mesenchymal transition (EMT) plays a key role in the pathogenesis of HCC occurrence and metastasis. Phospholysine phosphohistidine inorganic pyrophosphate phosphatase (LHPP) is a novel tumor suppressor. There is little study about LHPP in human HCC development. In the present study, we aimed to investigate the role of LHPP in human HCC cell metastasis. We analyzed the LHPP expression level in human HCC tissues compared with normal tissues in the public database. We detected the mRNA level and protein level of LHPP in transformed liver cell line (LO2) and human HCC cell lines (MHCC-97 H, MHCC-97L, and HepG2). We performed genetic gain and loss of function experiments with LHPP using small interfering RNA (siRNA) and lentivirus infection. Then, we detected that LHPP suppressed proliferation and promoted apoptosis in hepatocellular carcinoma cell lines. Also, we investigated the role of LHPP in the EMT process. Finally, we examined the effect of LHPP on TGF-β-induced EMT. Interestingly, we also found that LHPP expression is positively regulated tumor suppressor p53. Our data showed that LHPP is significantly decreased in the human HCC tissues and human HCC cell lines compared with normal liver tissues and transformed liver cells. Knockdown of LHPP promotes HCC cell proliferation and metastasis, and LHPP expression levels negatively correlate with EMT-related genes. Furthermore, LHPP inhibits TGF-β-induced EMT in HCC cell lines. These studies validate LHPP as a tumor suppressor in liver cancer and provide a new genetic target for HCC diagnosis and treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Liver cancer is the fourth leading cause of cancer-related death and ranks sixth in terms of new cases across the world [21, 27]. Hepatocellular carcinoma (HCC), the most common form of primary liver malignancy worldwide, is a deadly disease that normally arises on the background of chronic liver disease such as cirrhosis [5, 25]. HCC development is complicated and occurs through a multistep biological process of malignant transformation of normal hepatocytes in which various factors, including genetic and epigenetic alterations, regulation of oxidative stress, inflammation, and immunity are involved [1, 19]. Although there are several conventional methods like surgical resection, liver transplantation, and radiofrequency ablation (RFA) already widely used to treat HCC, the amount of HCC mortality is still increasing [20]. The underlying molecular mechanisms for HCC occurrence and development are still unclear. Therefore, it is extremely urgent to elucidate the underlying molecular mechanisms and develop new therapeutic strategies to prevent HCC.
Epithelial-mesenchymal transition (EMT) is the mechanism that drives a transient and reversible de-differentiation of epithelial cells to a mesenchymal-like or a mesenchymal phenotype. EMT plays a critical role in tumor progression [14]. For HCC progression, EMT is usually a crucial prerequisite for its invasive and metastatic potential [2]. Therefore, investigation of potential key molecules involved in EMT properties may contribute to developing new therapeutic strategies against HCC invasion and metastasis.
Phosphohistidine phosphate inorganic pyropho-sphatase (LHPP), a type of histidine phosphatase protein, is a member of the haloacid dehalogenase like hydrolase domain–containing (HDHD) gene family. LHPP is located on chromosome 10q26.13 and encodes a 29 kDa enzyme which consists of three leucine zipper domains [32]. LHPP is a unique enzyme that hydrolyzes not only oxygen-phosphorus bonds in inorganic pyrophosphate but also nitrogen-phosphorus bonds in phospholysine, phosphohistidine, and imidodiphosphate in vitro [32]. LHPP has been purified from bovine liver and LHPP protein is able to hydrolyze imidodiphosphate, 3-phosphohistidine, and 6-phospholysine [9, 15]. The early studies about LHPP mainly focus on its psychiatric phenotypes caused by two SNPs, one SNP (rs35936514) is correlated with major depressive disorder (MDD) and the other SNP (rs34997829) is correlated with Risky sexual behaviors (RSB) [4, 6, 22]. Interestingly, another genome-wide association study (GWAS) identifies LHPP (rs201982221) acts as the significantly associated loci in oral and pharyngeal cancers [8, 14]. Recently, LHPP has been demonstrated as a tumor suppressor in HCC and other cancers [11, 16, 17, 24, 29, 30, 34]. Hindupur et al. [9] found that LHPP inhibited HCC progression by regulating AKT expression level and activity in mice. Liao et al. [18] identified that overexpression of LHPP attenuated the cell viability, colony formation, and invasion in human HCC cells. However, whether LHPP affects epithelial-mesenchymal transition in hepatocellular carcinoma is still unknown.
In this study, we aimed to investigate the roles of LHPP in human HCC cell metastasis. We found that LHPP was downregulated in human HCC tissues and human HCC cell lines. Also, the expression of LHPP is negatively correlated with the progression and poor prognosis of HCC. Functional inactivation of LHPP can promote the proliferation and metastasis of HCC cells, whereas the overexpression of LHPP has the opposite effect. In addition, LHPP can inhibit the transforming growth factor β (TGF-β)–induced EMT. Collectively, our results provide new insights into the role and molecular mechanism of LHPP in HCC progression.
Materials and methods
Cell culture
The human fetal hepatocyte cell line L02 and HCC cell line HepG2 were purchased from Cell Resource Center, Shanghai Institutes for Biological Sciences. Two cell lines (MHCC97L and MHCC97H) were chosen to represent low and high metastatic potential of HCC, respectively. Although both MHCC97L and MHCC97H cell lines are from the same clone origin and have high metastatic ability, MHCC97H exhibits stronger metastatic potential than MHCC97L in a physiological aspect. They were kindly provided by Dr. Song Guanbin (Chongqing University). All these cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), and 100 U/ml of penicillin and streptomycin in a humidified incubator with 5% CO2.
Plasmids and siRNAs
Plenti-CMV-EGFP vector was purchased from Addgene. The Plenti-CMV-LHPP vector was generated by adding LHPP CDS regions to Plenti-CMV-EGFP instead of EGFP position. These lentiviruses were produced in HEK293T using a mixture of lentivirus packaging plasmids (pCMV-dR8.2 dvpr and pCMV-VSV-G). The siRNA of LHPP target sequences designed by GenePharma (Shanghai) were the following:
Negative scrambled siRNA forward: 5′-UUCUCCGAAGGUGUCACGUTT-3′
Negative scrambled siRNA reverse: 5′-ACGUGACACGUUCGGAGAATT-3′
LHPP siRNA 1#: 5′-TGACGGAGTCCGCTCAGAATTTGAT-3′
LHPP siRNA 2#: 5′-CATCCAACCCAAACTGTGTGGTAA-3′
LHPP siRNA 3#: 5′-CAACCCAAACTGTGTGGTAATTGCA-3′
Reagents and primary antibodies
Polybrene and Puromycin were purchased from Sigma. The primary antibodies used in this study: LHPP (15,759–1-AP), p53 (10,442–1-AP), and GAPDH (60,004–1-Ig) were purchased from Proteintech. E-cadherin (24E10) and N-cadherin (13A9) were purchased from Cell Signaling Technology.
Quantitative real-time RT-PCR
RNA was extracted from cells using the RNAiso Plus kit (Takara Bio Inc.) and cDNA preparation was performed according to the manufacturer’s instructions using primeScript RT Master kit (Takara Bio Inc.). Real-time RT-qPCR was performed with SYBR qPCR SuperMix Plus (annoronbio, China) using the 7300 Real-Time PCR System or ViiA7 System (AB Applied Biosystems). β-actin was used as control. The primers used were as follows:
human LHPP-F: 5′-ATGACGGAGTCCGCTCAGAAT-3′
human LHPP-R: 5′-ACAGGTTTTTCCAGCTCCATGAG-3′
human β-actin-F: 5′-CTCCATCCTGGCCTCGCTGT-3′
human β-actin-R: 5′-GCTGTCACCTTCACCGTTCC-3′
human Bax-F: CGAACTGGACAGTAACATGGAG.
Human Bax-R: CATCTGGTCTGGAGTACGTATC.
Human Caspase3-F: TCCTAGCGGATGGGTGCTAT.
Human Caspase3-R: TGAGGTTTGCTGCATCGACA.
Western blot
Cells were lysed with RIPA buffer containing a protease inhibitor cocktail (Roche Diagnostics, 05,892,970,001) and a phosphatase inhibitor cocktail (Roche Diagnostics, 04,906,845,001). Then, the lysates were centrifugated at 13523 g for 20 min at 4 °C. The total protein concentration was measured by BCA protein assay kit (23,225, Thermo Fisher Scientific). Equal amounts of protein samples were separated by 10% SDS/PAGE and transferred to nitrocellulose membranes. After blocking with 5% milk, the membrane was incubated with the primary antibodies. After washing, the membranes are incubated with secondary antibodies. Signals were detected using the Bio-Rad gel documentation system with the Super Signal West Dura Extended Duration Substrate (Bioscience, Shanghai).
Cell proliferation assay
At the indicated times (12, 24, and 48 h), MHCC97H, MHCC97L, and HepG2 cell lines were treated with CCK8 (Beyotime, C0037) in 96-well plates, and the wells were incubated for an additional 1 h. Finally, absorbance at 450 nm was evaluated using a Multiscan MS spectrophotometer.
Statistical analyses
All statistical analyses were performed using GraphPad Prism6. The differences between the groups were compared by the Group t-test (comparing two variables) and one-way ANOVA analysis (comparing multiple variables). All data were presented as the means ± SD. Survival analysis was performed using the log-rank test. All P values < 0.05 were considered statistically significant.
Results
LHPP was downregulated in human HCC tissues and cell lines
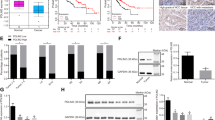
The GSE14520 dataset [23] (https://doi.org/10.1158/0008-5472) of the Gene Expression Omnibus (GEO) database and The Cancer Genome Atlas-Liver Hepatocellular Carcinoma (TCGA-LIHC, https://doi.org/10.1038/ng.2764 ) dataset [3] were used as data source. We evaluated the mRNA level of LHPP in human HCC samples from the GEO database and TCGA-LIHC dataset. The GSE14520 dataset contains data from 486 individuals (containing 247 tumors and 239 adjacent normal liver tissues). The TCGA-LIHC dataset contains 50 normal samples and 371 tumor samples. According to Fig. 1A and B, there is a highly significant decrease (****P < 0.0001) in the mRNA levels of LHPP between the N and the T samples from the database. Furthermore, we analyzed the LHPP mRNA expression among different stages of HCC (Fig. 1C). It shows that LHPP is downregulated with development of HCC. The decreased LHPP mRNA level detected in a subset of HCC tissues was also consistent with LHPP protein expression pattern in HCC patients. HCC patients revealed a relatively lower LHPP protein level compared with normal liver tissues based on the Human Protein Atlas dataset (https://www.proteinatlas.org) (Fig. 1D) [26]. LHPP was downregulated in multiple types of cancer tissues (Fig. 1E). Additionally, LHPP has a relatively lower promoter methylation level in HCC compared with normal conditions (Fig. 1F).
LHPP expression status in human HCC samples. A In comparison with the LHPP mRNA expression in non-tumor tissues (n = 239) and HCC tissues (n = 247) using GEO database. B In comparison with the LHPP mRNA expression in non-tumor tissues (n = 50) and HCC tissues (n = 371) using the TCGA database. C The LHPP mRNA expression between different stages (I: not advanced; II: moderately advanced; III: very advanced) of HCC in the TCGA database. D Immuno-histochemistry images of LHPP in human HCC and normal liver tissues are downloaded from the Human Protein Atlas website. The accession number of NL is Normal tissue, NOS (M-00100) Patient id: 2429. The accession number of LIHC is Hepatocellular, NOS (M-81703) Patient id: 3215. The LIHC sample has a lower LHPP level compared with the NL sample. E Expression of LHPP in TCGA cancers with tumor and normal tissue samples. F Analysis of LHPP promoter methylation level in human HCC samples and normal liver samples based on the TCGA database
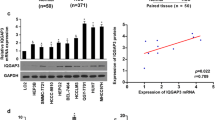
To investigate the tumor-suppressor function of LHPP in liver cancer development in vitro, we detected the LHPP expression level in transformed liver cell line (L02) and HCC cell lines (MHCC97H, MHCC97L, and HepG2). The RT-qPCR results showed that the LHPP mRNA expression in the HCC cell lines was significantly decreased compared with the transformed liver cell line (Fig. 2A). Notably, LHPP mRNA expression level is higher in the MHCC97L cell line compared with the MHCC97H cell line. It indicated that LHPP may play a role in HCC metastasis in vitro. The same protein expression pattern was detected in the above cell lines by Western blots (Fig. 2B and C). Taken together, these results suggest that LHPP as a tumor suppressor may play a major role in HCC progression.
LHPP expression status in human HCC cell lines. A The mRNA expression level of LHPP in transformed liver cell line (LO2) and HCC cell lines (MHCC97H, MHCC97L, and HepG2) by RT-qPCR. B The protein expression level of LHPP in transformed liver cell line (LO2) and HCC cell lines (MHCC97H, MHCC97L, and HepG2) by Western blots. C Statistic analysis result of Western blots. Significant differences were determined using an Independent Group t-test analysis, *P < 0.05, **P < 0.01
Downregulated LHPP contributes to HCC cell proliferation and inhibits LHPP apoptosis
We investigated the role of LHPP in HCC cell proliferation using small interfering RNA (siRNA)–mediated knockdown. We knocked down LHPP in MHCC97H, MHCC97L, and HepG2 cells by using three different siRNA. Western blot results showed that LHPP was efficiently downregulated in MHCC97H and HepG2 cells by using siRNA 3# (Fig. 3A, C and E). Our results revealed that knockdown LHPP increased the proliferation of MHCC97H and HepG2 cells (Fig. 3B and F). While we also find that knockdown LHPP also has a trend to promote MHCC97L cell proliferation, there has been no significant difference. Taken together, downregulated LHPP promotes HCC cell proliferation. Furthermore, we detected the mRNA expression of Apoptosis Regulator (Caspase3 and Bax) in HCC cell lines by RT-qPCR. As shown in Fig. 3G and I, knockdown LHPP significantly decreased Caspase3 and Bax mRNA levels in MHCC97H and HepG2 cell lines. Consistently, knockdown LHPP significantly decreased Caspase3 expression in MHCC97H cells (Fig. 3H). The Bax mRNA level is also lower in the LHPP knockdown group compared with the control group (Fig. 3H).
LHPP reduction promotes the proliferation of HCC cells. A Detect the LHPP protein level after siRNA knockdown in MHCC97H cells by Western blots. B CCK-8 analysis of MHCC97H cell proliferation after siRNA knockdown LHPP. C Detect the LHPP protein level after siRNA knockdown in MHCC97L cells by Western blots. D CCK-8 analysis of MHCC97L cell proliferation after siRNA knockdown LHPP. E Detect the LHPP protein level after siRNA knockdown in HepG2 cells by Western blots. F CCK-8 analysis of HepG2 cell proliferation after siRNA knockdown LHPP. G The relative mRNA expression of Caspase3 and Bax in MHCC97H cells by RT-qPCR. H The relative mRNA expression of Caspase3 and Bax in MHCC97L cells by RT-qPCR. I The relative mRNA expression of Caspase3 and Bax in HepG2 cells by RT-qPCR. Significant differences were determined using an Independent Group t-test analysis, *P < 0.05
LHPP serves an important role in the EMT process in HCC cell lines
As EMT is one of the important mechanisms for cell migration and invasion, we explore the effect of LHPP on the progression of EMT. Epithelial markers (E-cadherin) and mesenchymal markers (N-cadherin) were detected by Western blot in MHCC97H and HepG2 cells. Western blot results revealed that compared with NS control cells, E-cadherin protein level was downregulated in MHCC97H cell line where LHPP was knocked down. By contrast, N-cadherin protein level was upregulated (Fig. 4A and B). In addition, a similar result was shown in HepG2 cells (Fig. 4C and D). By using two different liver cancer cell lines, we found that LHPP has a better effect on EMT-related protein expression in HepG2 cell lines. Hence, to further confirm our result, lentivirus-mediated overexpression of LHPP was used in HepG2 cells. As shown in Fig. 5, we efficiently overexpressed LHPP in HepG2 cells. Our results showed that overexpressed LHPP in HepG2 cells increased E-cadherin protein level and decreased N-cadherin protein level (Fig. 6A). We further explored the effect of LHPP on the metastasis of HepG2 cells. Our data showed that overexpression of LHPP significantly decreased the migration ability in HepG2 cells (Fig. 6B). These results indicated that LHPP could inhibit HCC metastasis though EMT.
Knockdown of LHPP altered the expression of EMT-related proteins. A–B The expressions of E-cadherin and N-cadherin were detected by Western blot in MHCC97H cells transfected with NS control siRNA and LHPP siRNA 3#. **P < 0.01. C–D The expressions of E-cadherin and N-cadherin were detected by Western blot in HepG2 cells transfected with NS control siRNA and LHPP siRNA 3#. Significant differences were determined using an Independent Group t-test analysis, *P < 0.05, **P < 0.01
Stable overexpression of LHPP in HepG2 cell line. A The pictures of HepG2-Lenti-EGFP (Ctrl) and HepG2-Lenti-LHPP cells in light field and green fluorescence (scale bar: 50 μm). B qRT-PCR detection of LHPP mRNA expression in HepG2-Lenti-EGFP (Ctrl) and HepG2-Lenti-LHPP cells (n = 3, **P < 0.01). C–D Western blot analysis of the expression of LHPP in HepG2-Lenti- EGFP (Ctrl) and HepG2-Lenti-LHPP cells (n = 3, *P < 0.05)
Overexpression of LHPP altered HepG2 cell migration and expression of EMT-related proteins. A The expressions of E-cadherin and N-cadherin were detected by Western blot in HepG2 cells transfected with Plenti-CMV-EGFP and Plenti-CMV-LHPP vector. B Transwell assay of the migration ability of HepG2-EGFP cells and control HepG2-LHPP cells. 104 cells per chamber were seeded. C–D The expressions of E-cadherin and N-cadherin were detected by Western blot in TGF-β treated HepG2 cell line transfected with Plenti-CMV-EGFP and Plenti-CMV-LHPP vector. Significant differences were determined using an Independent Group t-test analysis, **P < 0.01
We have determined that LHPP inhibited HCC cell proliferation and inhibited HCC metastasis though EMT, but whether it played roles in TGF-β-induced EMT was not clear. To observe the role of LHPP in TGF-β-induced EMT, we observed EMT-related protein levels after exposure to TGF-β followed by performing genetic gain of function experiments with LHPP. First, we established a TGF-β-induced EMT model in HepG2 cells. We overexpressed LHPP in HepG2 cell line to detect the EMT-related protein level after 10 ng/ml TGF-β1 was treated for 48 h. As shown in Fig. 6C and D E-cadherin protein level increased, and N-cadherin protein level decreased slightly. These data suggested that LHPP prevented TGF-β-induced EMT in HCC cell lines.
HPP positively regulates the expression of p53 protein
p53 is a well-known transcription factor that suppresses tumor growth through regulation of dozens of target genes with diverse biological functions. We wonder whether the cancer-inhibiting effect of LHPP in HCC has any effect on p53. So, we studied the effect of LHPP on the expression of p53 protein by gaining and losing the function of LHPP. As shown in Fig. 7A and B, p53 protein level increased after overexpression of LHPP. By contrast, p53 protein level decreased after knockdown LHPP (Fig. 7C and D). These results indicated that there is an internal mechanism of positive regulation between LHPP and p53.
Overexpression and knockdown of LHPP alters p53 protein expression. A–B The expressions of p53 and LHPP were detected by Western blot in HepG2 cells transfected with Plenti-CMV-EGFP and Plenti-CMV-LHPP vector. C–D The expressions of P53 and LHPP were detected by Western blot in HepG2 cells transfected with NS control siRNA and LHPP siRNA 3#. Significant differences were determined using an Independent Group t-test analysis, *P < 0.05, **P < 0.01
Discussion
LHPP is a novel tumor-suppressor gene and its anti-tumor function has been implicated in multiple tumor types since 2018 [9]. However, there are few studies on its function in liver cancer. In the present study, we provide a comprehensive analysis of LHPP status in human HCC using public datasets. The GEO database, TCGA database, and UALCAN database (http://ualcan.path.uab.edu/index.html) showed that LHPP was downregulated in multiple types of cancer tissues (Fig. 1E). It is well known that inactivation of certain tumor-suppressor genes occurs as a consequence of hypermethylation within the promoter regions and numerous studies have demonstrated a broad range of genes being silenced by DNA methylation in different cancer types [12, 13]. There is a decreased LHPP promoter methylation observed in HCC compared with normal liver (Fig. 1F). More research is needed into its promoter methylation function. The Kaplan–Meier overall survival and disease-free survival curves based on LHPP expression in the GEPIA database and TCGA database also showed that high expression of LHPP was associated with better clinical outcomes in HCC (Fig. 8A). In addition, we found that the mRNA and protein levels of LHPP were reduced in human HCC cells compared with normal liver cells.
Survival curves and negative correlation were found between the levels of LHPP and EMT-related genes. A Kaplan–Meier overall survival and disease-free survival curves based on LHPP expression in the GEPIA database using TCGA database (stratified by median). B The LHPP score is negatively correlated with CDH1, CDH2, ZEB1, VIM, and SNAL1 in the TCGA cohort. Using Pearson’s correlation test (r and p values are indicated in the graphs)
There are many studies reporting that LHPP can attenuate cell proliferation in colorectal cancer [11], renal cell carcinoma (RCC) [33], bladder cancer [16], pancreatic cancer [30], and so on. Recently, Liao et al. [18] showed that LHPP overexpression of LHPP inhibits the growth and metastasis of HCC cells. Consistent with this finding in HCC, we found that knockdown of LHPP can enhance HCC cell proliferation. However, there are still few studies that describe the role of LHPP in human HCC metastasis and its underlying mechanisms are still unclear. In our study, we found that LHPP can affect EMT-related protein levels in HCC cell lines. We detected EMT-related protein expression in the LHPP overexpressed and down expressed HCC cell lines. Our data showed that overexpressed LHPP in HepG2 cells increased E-cadherin protein level and decreased N-cadherin protein level. In addition, E-cadherin protein level was downregulated in HCC cell lines where LHPP was knocked down. Also, data analysis revealed that the LHPP score is negatively correlated with EMT-related genes (CDH1, CDH2, and VIM) and EMT-activating transcription factors (ZEB1 and SNAL1) in the TCGA cohort (Fig. 8B). Thus, we concluded that there is a certain relationship between LHPP and EMT, which may represent a new therapeutic target for HCC metastasis (Scheme 1).
EMT also plays a key role in the pathogenesis of HCC occurrence and metastasis [7, 31]. In this process, expressions of epithelial cell markers such as E-cadherin decrease and those of mesenchymal cell markers such as N-cadherin and vimentin as well as the transcription factors snail, slug, and twist increase. Meanwhile, the cell gains more mobility and invasive ability with marked morphological changes. In this study, we successfully used TGF-β to establish an EMT model in human HCC cell lines. Our data showed that N-cadherin and vimentin protein levels were increased after the knockdown of LHPP in MHCC97H cell line, E-cadherin protein level increased in LHPP overexpressed HepG2 cell line. These data suggested that LHPP inhibited TGF-β-induced EMT in vitro.
Our results in this study showed that LHPP is a novel tumor suppressor that directly regulates p53 protein levels in HepG2 cells. As we all know, p53 is a well-known tumor suppressor, and upregulation of p53 protein in response to ribosomal stress is largely due to the disruption of interaction between p53 and MDM2. Mdm2 binds the transcriptional activation domain of p53 and blocks its ability to regulate target genes and to exert antiproliferative effects [8, 28]. Currently, the underlying mechanism of LHPP regulating p53 is still unclear, which should be addressed by our future studies. We will explore if there is an underline pathway that LHPP regulates the interaction of p53 and MDM2.
In summary, we found that LHPP, as a novel tumor suppressor, could not only inhibit HCC cell proliferation, but also reshape the EMT process in HCC. Our studies validate LHPP as a tumor suppressor in liver cancer, supporting its inhibition as a potential therapeutic strategy for HCC metastasis. These data provide a new insight for the future study of human HCC.
References
Alqahtani, A., Z. Khan, A. Alloghbi, T. S. Said Ahmed, M. Ashraf & D. M. Hammouda (2019) Hepatocellular carcinoma: molecular mechanisms and targeted therapies. Medicina (Kaunas), 55.
Bakir B, Chiarella AM, Pitarresi JR, Rustgi AK (2020) EMT, MET, plasticity, and tumor metastasis. Trends Cell Biol 30:764–776
Cancer Genome Atlas Research Network, Weinstein JN, Collisson EA, Mills GB, Shaw KR, Ozenberger BA, Ellrott K, Shmulevich I, Sander C, Stuart JM (2013) The Cancer Genome Atlas Pan-Cancer analysis project. Nat Genet 45(10):1113–1120
Consortium C (2015) Sparse whole-genome sequencing identifies two loci for major depressive disorder. Nature 523:588–591
Craig AJJAO, von Felden T, Garcia-Lezana S, Sarcognato AAO (2020) Villanueva Tumour evolution in hepatocellular carcinoma.
Cui L, Gong X, Tang Y, Kong L, Chang M, Geng H, Xu K, Wang F (2016) Relationship between the LHPP gene polymorphism and resting-state brain activity in major depressive disorder. Neural Plast 2016:9162590
Giannelli G, Koudelkova P, Dituri F, Mikulits W (2016) Role of epithelial to mesenchymal transition in hepatocellular carcinoma. J Hepatol 65:798–808
Haupt Y, Maya R, Kazaz A, Oren M (1997) Mdm2 promotes the rapid degradation of p53. Nature 387(6630):296–299
Hindupur SK, Colombi M, Fuhs SR, Matter MS, Guri Y, Adam K, Cornu M, Piscuoglio S, Ng CKY, Betz C, Liko D, Quagliata L, Moes S, Jenoe P, Terracciano LM, Heim MH, Hunter T, Hall MN (2018) The protein histidine phosphatase LHPP is a tumour suppressor. Nature 555:678–682
Hiraishi H, Yokoi F, Kumon A (1998) 3-phosphohistidine and 6-phospholysine are substrates of a 56-kDa inorganic pyrophosphatase from bovine liver. Arch Biochem Biophys 349:381–387
Hou B, Li W, Li J, Ma J, Xia P, Liu Z, Zeng Q, Zhang X, Chang D (2020) Tumor suppressor LHPP regulates the proliferation of colorectal cancer cells via the PI3K/AKT pathway. Oncol Rep 43:536–548
Klutstein M, Nejman D, Greenfield R, Cedar H (2016) DNA methylation in cancer and aging. Cancer Res 76:3446–3450
Kulis M, Esteller M (2010) DNA methylation and cancer. Adv Genet 70:27–56
Lee GA, Hwang KA, Choi KC (2016) Roles of dietary phytoestrogens on the regulation of epithelial-mesenchymal transition in diverse cancer metastasis. Toxins (Basel), 8.
Lesseur C, Diergaarde B, Olshan AF, Wünsch-Filho V, Ness AR, Liu G, Lacko M, Eluf-Neto J, Franceschi S, Lagiou P, Macfarlane GJ, Richiardi L, Boccia S, Polesel J, Kjaerheim K, Zaridze D, Johansson M, Menezes AM, Curado MP, Robinson M, Ahrens W, Canova C, Znaor A, Castellsagué X, Conway DI, Holcátová I, Mates D, Vilensky M, Healy CM, Szeszenia-Dąbrowska N, Fabiánová E, Lissowska J, Grandis JR, Weissler MC, Tajara EH, Nunes FD, de Carvalho MB, Thomas S, Hung RJ, Peters WH, Herrero R, Cadoni G, Bueno-de-Mesquita HB, Steffen A, Agudo A, Shangina O, Xiao X, Gaborieau V, Chabrier A, Anantharaman D, Boffetta P, Amos CI, McKay JD, Brennan P (2016) Genome-wide association analyses identify new susceptibility loci for oral cavity and pharyngeal cancer. Nat Genet 48:1544–1550
Li Y, Zhang X, Zhou X (2019) LHPP suppresses bladder cancer cell proliferation and growth via inactivating AKT/p65 signaling pathway. Biosci Rep, 39.
Li Z, Zhou X, Zhu H, Song X, Gao H, Niu Z, Lu J (2021) Purpurin binding interacts with LHPP protein that inhibits PI3K/AKT phosphorylation and induces apoptosis in colon cancer cells HCT-116. J Biochem Mol Toxicol 35:e22665
Liao L, Duan D, Liu Y, Chen L (2020) LHPP inhibits hepatocellular carcinoma cell growth and metastasis. Cell Cycle 19:1846–1854
Liu M, Jiang L, Guan XY (2014) The genetic and epigenetic alterations in human hepatocellular carcinoma: a recent update. Protein Cell 5:673–691
Liver EAFTSOT, Cancer EOFRATO (2012) EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 56:908–943
Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS (2021) Hepatocellular carcinoma Nat Rev Dis Primers 7:6
Polimanti R, Wang Q, Meda SA, Patel KT, Pearlson GD, Zhao H, Farrer LA, Kranzler HR, Gelernter J (2017) The interplay between risky sexual behaviors and alcohol dependence: genome-wide association and neuroimaging support for LHPP as a risk gene. Neuropsychopharmacology 42:598–605
Roessler S, Jia HL, Budhu A, Forgues M, Ye QH, Lee JS, Thorgeirsson SS, Sun Z, Tang ZY, Qin LX, Wang XW (2010) A unique metastasis gene signature enables prediction of tumor relapse in early-stage hepatocellular carcinoma patients. Cancer Res 70(24):10202–10212
Sun W, Qian K, Guo K, Chen L, Xiang J, Li D, Wu Y, Ji Q, Sun T, Wang Z (2020) LHPP inhibits cell growth and migration and triggers autophagy in papillary thyroid cancer by regulating the AKT/AMPK/mTOR signaling pathway. Acta Biochim Biophys Sin (Shanghai) 52:382–389
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics, 2012. CA Cancer J Clin 65:87–108
Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C, Sjöstedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Pontén F (2015) Proteomics Tissue-based map of the human proteome. Science 347(6220):1260419
Villanueva A (2019) Hepatocellular carcinoma. N Engl J Med 380:1450–1462
Wade M, Wang YV, Wahl GM (2010) The p53 orchestra: Mdm2 and Mdmx set the tone. Trends Cell Biol 201020(5):299–309
Wang D, Ning Z, Zhu Z, Zhang C, Wang P, Meng Z (2021) LHPP suppresses tumorigenesis of intrahepatic cholangiocarcinoma by inhibiting the TGFβ/smad signaling pathway. Int J Biochem Cell Biol 132:105845
Wu, F., Y. Chen & J. Zhu (2020) LHPP suppresses proliferation, migration, and invasion and promotes apoptosis in pancreatic cancer. Biosci Rep, 40.
Xu MY, Chen R, Yu JX, Liu T, Qu Y, Lu LG (2016) AZGP1 suppresses epithelial-to-mesenchymal transition and hepatic carcinogenesis by blocking TGFβ1-ERK2 pathways. Cancer Lett 374:241–249
Yokoi F, Hiraishi H, Izuhara K (2003) Molecular cloning of a cDNA for the human phospholysine phosphohistidine inorganic pyrophosphate phosphatase. J Biochem 133:607–614
Zhang X, Kang H, Xiao J, Shi B, Li X, Chen G (2020) LHPP inhibits the proliferation and metastasis of renal cell carcinoma. Biomed Res Int 2020:7020924
Zheng J, Dai X, Chen H, Fang C, Chen J, Sun L (2018) Down-regulation of LHPP in cervical cancer influences cell proliferation, metastasis and apoptosis by modulating AKT. Biochem Biophys Res Commun 503:1108–1114
Acknowledgements
We thank Dr. Guanbin Song (Chongqing University, Chongqing, China) for sharing the MHCC97H and MHCC97L cell lines.
Funding
This work was supported by the Natural Science Foundation of Chongqing (2021ycjh-bgzxm0169; cstc2021jcyj-cxttX0002cstc), Chongqing Graduate Scientific Research and Innovation Foundation of China (No. CYB19043), the National Natural Science Foundation of China (No. 82002909), Visiting Scholar Foundation of Key Laboratory of Biorheological Science and Technology (Chongqing University), Ministry of Education (CQKLBST-2021-002) and Sichuan Science and Technology Program (2022NSFSC1318).
Author information
Authors and Affiliations
Contributions
L.M. and S.F. executed this study; L.M. wrote the manuscript; X.X. assisted with the cell culture experiments; Y.C. and L.Z. assisted with the Western blots experiments; S.L. proofread the manuscript; L.T. supervised the study and edited the manuscript. The authors declare that all data was generated in-house and that no paper mill was used.
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
1. LHPP acts as a tumor suppressor in HCC and LHPP expression is lower in HCC compared with in normal conditions.
2. LHPP suppresses cell proliferation and migration in HCC cell lines.
3. LHPP suppresses the EMT process in HCC.
4. p53 protein expression is positively regulated by LHPP in HCC.
Rights and permissions
About this article
Cite this article
Ma, L., Sun, H., Xu, X. et al. Tumor suppressor LHPP suppresses cell proliferation and epithelial-mesenchymal transition in hepatocellular carcinoma cell lines. J Physiol Biochem 78, 807–817 (2022). https://doi.org/10.1007/s13105-022-00903-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-022-00903-7