Abstract
Opa-interacting protein 5 antisense RNA 1 (OIP5-AS1), a long non-coding RNA (lncRNA), has been reported to link with the progression of some cancers. However, its biological functions and underlying molecular mechanisms in pancreatic cancer are largely unknown. The aim of this study was to investigate the role of lncRNA OIP5-AS1 in pancreatic cancer. Quantitative real-time PCR analysis revealed that OIP5-AS1 is highly expressed in pancreatic cancer tissues versus adjacent non-tumor tissues. In vitro functional assays showed that downregulation of OIP5-AS1 or overexpression of miR-342-3p inhibited the proliferation, decreased Ki67 expression, and induced cell cycle arrest in pancreatic cancer cells. The expression of cyclinD1, CDK4, and CDK6 was decreased by knockdown of OIP5-AS1. Moreover, we found that OIP5-AS1 acted as a miR-342-3p sponge to suppress its expression and function. Dual-luciferase assay confirmed the interaction of OIP5-AS1 and miR-342-3p and verified anterior gradient 2 (AGR2) as a direct target of miR-342-3p. Results showed that depletion of miR-342-3p abolished the inhibitory effects of OIP5-AS1 knockdown on pancreatic cancer cell growth. The expression of Ki67, AGR2, cyclinD1, CDK4, CDK6, p-AKT, and p-ERK1/2 was reversed by silencing of miR-342-3p in pancreatic cancer cells with OIP5-AS1 knockdown. Further, knockdown of OIP5-AS1 suppressed tumor growth in a xenograft mouse model of pancreatic cancer. OIP5-AS1 induced pancreatic cancer progression via activation of AKT and ERK signaling pathways. Therefore, we demonstrate that OIP5-AS1 functions as oncogene in pancreatic cancer and its downregulation inhibits pancreatic cancer growth by sponging miR-342-3p via targeting AGR2 through inhibiting AKT/ERK signaling pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic cancer is one of the deadliest cancers with poor diagnosis. It is the leading cancerous cause of death among both men and woman globally [33]. It is reported that the trend of pancreatic cancer incidence and mortality rates is increasing in China, as in most other countries in the world [3]. According to GLOBOCAN 2012 estimates, there are about 19.45% of 337,872 cases of newly diagnosed pancreatic cancer and 19.27% of 330,391 deaths from pancreatic cancer each year in China [17]. Currently, the standard treatment of pancreatic cancer is surgery followed by chemotherapeutics, yet there are only 20% of overall 5-year survival rate after pancreatic resection [8]. Despite great efforts devoted to improving the outcome of pancreatic cancer, its high invasiveness and metastasis result in poor progress in disease prognosis [30]. There is an urgent need to better understand the pathogenesis of pancreatic cancer and improve current therapies for these patients.
Long non-coding RNAs (lncRNAs) with more than 200 bp in length and microRNAs (miRNAs) with a length of about 22–25 bp both are a class of RNA transcripts and characterized by non-protein coding capability. Mounting studies have reported that both lncRNAs and miRNAs play an important role in biological processes such as cell cycle, proliferation, apoptosis, differentiation, invasion, and migration [27]. Abnormalities in lncRNA and miRNA expression are common in most of human cancers, supporting their involvement in cancer occurrence and development [14]. Opa-interacting protein 5 antisense RNA 1 (OIP5-AS1), a novel identified lncRNA, is transcribed in the antisense of the gene encoding opa-interacting protein 5 (OIP5) [13]. OIP5-AS1 exerts oncogenic function in multiple cancers, such as cervical cancer [40], osteosarcoma [5], and lung cancer [44]. To the best of our knowledge, the role of OIP5-AS1 in pancreatic cancer has not been reported yet. It is identified that there is miRNA dysregulation in most of human cancers including pancreatic cancer with acting as oncogenes or tumor suppressors. miR-342-3p has been showed to inhibit the progression of various cancers such as breast cancer [26], hepatocellular carcinoma [20], and glioma [43]. In addition, it was found downregulated in pancreatic cancer patients and related to their overall survival [15]. However, still little is known about its role in pancreatic cancer.
In the present study, we found that lncRNA OIP5-AS1 is highly expressed in pancreatic cancer patients and acts as a competing endogenous RNA (ceRNA) of miR-342-3p to inhibit its expression and function. Downregulation of OIP5-AS1 decreased cell proliferation and cell cycle progression in human pancreatic cancer cells and suppressed xenograft tumor growth in nude mice. We demonstrated the oncogenic function of OIP5-AS1 via targeting miR-342-3p in pancreatic cancer.
Materials and methods
Human pancreatic carcinoma samples
A total of 28 pairs of pancreatic cancer tissues and adjacent non-tumor tissues were obtained from patients at Shengjing Hospital of China Medical University between September 2015 and May 2018. Informed consent was obtained from each participant. None of the patients underwent any chemotherapy or radiotherapy. All tissue samples were collected, immediately snap-frozen using liquid nitrogen and stored at − 80 °C until RNA extraction. This study was approved by the Ethic Committee of Shengjing Hospital of China Medical University and performed in accordance with the Declaration of Helsinki.
Cell culture and transfection
Three different human pancreatic cancer cell lines including ASPC-1, PANC-1, and BXPC-3 purchased from Procell Life Science & Technology Co., Ltd., Wuhan, China were used in this study. ASPC-1 and BXPC-3 cells were cultured in RPMI-1640 medium (Gibco Life Technologies, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS) (Biological Industries, Kibbutz Beit Haemek, Israel), and PANC-1 cells were cultured in DMEM medium (Gibco) supplemented with 10% FBS at 37 °C under 5% CO2 in a humidified incubator.
According to polymerase chain reaction (PCR) screening results, ASPC-1 and PANC-1 were selected for OIP5-AS1 knockdown, and ASPC-1 and BXPC-3 were used for miR-342-3p overexpression. Cells were seeded into 6-well plates at a density of 4 × 105 per well. After incubation with serum-free culture medium for 1 h, ASPC-1 and PANC-1 cells were transfected with OIP5-AS1 siRNA or NC siRNA or co-transfected with miR-342-3p inhibitor or NC inhibitor. ASPC-1 and BXPC-3 were transfected with miR-342-3p mimic or NC mimic. All transfections were performed with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) in accordance with the manufacturer’s instruction. After transfection, cells were treated with 5 ng/mL fibroblast growth factor (FGF)-2 for 48 h.
Animals and treatment
Healthy male nude mice (2 months old, weighting 18–20 g) were purchased from Beijing HFK Bioscience Co. Ltd. and housed at 22 ± 1 °C and 45–55% humidity under an alternating 12-h light/dark cycle with free access to food and water. Mice are divided into three groups: Control group, NC shRNA group, and OIP5-AS1 shRNA group. After adaptive feeding for 1 week, 5 × 106 ASPC-1 tumor cells were subcutaneously injected into the right flank area of all groups of nude mice. When tumors grew to 50–100 mm3 (day 16), mice were injected through tail vein with 10 μg of OIP5-AS1 shRNA plasmids or NC shRNA plasmids three times a week for 2 weeks. The tumor volume was examined every third day during the treatment. At day 30, the tumor was excised and its weight was measured.
All animal experiments were approved by Institutional Animal Care and Use Committee and performed in accordance with Guide for the Care and Use of Laboratory Animals.
Real-time PCR and quantitative reverse transcription PCR (qRT-PCR)
Total RNA was isolated from pancreatic cancer tissues, matched non-tumor tissues or tumor cells with TRIpure lysis buffer (BioTeke Corporation, Beijing, China). After reverser transcription, the first-strand cDNA was synthesized using Super M–MLV transcriptase (BioTeke) following the instructions. The qRT-PCR was performed on a real-time PCR system using the cDNA template, gene specific primers, SYBR Green (Sigma-Aldrich, St Louis, MO, USA), and 2 × Power Taq PCR MasterMix (BioTeke). The primer sequences were as follows: lncRNA OIP5-AS1 forward, 5’-TACTCAGATGGACCAGGAT-3′; lncRNA OIP5-AS1 reversed, 5’-TTACGGGACATAACAAGG-3′; anterior gradient 2 (AGR2) forward, GCATTCTTGCTCCTTGTGG; AGR2 reversed, GACTGTGTGGGCACTCATCC; β-actin forward, CACTGTGCCCATCTACGAGG; β-actin reversed, TAATGTCACGCACGATTTCC; miR-342-3p RT Primer, 5’-GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGCACCAGAGCCAACACGGGT-3′; miR-342-3p forward, 5’-TCTCACACAGAAATCGCACCCGT-3′; miR-342-3p, reversed, 5’-GCAGGGTCCGAGGTATTC-3′; U6 RT Primer, 5’-GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGCACCAGAGCCAACAAAATATGG-3′; U6 forward, 5’-GCTTCGGCAGCACATATACT-3′; U6 reversed, 5’-GCAGGGTCCGAGGTATTC-3′. The relative expression levels were analyzed by 2−ΔΔCt method and normalized against β-actin or U6 expression.
Cell counting Kit-8 (CCK-8) assay
Cell proliferation was examined by CCK-8 assay according to the manufacturer’s protocols. Briefly, cells were cultured in 96-well plates at a density of 3 × 103 per well for 0, 24, 48, 72, or 96 h following transfection. At each time point, 10 μl CCK-8 solution (Sigma) was added into each well and incubated for 1 h. Subsequently, the absorbance at 450 nm was measured with a microplate reader.
Immunohistochemistry
Paraformaldehyde-fixed and paraffin-embedded tumor tissues were sectioned, deparaffinized, and rehydrated. After microwave-stimulated antigen retrieval, sections were incubated with 3% H2O2 (Sinopharm Chemical Regent Co., Ltd. Shanghai, China) for 15 min. Then, sections were blocked with goat serum (Solarbio Science & Technology, Co., Ltd., Beijing, China) for 15 min, probed with primary antibody against Ki67 (1:200; Abcam, Cambridge, MA, USA) at 4 °C overnight, and incubated with secondary antibody HRP-conjugated goat-anti rabbit IgG (1:500; Thermo Fisher Scientific, Waltham, MA, USA) at 37 °C for 1 h. After DAB treatment and hematoxylon redyeing, sections were finally analyzed under a microscope at 400×.
Immunofluorescence
Cells grown on glass coverslips were fixed with 4% paraformaldehyde (Sinopharm Chemical Reagent) and then treated with 0.1% tritonX-100 (Beyotime, Haimen, Jiangsu, China) for 30 min. After PBS washing, cells were blocked with goat serum (Solarbio Science & Technology) for 15 min. Then they were probed with primary antibody against Ki67 (1:200; Abcam) at 4 °C overnight and incubated with goat-anti rabbit IgG secondary antibody labeled with Cy3 (1:200, Beyotime), followed by nuclei counterstaining using DAPI (Beyotime). The staining results were analyzed under a fluorescence microscope at 400×.
Flow cytometry
Cell cycle distribution was determined by Cell Cycle Analysis Kit using propidium iodide staining (Beyotime). After transfection, cells were collected and seeded into 6-well plates. Then cells were fixed with 70% cold ethanol at 4 °C for 12 h, centrifuged to remove the supernatant, and resuspended in 500 μl staining buffer. After addition of 10 μl RNase A and 25 μl propidium iodide to each well, the cells were incubated in the dark at 37 °C for 30 min. Subsequently, the cell cycle analysis was performed with the use of a flow cytometer (ACEA Biosciences, San Diego, California, USA).
Western blot
Total protein of tissues or cells was extracted utilizing RIPA buffer (Beyotime). A BCA protein assay kit was used to determine the protein concentration. Protein samples were separated by SDS-PAGE and then blotted onto PVDF membranes (Thermo Fisher Scientific). After blocking with 5% (M/V) bovine serum albumin (BSA) (Biosharp, Hefei, China) for 1 h, membranes were probed with primary antibodies against cyclinD1 (1:1000; Cell Signaling Technology (CST), Danvers, MA, USA), CDK4 (1:1000; CST), CDK6 (1:1000; Proteintech Group, Wuhan, China), AGR2 (1:500; Abclonal Technology, Wuhan, China), protein kinase B (AKT) (1:500; CST), phospho-AKT at ser473 (p-AKTser473) (1:1000; CST), extracellular signal-regulated kinase (ERK)1/2 (1:2000; CST), or phospho-ERK1/2 at Thr202/Tyr204 (p-ERK1/2Thr202/Tyr204) (1:1000; CST) at 4 °C overnight. β-Actin (1:2000, Proteintech) acted as the internal control. Membranes were then incubated with goat anti-rabbit (1:10000; Proteintech) or goat anti mouse IgG (1:10000, Proteintech). Protein blots were finally visualized using ECL kit (7 Sea Pharmtech, Shanghai, China).
Dual-luciferase reporter assay
The sequences of lncRNA OIP5-AS1 or AGR2 containing miR-342-3p binding sites or respective mutant sits, after amplification, were cloned into luciferase reporter vectors pGL3 (Promega, Madison, WI, USA), which were named as wild-type (wt)-OIP5-AS1, mutant (mut)-OIP5-AS1, wt-AGR2, or mut-AGR2. 293 T cells; common cells used for transfection experiments including luciferase assay [11, 12], and were co-transfected with the constructed wt-plasmid or mut-plasmid and miR-342-3p mimic or NC mimic using Lipofectamine 2000 (Invitrogen). Forty-eight hours after transfection, cells were harvested, and the Firefly and Renilla luciferase activities were detected using the Firefly Luciferase Assay kit (Promega) following the manufacturer’s protocols. The relative luciferase activity was calculated by normalizing Firefly activity against Renilla activity.
Statistical analysis
All data are presented as mean ± standard deviation (SD). Statistical significance was determined with GraphPad Prism 7.0 using two-tailed Student’s t test and one-way or two-way analysis of variance (ANOVA). A P value less than 0.05 was considered statistically significant.
Results
The expression of lncRNA OIP5-AS1 and miR-342-3p in pancreatic cancer
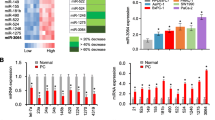
A total of 28 pancreatic cancer tissue samples and 30 adjacent non-tumor tissue samples were collected from patients diagnosed with pancreatic cancer. The expression of lncRNA OIP5-AS1 (Fig. 1a) and miR-342-3p (Fig. 1b) was detected by real-time PCR and qRT-PCR. We found an upregulation of OIP5-AS1 and a downregulation of miR-342-3p in pancreatic carcinoma, compared with that in adjacent non-tumor tissues. The correlation analysis showed a negative correlation between OIP5-AS1 and miR-342-3p in pancreatic cancer tissues versus normal ones (Fig. 1c). Moreover, GEPIA database provides survival data and shows that pancreatic cancer patients with higher OIP5-AS1 had poor prognosis than that with lower expression (Fig. 1d). We further analyzed the clinical relevance OIP5-AS1 in pancreatic cancer. We found higher OIP5-AS1 expression in metastatic tumor as well as in advanced stage (Supplementary Fig. 1). The results implied a possibility that OIP5-AS1 expression was positively correlated with lymph node metastasis and tumor stage, though no significant difference due to limitation of clinical samples. These data suggested the involvement of OIP5-AS1 and miR-342-3p in pancreatic cancer progression.
The expression of lncRNA OIP5-AS1 and miR-342-3p in pancreatic cancer. The expression of lncRNA OIP5-AS1 (a) and miR-342-3p (b) was measured by quantitative RT-PCR in pancreatic carcinoma and adjacent non-tumor tissues. (c) Negative correlation between OIP5-AS1 and miR-342-3p in pancreatic cancer tissues versus normal ones. (d) GEPIA database provides overall survival data in pancreatic cancer patients with high and low OIP5-AS1 expression levels. Data were present as 2-ΔCT. Data were shown as mean ± SD (n = 28) and analyzed by Student’s t test. ††P < 0.01 vs. pancreatic carcinoma
Knockdown of lncRNA OIP5-AS1 inhibits pancreatic cancer growth
Four pancreatic cancer cell lines including ASPC-1, PANC-1, BXPC-3, and Capan-2 were examined in this study. Quantitative real-time PCR analysis showed that the expression of OIP5-AS1 was relatively higher in ASPC-1 and PANC-1 cell lines than that in BXPC-3 and Capan-2 cell lines (Fig. 2a). According to PCR results, OIP5-AS1 knockdown was carried out in ASPC-1 and PANC-1. After transfection with OIP5-AS1 siRNAs or NC siRNAs, the transfection efficiency was determined by real-time PCR (Fig. 2b). In comparison with negative controls, the expression of OIP5-AS1 was significantly downregulated in both ASPC-1 and PANC-1 cells transfected with OIP5-AS1 siRNAs. Cell proliferation was decreased by OIP5-AS1 knockdown at 48, 72, or 96 h post-transfection as detected by CCK-8 assay (Fig. 2c). Immunofluorescence staining revealed that Ki67 expression was obviously decreased in pancreatic cancer cells with OIP5-AS1 silencing (Fig. 2d). Additionally, knockdown of OIP5-AS1 was found to induce cell cycle arrest, as evidenced by increased proportion of cells in G1 phase and reduced cell proportion in S and G2 phases (Fig. 2e). Western blotting analysis showed that the expression levels of cyclinD1, CDK4, and CDK6 were reduced in pancreatic cancer cell ASPC-1 and PANC-1 following downregulation of OIP5-AS1 (Fig. 2f–i).
Downregulation of lncRNA OIP5-AS1 inhibits pancreatic cancer growth. (a) The expression of OIP5-AS1 was detected in four pancreatic cancer cell lines including ASPC-1, BXPC-3, PANC-1, and Capan-2. OIP5-AS1 siRNAs or negative control (NC) siRNAs were transfected into ASPC-1 and PANC-1 cells, respectively. (b) Forty-eight hours after transfection, lncRNA OIP5-AS1 expression was detected. (c) CCK-8 analysis of cell proliferation at 0, 24, 48, 72, and 96 h post-transfection. (d) Immunostaining for Ki67 and counterstaining with DAPI in ASPC-1 and PANC-1 cells with OIP5-AS1 knockdown or not. (e) Cell cycle distribution was examined using flow cytometry. (f) Western blotting analysis of the protein expression of cyclinD1 (g), CDK4 (h), and CDK6 (i). Data were shown as mean ± SD (n = 3) and analyzed by two-way ANOVA or Student’s t test. **P < 0.01 vs. NC siRNA. Scale bar = 50 μm
LncRNA OIP5-AS1 exerts its functions by sponging miR-342-3p via targeting AGR2
Dual-luciferase assay was performed to evaluate the binding activity between miR-342-3p and OIP5-AS1 and miR-342-3p and AGR2. The sequences of OIP5-AS1 or AGR2 containing miR-342-3p binding sites or corresponding mutant sites are displayed in Fig. 3a. The relative luciferease activity was significantly lowered in cells co-transfected with wt-OIP5-AS1 or wt-AGR2 and miR-342-3p mimic than that with mut-OIP5-AS1 or mut-AGR2 and miR-342-3p mimic. Additionally, the expression of miR-342-2p was significantly increased by silencing OIP5-AS1 in both ASPC-1 and PANC-1 cells (Fig. 3b), indicating that OIP5-AS1 negatively regulated miR-342-3p expression in pancreatic cancer. The mRNA and protein expression levels of AGR2 were found decreased by miR-342-3p overexpression and increased by miR-342-3p knockdown in both ASPC-1 and PANC-1 cells (Fig. 3c, d). These results suggested that miR-342-3p is a direct target of OIP5-AS1 and AGR2 was a target gene of miR-342-3p in pancreatic cancer cells, which implied a possible OIP5-AS1/miR-342-3p/AGR2 mechanism in regulation of pancreatic cancer progression.
LncRNA OIP5-AS1 exerts its functions by sponging miR-342-3p via targeting AGR2. (a) The sequences of OIP5-AS1 or AGR2 containing miR-342-3p binding sites (wt-OIP5-AS1 and wt-AGR2) or corresponding mutant sites (mut-OIP5-AS1 and mut-AGR2). The relative luciferase activity was measured to evaluate the binding activity of OIP5-AS1 or AGR2 with miR-342-3p using dual-luciferase assay. (b) The expression of miR-342-3p was detected in ASPC-1 and PANC-1 cells with downregulation of OIP5-AS1. (c, d) The mRNA and protein expression levels of AGR2 were detected in pancreatic cancer cells transfected with miR-342-3p mimics or inhibitor using real-time PCR or western blot. Data were shown as mean ± SD (n = 3) and analyzed by Student’s t test. **P < 0.01 vs. NC siRNA; ##P < 0.01 vs. mut-OIP5-AS1 + miR-342-3p mimic; ^^P < 0.01 vs. mut-AGR2 + miR-342-3p mimic; &&P < 0.01 vs. NC mimic; ++P < 0.01 vs. NC inhibitor
Overexpression of miR-342-3p suppresses pancreatic cancer cell growth
The expression of miR-342-3p was examined in four pancreatic cancer cell lines. Analysis of qRT-PCR showed that miR-342-3p expression was relatively lower in ASPC-1 and BXPC-3 than that in PANC-1 and Capan-2 (Fig. 4a). Thus, miR-342-3p was overexpressed in ASPC-1 and BXPC-3 cells by transfection with miR-342-3p mimc, and NC mimic was served as negative control. Forty-eight hours after transfection, the transfection efficiency was determined by qRT-PCR as shown in Fig. 4b. CCK-8 analysis revealed that cell proliferation was inhibited by upregulation of miR-342-3p (Fig. 4c). Moreover, immunostaining for Ki67 showed a decrease in Ki67-positive cells following miR-342-3p overexpression (Fig. 3d). Flow cytometric analysis revealed that upregulation of miR-342-3p inhibited cell cycle progression along with higher proportion of cells in G1 phase and lower proportion of cells in S and G2 phases (Fig. 4e). Besides, the expression levels of cyclinD1, CDK4, and CDK6 were significantly decreased in pancreatic cancer cells transfected with miR-342-3p mimics as detected by western blot (Fig. 4f–i).
Overexpression of miR-342-3p suppresses pancreatic cancer cell growth. (a) The expression of miR-342-3p was detected in four pancreatic cancer cell lines including ASPC-1, BXPC-3, PANC-1, and Capan-2. According to PCR screening results, miR-342-3p mimics or negative control (NC) mimics were transfected into ASPC-1 and BXPC-3 cells. (b) RT-PCR analysis of miR-342-3p expression at 48 h post-transfection. (c) Cell proliferation at 0, 24, 48, 72, and 96 h post-transfection was measured by CCK-8 assay. (d) Immunostaining for Ki67 and counterstaining with DAPI in ASPC-1 and BXPC-3 cells after transfection. (e) Flow cytometric analysis of cell cycle distribution following transfection with miR-342-3p mimics or NC controls. (f) Western blotting analysis of the expression of cyclinD1 (g), CDK4 (h), and CDK6 (i). Data were shown as mean ± SD (n = 3) and analyzed by two-way ANOVA or Student’s t test. &&P < 0.01 vs. NC mimic. Scale bar = 50 μm
OIP5-AS1 inhibits pancreatic cancer cell growth via regulation of miR-342-3p
To explore whether OIP5-AS1 functions via regulation of miR-342-3p, OIP5-AS1 siRNAs or NC siNRAs and miR-342-3p inhibitors or NC inhibitors were co-transfected into pancreatic cancer cell lines ASPC-1 and PANC-1. At 48 h post-tranfection, cell proliferation was decreased by knockdown of OIP5-AS1 while increased with further depletion of miR-342-3p (Fig. 5a). Downregulation of miR-342-3p partially counteracted the inhibitory effect of OIP5-AS1 knockdown on AGR2 expression (Fig. 5b). The similar result was observed in immunofluorescence staining for Ki67 (Fig. 5c). Ki67-positive cells were found reduced after transfection with OIP5-AS1 siRNAs, while elevated following co-transfection with OIP5-AS1 siRNAs and miR-342-3p inhibitors. Western blotting analysis showed that the expression levels of cyclinD1, CDK4, CDK6, AGR2, p-AKT, and p-ERK1/2 were decreased by OIP5-AS1 knockdown while increased by silencing both OIP5-AS1 and miR-342-3p (Fig. 5d–l).
OIP5-AS1 inhibits pancreatic cancer cell growth via regulation of miR-342-3p. Pancreatic cancer cell lines, ASPC-1 and PANC-1, were co-transfected with OIP5-AS1 siRNAs or negative control (NC) siRNAs and miR-342-3p inhibitors or NC inhibitors. (a) CCK-8 analysis of cell proliferation at 48 h post-transfection. (b) The expression of AGR2 was detected by RT-PCR. (c) Immunostaining for Ki67 and counterstaining with DAPI in transfected ASPC-1 and PANC-1 cells. The expression of cyclinD1 (d), CDK4 (e), CDK6 (f), AGR2 (g), p-AKT (h), AKT (i), p-ERK1/2 (j), and ERK1/2 (k) was determined by western blot. Data were shown as mean ± SD (n = 3) and analyzed by one-way ANOVA. **P < 0.01 vs. NC siRNA; $P < 0.05 vs. OIP5-AS1 siRNA+NC inhibitor, $$P < 0.01 vs. OIP5-AS1 siRNA+ NC inhibitor. Scale bar = 50 μm
OIP5-AS1 functions through AKT/ERK signaling pathway
We further investigated whether OIP5-AS1 took effects on pancreatic cancer’s malignant behaviors through AKT/ERK signaling. Pancreatic cancer cells were transfected with OIP5-AS1 siRNA followed by treatment with 5 ng/mL FGF-2. FGF-2 is known to induce AKT and ERK phosphorylation [31]. Western blotting analysis showed that OIP5-AS1 silencing-induced inhibition of AKT and ERK phosphorylation was partially reversed by FGF-2 treatment in pancreatic cancer cells (Fig. 6a–b). Moreover, CCK8 assay showed that cell viability was reduced by knockdown of OIP5-AS1 and increased by FGF-2-induced activation of AKT/ERK signaling (Fig. 6c). Flow cytometric analysis revealed that suppression of cell cycle progression induced by OIP5-AS1 siRNA was relieved by FGF-2 treatment (Fig. 6d). These results suggested that OIP5-AS1 regulated pancreatic cancer cell viability and cell cycle through AKT/ERK signaling.
OIP5-AS1 functions through AKT/ERK signaling pathway. To investigate OIP5-AS1 functions through AKT/ERK signaling in regulation of pancreatic cancer’s malignant behaviors, pancreatic cancer cells were transfected with OIP5-AS1 siRNA followed by treatment with 5 ng/mL FGF-2. (a, b) Western blotting analysis of AKT, p-AKT, ERK1/2, and p-ERK1/2 expression in ASPC-1 and PANC-1 pancreatic cancer cells. (c) CCK8 assay for cell viability. (d) Flow cytometric analysis of cell cycle distribution. Data were shown as mean ± SD (n = 3) and analyzed by one-way ANOVA. **P < 0.01 vs. NC siRNA; xP < 0.05 vs. OIP5-AS1 siRNA; xxP < 0.01 vs. OIP5-AS1 siRNA
Downregulation of OIP5-AS1 suppresses xenograft tumor growth in nude mice
To advance study the effects of OIP5-AS1 knockdown on tumor growth in vivo, OIP5-AS1 shRNA plasmids or negative control (NC) shRNA plasmids were intravenously injected into tumor-bearing mice. Tumor volume was measured every third day after injection, and tumor weight at the end of treatment (day 30) was determined. The data showed that downregulation of OIP5-AS1 significantly suppressed tumor growth since day 22 throughout the experiment (Fig. 7a), accompanied by decreased tumor weight (Fig. 7b). Quantitative real-time PCR analysis revealed a downregulation of OIP5-AS1 and an upregulation of miR-342-3p following knockdown of OIP5-AS1 in xenograft pancreatic tumors of nude mice (Fig. 7c–d). Histological analysis of Ki67 revealed that OIP5-AS1 knockdown lowered Ki67 expression in pancreatic tumors, compared with that in controls (Fig. 7e). The expression of cyclinD1, CDK4, CDK6, AGR2, p-AKT, and p-ERK1/2 was significantly decreased by silencing of OIP5-AS1 in pancreatic tumors of nude mice (Fig. 7f–m).
Downregulation of OIP5-AS1 suppresses xenograft tumor growth in nude mice. The tumors were allowed to grow in the nude mice by subcutaneous injection of pancreatic cancer cells into their right armpits. OIP5-AS1 shRNAs or negative control (NC) shRNAs were then intravenously injected into tumor-bearing mice 3 times weekly (a) Tumor volume was measured every third day after injection since day 7. (b) At the end of treatment (day 30), the tumor was excised and its weight was determined. The expression of OIP5-AS1 (c) and miR-342-3p (d) in tumors was measured by RT-PCR. (e) Immunohistochemistry of Ki67 in tumor tissues. The expression of cyclinD1 (f), CDK4 (g), CDK6 (h), AGR2 (i), AKT (j), p-AKT (k), ERK1/2 (l), and p-ERK1/2 (m) was detected by western blot. Data were shown as mean ± SD (n = 6) and analyzed by two-way ANOVA or Student’s t test. %P < 0.05 vs. NC shRNA; %%P < 0.01 vs. NC shRNA. Scale bar = 50 μm
Discussion
Pancreatic cancer is a devastating cancer worldwide, and non-coding RNAs are emerging to be novel biomarkers for diagnosis, prognosis, and therapeutic targets of pancreatic cancer [24]. A study found downregulation of miR-342-3p in high-risk versus low-risk intraductal papillary mucinous neoplasms, which are pancreatic ductal adenocarcinoma precursors [23]. The expression of miR-342-3p was markedly lower in pancreatic tissue than in normal ones [4]. Another study reported that miR-342-3p expression was closely related to postoperative overall survival of pancreatic cancer patients [15]. These previous studies strongly suggested miR-342-3p correlated with pancreatic cancer development. Further, bioinformatic predictive analysis reveals that miR-342-3p is a direct target of lncRNA OIP5-AS1. Besides, studies have showed the oncogenic function of lncRNA OIP5-AS1 in various cancers [16, 29, 35]. However, there is no report of OIP5-AS1 in pancreatic cancer yet. In the present study, OIP5-AS1 was found upregulated and miR-342-3p was downregulated in the tumor tissues of pancreatic cancer patients. Correlation analysis confirmed the negative correlation between OIP5-AS1 and miR-342-3p in pancreatic cancer. Moreover, GEPIA database reveals that pancreatic cancer patients with higher OIP5-AS1 had poor prognosis than that with lower expression. These findings indicated the possible correlation of OIP5-AS1/miR-342-3p and pancreatic cancer progression. Here we further investigated the regulatory mechanism of OIP5-AS1/miR-342-3p in pancreatic cancer. Downregulation of OIP5-AS1 or overexpression of miR-342-3p inhibited the proliferation and induced cell cycle arrest in pancreatic cancer cells, and suppress tumor growth in vivo. OIP5-AS1 was identified as miR-342-3p sponge and negatively regulated its expression. Depletion of miR-342-3p reversed the inhibitory effects of OIP5-AS1 knockdown on pancreatic cancer cell growth. Thus, OIP5-AS1 promotes pancreatic cancer cell growth via sponging miR-342-3p.
Increased cell proliferation and decreased cell death are features of malignant cell growth in cancer. It is reported that OIP5-AS1 positively regulates cell proliferation and its silencing induces cell cycle arrest [21]. Knockdown of OIP5-AS1 inhibited cell proliferation and malignancy processes [42, 44]. In accordance with these data, we found that downregulation of OIP5-AS1 inhibited pancreatic cancer cell proliferation along with decreased expression of Ki67, a proliferation marker. Besides, cell proliferation is regulated by cell cycle activators and repressors. Cyclin-dependent-kinases (CDK) and cyclins are regulators of cell cycle [7]. CyclinD1, CDK4, and CDK6 are considered as members involved in cell cycle regulatory pathway. Inhibition of CDK4/CDK6 induced cell cycle arrest [25]. The results showed that OIP5-AS1 suppression blocked cell cycle progression at G1/S phase in pancreatic cancer cells. Western blotting analysis showed that the expression of cyclinD1, CDK4, and CDK6 was decreased in pancreatic cancer cells with OIP5-AS1 knockdown. Moreover, depletion of OIP5-AS1 inhibited pancreatic tumor growth in nude mice, accompanied with a decrease in the expression of Ki67, cyclinD1, CDK4, and CDK6. Therefore, OIP5-AS1 appears to function as an oncogene and its inhibition suppressed cell proliferation and cell cycle progression in pancreatic cancer.
The crosstalk between lncRNAs and miRNAs has been demonstrated to play an important role during the pathogenesis of cancer [1]. LncRNAs could serve as miRNA sponge that suppress its expression and function and thus de-repress their targeting mRNAs [6, 22]. For example, Cheng et al. [4] showed that lncRNA SNHG7 promoted cell proliferation by regulating ID4 via sponging miR-342-3p in pancreatic cancer. Xu et al. [38] revealed that lncRNA DLEU2 enhanced the proliferation and invasion of pancreatic cancer cells through acting as a miR-455 sponge to modulate SMAD2 expression. In the present study, the expression of miR-342-3p was found to be negatively correlated with OIP5-AS1 expression in pancreatic cancer. Based on bioinformatics predictive analysis, we hypothesized that lncRNA OIP5-AS1 might function as a sponge of miR-342-3p to negatively regulate its expression. To explore the underlying regulatory mechanism involving the tumor promoting role of OIP5-AS1 in pancreatic cancer, the possible binding activity of OIP5-AS1 and miR-342-3p was identified by dual-luciferase assay. Functional assay showed that inhibition of miR-342-3p abolished the inhibitory effects of OIP5-AS1 knockdown on pancreatic cancer cell growth. These findings demonstrate that OIP5-AS1 promotes cell proliferation via sponging miR-342-3p in pancreatic cancer.
MiRNAs are known to play a key role in regulation of gene expression at posttranscriptional level, and their abnormalities are considered as cancer hallmarks [34]. Studies have verified that miR-342-3p exerts tumor suppressive function to inhibit tumor growth in multiple human tumors. Xie et al. [36] found that miR-342-3p suppressed non-small cell lung cancer cell proliferation and invasion. A recent study showed that miR-342-3p acted as tumor suppressor to inhibit cell proliferation in liver cancer [45]. The expression miR-342-3p expression was observed to be decreased in hepatocellular carcinoma tissues, and patients with high miR-342-3p expression showed better overall survival [10]. In this study, miR-342-3p expression was found downregulated in human pancreatic cancer tissues versus normal tissues. In line with previous studies, the tumor suppressive role of miR-342-3p was evidenced in pancreatic cancer. The results showed that overexpression of miR-342-3p exerted inhibitory effects on cell proliferation and cell cycle progression in pancreatic cancer cells, accompanied with a decrease in the expression of proliferation-associated proteins including Ki67, cyclinD1, CDK4, and CDK6. It is demonstrated that AGR2 is overexpressed in multiple human cancers and contributes to cancer malignant behaviors [32, 37]. A previous study has showed that AGR2 is involved in pancreatic cancer initiation [9]. Cell apoptosis was induced by AGR2 knockdown in pancreatic cancer cells [19]. In the present study, miR-342-3p negatively regulated AGR2 expression in vitro and in vivo, suggesting that miR-342-3p might suppress pancreatic cancer progression via targeting AGR2. Xue et al. demonstrated miR-342-3p/AGR2 in suppression of cell proliferation and migration in non-small cell lung cancer [39], which was in accordance with our results. Taken together, these results suggest an OIP5-AS1/miR-342-3p/AGR2 axis in regulation of cell proliferation in pancreatic cancer.
AKT and ERK signaling pathways have been demonstrated to play an important role in regulating tumor progression, metastasis, and chemoresistance in human cancers [41]. A study showed that non-small cell lung cancer cell malignancies including proliferation, invasion, and migration capacity were inhibited via downregulating AKT/ERK pathway [2]. In the present study, as verified by western blotting, the phosphorylation of p-AKT and p-ERK1/2 was inhibited by OIP5-AS1 knockdown in pancreatic cancer cells, while reversed by FGF-2 treatment. Further, FGF-2-induced phosphorylation of p-AKT and p-ERK1/2 partially counteracted OIP5-AS1 silencing-induced suppression of cell viability and cell cycle progression in pancreatic cancer cells. Inhibition of AKT/ERK signaling resulted from knockdown of OIP5-AS1 was validated in vivo accompanied with reduced tumor proliferation. These findings indicated that OIP5-AS1 might exert its functions in regulation of pancreatic cancer’s malignant behaviors through activation of AKT/ERK signaling. Our results were in agreement with previous studies that pancreatic cancer cell proliferation is mediated by both AKT and ERK signaling [18, 28]. As discussed above, the oncogenic role of OIP5-AS1 in pancreatic cancer has been demonstrated in vitro and in vivo via functional experiments. Therefore, we suggest that OIP5-AS1/miR-342-3p axis may involve in tumor progression via AKT/ERK signaling pathway in pancreatic cancer.
Conclusions
The present study demonstrates that downregulation of lncRNA OIP5-AS1 inhibited pancreatic cancer growth by sponging miR-342-3p via targeting AGR2 through AKT/ERK signaling pathway. Therefore, we suggest that OIP5-AS1 could be a potential therapeutic target for pancreatic cancer and the crosstalk between OIP5-AS1 and miR-342-3p as well as miR-342-3p and AGR2 provides better understanding of pathogenesis of pancreatic cancer as well the novel treatment of the disease.
References
Abdollahzadeh R, Daraei A, Mansoori Y, Sepahvand M, Amoli MM, Tavakkoly-Bazzaz J (2019) Competing endogenous RNA (ceRNA) cross talk and language in ceRNA regulatory networks: a new look at hallmarks of breast cancer. J Cell Physiol 234(7):10080–10100. https://doi.org/10.1002/jcp.27941
Chang L, Fang S, Chen Y, Yang Z, Yuan Y, Zhang J, Ye L, Gu W (2019) Inhibition of FASN suppresses the malignant biological behavior of non-small cell lung cancer cells via deregulating glucose metabolism and AKT/ERK pathway. Lipids Health Dis 18(1):118–110. https://doi.org/10.1186/s12944-019-1058-8
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J (2016) Cancer statistics in China, 2015. CA Cancer J Clin 66(2):115–132. https://doi.org/10.3322/caac.21338
Cheng D, Fan J, Ma Y, Zhou Y, Qin K, Shi M, Yang J (2019) LncRNA SNHG7 promotes pancreatic cancer proliferation through ID4 by sponging miR-342-3p. Cell Biosci 9:28. https://doi.org/10.1186/s13578-019-0290-2
Dai J, Xu L, Hu X, Han G, Jiang H, Sun H, Zhu G, Tang X (2018) Long noncoding RNA OIP5-AS1 accelerates CDK14 expression to promote osteosarcoma tumorigenesis via targeting miR-223. Biomed Pharmacother 106:1441–1447. https://doi.org/10.1016/j.biopha.2018.07.109
Denzler R, McGeary SE, Title AC, Agarwal V, Bartel DP, Stoffel M (2016) Impact of microRNA levels, target-site complementarity, and cooperativity on competing endogenous RNA-regulated gene expression. Mol Cell 64(3):565–579. https://doi.org/10.1016/j.molcel.2016.09.027
Draney C, Austin MC, Leifer AH, Smith CJ, Kener KB, Aitken TJ, Hess KH, Haines AC, Lett LA, Hernandez-Carretero A, Fueger PT, Arlotto M, Tessem JS (2018) HDAC1 overexpression enhances beta-cell proliferation by down-regulating Cdkn1b/p27. Biochem J 475(24):3997–4010. https://doi.org/10.1042/bcj20180465
Dreyer SB, Chang DK, Bailey P, Biankin AV (2017) Pancreatic cancer genomes: implications for clinical management and therapeutic development. Clin Cancer Res 23(7):1638–1646. https://doi.org/10.1158/1078-0432.ccr-16-2411
Dumartin L, Alrawashdeh W, Trabulo SM, Radon TP, Steiger K, Feakins RM, di Magliano MP, Heeschen C, Esposito I, Lemoine NR, Crnogorac-Jurcevic T (2017) ER stress protein AGR2 precedes and is involved in the regulation of pancreatic cancer initiation. Oncogene 36(22):3094–3103. https://doi.org/10.1038/onc.2016.459
Gao Y, Zhang SG, Wang ZH, Liao JC (2017) Down-regulation of miR-342-3p in hepatocellular carcinoma tissues and its prognostic significance. Eur Rev Med Pharmacol Sci 21(9):2098–2102
Greenblatt MB, Park KH, Oh H, Kim JM, Shin DY, Lee JM, Lee JW, Singh A, Lee KY, Hu D, Xiao C, Charles JF, Penninger JM, Lotinun S, Baron R, Ghosh S, Shim JH (2015) CHMP5 controls bone turnover rates by dampening NF-kappaB activity in osteoclasts. J Exp Med 212(8):1283–1301. https://doi.org/10.1084/jem.20150407
Iannotti FA, Pagano E, Guardiola O, Adinolfi S, Saccone V, Consalvi S, Piscitelli F, Gazzerro E, Busetto G, Carrella D, Capasso R, Puri PL, Minchiotti G, Di Marzo V (2018) Genetic and pharmacological regulation of the endocannabinoid CB1 receptor in Duchenne muscular dystrophy. Nat Commun 9(1):3950. https://doi.org/10.1038/s41467-018-06267-1
Kim J, Abdelmohsen K, Yang X, De S, Grammatikakis I, Noh JH, Gorospe M (2016) LncRNA OIP5-AS1/cyrano sponges RNA-binding protein HuR. Nucleic Acids Res 44(5):2378–2392. https://doi.org/10.1093/nar/gkw017
Klinge CM (2018) Non-coding RNAs in breast Cancer: intracellular and intercellular communication. Non-coding RNA 4(4). https://doi.org/10.3390/ncrna4040040
Lee KH, Lee JK, Choi DW, Do IG, Sohn I, Jang KT, Jung SH, Heo JS, Choi SH, Lee KT (2015) Postoperative prognosis prediction of pancreatic cancer with seven microRNAs. Pancreas 44(5):764–768. https://doi.org/10.1097/mpa.0000000000000346
Li M, Ning J, Li Z, Fei Q, Zhao C, Ge Y, Wang L (2019) Long noncoding RNA OIP5-AS1 promotes the progression of oral squamous cell carcinoma via regulating miR-338-3p/NRP1 axis. Biomed Pharmacother 118:109259. https://doi.org/10.1016/j.biopha.2019.109259
Lin QJ, Yang F, Jin C, Fu DL (2015) Current status and progress of pancreatic cancer in China. World J Gastroenterol 21(26):7988–8003. https://doi.org/10.3748/wjg.v21.i26.7988
Liu Y, Bi T, Wang G, Dai W, Wu G, Qian L, Gao Q, Shen G (2015) Lupeol inhibits proliferation and induces apoptosis of human pancreatic cancer PCNA-1 cells through AKT/ERK pathways. Naunyn Schmiedeberg's Arch Pharmacol 388(3):295–304. https://doi.org/10.1007/s00210-014-1071-4
Liu QG, Li YJ, Yao L (2018) Knockdown of AGR2 induces cell apoptosis and reduces chemotherapy resistance of pancreatic cancer cells with the involvement of ERK/AKT axis. Pancreatology 18(6):678–688. https://doi.org/10.1016/j.pan.2018.07.003
Liu W, Kang L, Han J, Wang Y, Shen C, Yan Z, Tai Y, Zhao C (2018) miR-342-3p suppresses hepatocellular carcinoma proliferation through inhibition of IGF-1R-mediated Warburg effect. OncoTargets Therapy 11:1643–1653. https://doi.org/10.2147/ott.s161586
Naemura M, Kuroki M, Tsunoda T, Arikawa N, Sawata Y, Shirasawa S, Kotake Y (2018) The long noncoding RNA OIP5-AS1 is involved in the regulation of cell proliferation. Anticancer Res 38(1):77–81. https://doi.org/10.21873/anticanres.12194
Paraskevopoulou MD, Hatzigeorgiou AG (2016) Analyzing MiRNA-LncRNA interactions. Methods Mol Biol (Clifton, NJ) 1402:271–286. https://doi.org/10.1007/978-1-4939-3378-5_21
Permuth-Wey J, Chen YA, Fisher K, McCarthy S, Qu X, Lloyd MC, Kasprzak A, Fournier M, Williams VL, Ghia KM, Yoder SJ, Hall L, Georgeades C, Olaoye F, Husain K, Springett GM, Chen DT, Yeatman T, Centeno BA, Klapman J, Coppola D, Malafa M (2015) A genome-wide investigation of microRNA expression identifies biologically-meaningful microRNAs that distinguish between high-risk and low-risk intraductal papillary mucinous neoplasms of the pancreas. PLoS One 10(1):e0116869. https://doi.org/10.1371/journal.pone.0116869
Previdi MC, Carotenuto P, Zito D, Pandolfo R, Braconi C (2017) Noncoding RNAs as novel biomarkers in pancreatic cancer: what do we know? Future Oncol (London, England) 13(5):443–453. https://doi.org/10.2217/fon-2016-0253
Rader J, Russell MR, Hart LS, Nakazawa MS, Belcastro LT, Martinez D, Li Y, Carpenter EL, Attiyeh EF, Diskin SJ, Kim S, Parasuraman S, Caponigro G, Schnepp RW, Wood AC, Pawel B, Cole KA, Maris JM (2013) Dual CDK4/CDK6 inhibition induces cell-cycle arrest and senescence in neuroblastoma. Clin Cancer Res 19(22):6173–6182. https://doi.org/10.1158/1078-0432.ccr-13-1675
Romero-Cordoba SL, Rodriguez-Cuevas S, Bautista-Pina V, Maffuz-Aziz A, D'Ippolito E, Cosentino G, Baroni S, Iorio MV, Hidalgo-Miranda A (2018) Loss of function of miR-342-3p results in MCT1 over-expression and contributes to oncogenic metabolic reprogramming in triple negative breast cancer. Sci Rep 8(1):12252. https://doi.org/10.1038/s41598-018-29708-9
Rynkeviciene R, Simiene J, Strainiene E, Stankevicius V, Usinskiene J, Miseikyte Kaubriene E, Meskinyte I, Cicenas J, Suziedelis K (2018) Non-coding RNAs in Glioma. Cancers 11(1). https://doi.org/10.3390/cancers11010017
Shen X, Artinyan A, Jackson D, Thomas RM, Lowy AM, Kim J (2010) Chemokine receptor CXCR4 enhances proliferation in pancreatic cancer cells through AKT and ERK dependent pathways. Pancreas 39(1):81–87. https://doi.org/10.1097/MPA.0b013e3181bb2ab7
Song L, Wang L, Pan X, Yang C (2020) lncRNA OIP5-AS1 targets ROCK1 to promote cell proliferation and inhibit cell apoptosis through a mechanism involving miR-143-3p in cervical cancer. Braz J Med Biol Res 53(1):e8883. https://doi.org/10.1590/1414-431x20198883
Steeg PS (2016) Targeting metastasis. Nat Rev Cancer 16(4):201–218. https://doi.org/10.1038/nrc.2016.25
Sun D, Liu Y, Yu Q, Zhou Y, Zhang R, Chen X, Hong A, Liu J (2013) The effects of luminescent ruthenium(II) polypyridyl functionalized selenium nanoparticles on bFGF-induced angiogenesis and AKT/ERK signaling. Biomaterials 34(1):171–180. https://doi.org/10.1016/j.biomaterials.2012.09.031
Tian S, Hu J, Tao K, Wang J, Chu Y, Li J, Liu Z, Ding X, Xu L, Li Q, Cai M, Gao J, Shuai X, Wang G, Wang L, Wang Z (2018) Secreted AGR2 promotes invasion of colorectal cancer cells via Wnt11-mediated non-canonical Wnt signaling. Exp Cell Res 364(2):198–207. https://doi.org/10.1016/j.yexcr.2018.02.004
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics, 2012. CA Cancer J Clin 65(2):87–108. https://doi.org/10.3322/caac.21262
Van Roosbroeck K, Calin GA (2017) Cancer hallmarks and microRNAs: the therapeutic connection. Adv Cancer Res 135:119–149. https://doi.org/10.1016/bs.acr.2017.06.002
Wang LW, Li XB, Liu Z, Zhao LH, Wang Y, Yue L (2019) Long non-coding RNA OIP5-AS1 promotes proliferation of gastric cancer cells by targeting miR-641. Eur Rev Med Pharmacol Sci 23(24):10776–10784. https://doi.org/10.26355/eurrev_201912_19780
Xie X, Liu H, Wang M, Ding F, Xiao H, Hu F, Hu R, Mei J (2015) miR-342-3p targets RAP2B to suppress proliferation and invasion of non-small cell lung cancer cells. Tumour Biol 36(7):5031–5038. https://doi.org/10.1007/s13277-015-3154-3
Xiu B, Chi Y, Liu L, Chi W, Zhang Q, Chen J, Guo R, Si J, Li L, Xue J, Shao ZM, Wu ZH, Huang S, Wu J (2019) LINC02273 drives breast cancer metastasis by epigenetically increasing AGR2 transcription. Mol Cancer 18(1):187. https://doi.org/10.1186/s12943-019-1115-y
Xu B, Gong X, Zi L, Li G, Dong S, Chen X, Li Y (2019) Silencing of DLEU2 suppresses pancreatic cancer cell proliferation and invasion by upregulating microRNA-455. Cancer Sci 110(5):1676–1685. https://doi.org/10.1111/cas.13987
Xue X, Fei X, Hou W, Zhang Y, Liu L, Hu R (2018) miR-342-3p suppresses cell proliferation and migration by targeting AGR2 in non-small cell lung cancer. Cancer Lett 412:170–178. https://doi.org/10.1016/j.canlet.2017.10.024
Yang J, Jiang B, Hai J, Duan S, Dong X, Chen C (2019) Long noncoding RNA opa-interacting protein 5 antisense transcript 1 promotes proliferation and invasion through elevating integrin alpha6 expression by sponging miR-143-3p in cervical cancer. J Cell Biochem 120(1):907–916. https://doi.org/10.1002/jcb.27454
Ye L, Pu C, Tang J, Wang Y, Wang C, Qiu Z, Xiang T, Zhang Y, Peng W (2019) Transmembrane-4 L-six family member-1 (TM4SF1) promotes non-small cell lung cancer proliferation, invasion and chemo-resistance through regulating the DDR1/Akt/ERK-mTOR axis. Respir Res 20(1):106. https://doi.org/10.1186/s12931-019-1071-5
Zeng H, Wang J, Chen T, Zhang K, Chen J, Wang L, Li H, Tuluhong D, Li J, Wang S (2019) Downregulation of long non-coding RNA Opa interacting protein 5-antisense RNA 1 inhibits breast cancer progression by targeting sex-determining region Y-box 2 by microRNA-129-5p upregulation. Cancer Sci 110(1):289–302. https://doi.org/10.1111/cas.13879
Zhang W, Bi Y, Li J, Peng F, Li H, Li C, Wang L, Ren F, Xie C, Wang P, Liang W, Wang Z, Zhu D (2017) Long noncoding RNA FTX is upregulated in gliomas and promotes proliferation and invasion of glioma cells by negatively regulating miR-342-3p. Lab Investig 97(4):447–457. https://doi.org/10.1038/labinvest.2016.152
Zhang Z, Liu F, Yang F, Liu Y (2018) Kockdown of OIP5-AS1 expression inhibits proliferation, metastasis and EMT progress in hepatoblastoma cells through up-regulating miR-186a-5p and down-regulating ZEB1. Biomed Pharmacother 101:14–23. https://doi.org/10.1016/j.biopha.2018.02.026
Zhao L, Zhang Y (2015) miR-342-3p affects hepatocellular carcinoma cell proliferation via regulating NF-kappaB pathway. Biochem Biophys Res Commun 457(3):370–377. https://doi.org/10.1016/j.bbrc.2014.12.119
Funding
This study was supported by a grant from the Foundation of Department of Science and Technology, Liaoning Province (No. CA19).
Author information
Authors and Affiliations
Contributions
Zhen Liu contributed to the conception of the study; Jia Ma and Baosheng Wang performed the experiments; Xin Wu performed the data analysis; Xiangpeng Meng drafted and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with ethical standards of Shengjing Hospital of China Medical University and with the Declaration of Helsinki and its later amendments or comparable ethical standards. All animal experiments were approved by Institutional Animal Care and Use Committee and performed in accordance with Guide for the Care and Use of Laboratory Animals.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
None
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
•OIP5-AS1 exerts oncogenic function in pancreatic cancer.
•miR-342-3p inhibits the progression of pancreatic cancer.
•OIP5-AS1 acts as a sponge of miR-342-3p in pancreatic cancer.
•AGR2 is a direct target gene of miR-342-3p.
•OIP5-AS1 contributes to pancreatic cancer progression via AKT and ERK pathways.
Electronic supplementary material
ESM 1
(PNG 40 kb)
Rights and permissions
About this article
Cite this article
Meng, X., Ma, J., Wang, B. et al. Long non-coding RNA OIP5-AS1 promotes pancreatic cancer cell growth through sponging miR-342-3p via AKT/ERK signaling pathway. J Physiol Biochem 76, 301–315 (2020). https://doi.org/10.1007/s13105-020-00734-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-020-00734-4