Abstract
The balance of pro-angiogenic and anti-angiogenic factors has a significant role in the development of diabetic cardiomyopathy. The purpose of this study was to examine the effect of 8 weeks of high-intensity interval training (HIIT) and moderate intensity continuous training (MICT) on the myocardial angiogenic factors and histological changes in male diabetic rats. Thirty-two male Wistar rats were randomly assigned into four groups: healthy non-exercised control, diabetic (D), D + HIIT, and D+ MICT groups. Diabetes type 2 was induced by a high-fat diet for 2 weeks and a single injection of streptozotocin. Following confirmation of diabetes, animals were subjected to HIIT (90 to 95% of VO2max) or MICT (50–65% of VO2max) protocols 5 days a week for 8 weeks. Western blotting was used for detection of protein expressions of vascular endothelial growth factor (VEGF), transforming growth factor-beta (TGF-β), matrix metalloproteinase-2 (MMP2), and tissue inhibitor of matrix metalloproteinase-2 (TIMP2) in the left ventricle. In addition, baseline and final blood glucose and body weight were measured. Histological changes were evaluated using H&E and Masson’s trichrome staining. The results showed that exercise increased protein levels of pro-angiogenic factors while reduced anti-angiogenic factors protein levels in diabetic animals. These changes were followed by increased capillary density and reduced interstitial fibrosis in the left ventricle. Moreover, the MICT was superior to HIIT in enhancing angiogenic factors and attenuation of blood glucose and fibrosis in the diabetic rats. These findings confirm the effectiveness of exercise, particularly MICT, in the improvement of diabetic cardiomyopathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes is a common metabolic disorder characterized by high blood glucose owing to a deficiency in insulin secretion, resistance to insulin, or both. Type2 diabetes mellitus (T2DM) is a highly prevalent disease worldwide, and estimated numbers show that 642 million people worldwide will suffer from T2DM by 2040 [1].
Diabetes is associated with several complications, such as neuropathy, nephropathy, retinopathy, and cardiovascular dysfunction. Cardiovascular disorders are a significant cause of death related to this disease [24]. Diabetic cardiomyopathy (DCM) is characterized by the morphological, functional, and metabolic changes in the heart produced as a complication of T2DM. This cardiac disorder is characterized by constant high blood glucose and lipids levels, which eventually generate oxidative stress, poor calcium handling, altered mitochondrial function, inflammation, and fibrosis [11]. Moreover, biological mechanisms linked to diabetes play an independent role in increasing the risk of DCM and myocardial infarction by inducing severe metabolic dysfunction [52].
DCM is associated with microvascular insufficiency and an impaired angiogenic response to chronic ischemia, decreasing myocardium perfusion, which finally contributes to tissue hypoxia, interstitial fibrosis, and heart failure [6, 22].
Myocardial angiogenesis is typically defined as the growth of new blood vessels from pre-existing vessels, leading to an improvement in myocardial perfusion. Angiogenesis is a multiple step process that includes activation of vascular endothelial cells by angiogenic factors: migration of endothelial cells, degradation of the extracellular matrix, and differentiation of endothelial cells into microvessels [8, 42].
Vascular endothelial growth factor (VEGF), the major pro-angiogenesis factor, lies in the center of the regulatory network controlling angiogenesis in both physiological and pathological settings [45]. Matrix metalloproteinases (MMPs) are required for the turnover of the extracellular matrix, degrading of connective tissue, and angiogenesis. Tissue inhibitors of metalloproteinases (TIMPs) inhibit protease activity of MMPs by binding to the catalytic site of activated MMPs [4]. On the other hand, transforming growth factor beta (TGF-β) can either stimulate or inhibit angiogenesis, depending on the tissue context and its expression levels [37, 47]. TGF-β is a potent inhibitor of angiogenesis with profibrotic property at high concentration by inhibiting endothelial cell proliferation and migration [3, 14, 18]. In diabetes high glucose levels could increase TGF-β expression levels, eventually resulting in cardiac fibrosis [51].
Physical activity is considered a method for improving metabolism [40]. Regular exercise increases glucose metabolism through an insulin-independent pathway, resulting in the enhancement of oxidative capacity in the muscle, and constitutes the most effective stimulus for improvement of cardiovascular metabolism [54]. Furthermore, in diabetic patients, regular exercise can prevent hypoglycemia, hyperglycemia, ketosis, and diabetes-associated complications [30, 46].
Positive effects of physical activity on endothelial function and cardiovascular system have been reported by several studies [43]. Exercise has also been shown to enhance vascular repair and angiogenesis, by decreasing the production of reactive oxygen species and pro-inflammatory cytokines, as well as improving the activation of VEGF pathway and the function of epithelial cells [13, 15, 19, 29, 48]. Additionally, tissue hypoxia by exercise promotes the formation of vascular sprouts composed of migratory tip cells and proliferating stalk cells and hence angiogenesis [53].
This study aimed to investigate the effect of two exercise protocols, high-intensity interval training (HIIT), and moderate intensity contentious training (MICT), on the expression of pro-angiogenic and anti-angiogenic factors, as well as histological changes in the heart tissue of T2DM rat model.
Materials and methods
Animals
Thirty-two male Wistar rats, weighing 180 ± 20 g, about 12-week age, were purchased from Pasteur Institute (Tehran, Iran). Animals were kept, four per cage in polyethylene cages (25*25*40 cm) with metal doors in the constant temperature of 24 ± 4 °C and humidity of 50–60% on a 12/12 light/dark cycle, with free access to food and water. All procedures of the current study were carried out according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH publication 86–23 revised 1985) and approved by the Ethical Committee of Tabriz University (EC-00212).
Following 1 week of adaptation to the laboratory condition, animals were randomly divided into two groups, including control (n = 8) and diabetic (n = 24) groups. For induction of diabetes, rats were fed a high-fat diet (HFD) (58% fat, 25%protein, 17% carbohydrate as a percentage of total kcal) were provided with commercially available rat pellet diet for a period of 2 weeks, and then were received intraperitoneal injection of 35 mg/kg streptozotocin (STZ, Sigma-Aldrich, American) dissolved in citrate buffer (0.1 M) with pH = 4.5 [28]. However, the control animals were fed a normal diet. Three days after STZ injection, blood samples were taken from the tail vein, and hyperglycemic status was assessed using a glucometer (Roche, Germany), and animals with blood glucose levels higher than 250 mg/dl were considered as diabetic animals [41]. After confirmation of diabetes, animals in the diabetic group were subdivided into three subgroups (n = 8/group) as follows: diabetic (D), D + HIIT, and D + MICT groups. Diabetic animals did not receive any exercise program, while HIIT and MICT groups were subjected to their respective exercise training protocols for 8 consecutive weeks.
Training programs
HIIT and MICT protocols
Animals were adapted to treadmill exercise for 2 weeks, gradually increasing from 10 to 30 min/day. An 8-week exercise protocol began, following this adaptation period. The rats in the exercise groups were subjected to either MICT or HIIT 5 days per week. The HIIT protocol included 10 bouts of 2 min high intensity running on a treadmill with 90% of VO2max with 60-s rest at a speed of 20 m/min in the first week, and the speed was gradually increased to 30 m/min in the 8th week (no slope). Warming and cooling down time was 5 min. The MICT group was performed at a constant running speed corresponding to 50–60% of maximal oxygen uptake (VO2max) throughout the training session [30]. Rat in the control and D groups were not subjected to any additional exercise. Running intensity for each protocol was based on a previous report outlining the association between running speed and VO2max.
Exercise capability was measured prior to and following the exercise training. The method was similar to that previously described by Moreira et al. [36]. Briefly, the rats ran on a graded treadmill at 15° inclination at an initial speed of 6 m/min. The speed was subsequently increased by 3 m/min every 3 min until the rats were unable to run. The total distance run by each rat was considered as exercise capability.
Sampling
Two days after the last training session and following 12 h of fasting, the rats were anesthetized by intraperitoneal injection of xylazine (10 mg/kg) and ketamine (100 mg/kg). Animals were sacrificed, and left ventricle tissues were removed. Tissue samples were washed with cold normal saline and immediately frozen in liquid nitrogen and stored at − 80 °C for further analysis [7].
Western blotting
The frozen left ventricle tissues were lysed in 1 ml of RIPA Lysis buffer containing 1% protease inhibitor (Sigma) and centrifuged at 13,000 rpm for 15 min, and then the supernatant was collected. The concentration of proteins in the acquired supernatant was determined using the Bradford assay kit. Subsequently, an equal amount of protein (60 μg) was separated on 10% polyacrylamide gel under reduced condition and transferred onto the PVDF membrane (Roche, UK). Next, the membrane was incubated overnight with primary antibodies including at 4 °C. measurement of biomarkers of angiogenesis consist of VEGF, TGF-β, pro-MMP2, cleaved MMP2, and TIMP2 in the heart homogenate were determined by western blotting assay [34, 39]. The membrane was washed three times with PBS then incubated with horseradish peroxidase-conjugated (HRP) goat anti-rabbit IgG secondary antibody for 1.5 h with shaking at room temperature. Enhanced chemiluminescence (ECL) detection kit was used for detection of antigen-antibody complexes. Images of the protein bands were obtained and calculated using Image J 1.62 software [16].
Histological analysis
Heart tissue samples were immersed in 10% buffered formalin in 0.1 M PBS for 1 week at 4 °C then washed in PBS, dehydrated in a series of graded ethanol, cleared in xylene, and embedded in paraffin wax. Paraffin-embedded tissues were cut at 5 μm thick transverse sections using a microtome and then mounted on slides. Next, slides were stained with Hematoxylin-Eosin (H&E) for detection of capillary density or Masson’s trichrome for detection of collagen deposit based on standard protocols. Three random sections from each animal were photographed through a 20x objective lens, using a light microscope (Leica, Germany), and histological changes were determined using Image J software [49].
Results
Effect of HIIT and MICT on protein expression of TGF-β and VEGF in diabetic animals
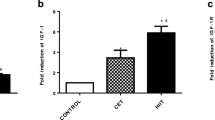
The results of one-way ANOVA showed a significant increase in the protein expression of TGF-β (p < 0.001, Fig. 1B) and a significant decrease in protein expression of VEGF (p < 0.001, Fig. 1C) in the left cardiac ventricle of diabetic animals compared to the control rats. However, HIIT and MICT protocols markedly down-regulated TGF-β protein expression, while up-regulated VEGF protein levels as compared to the D group. Meanwhile, MICT was greater than HIIT protocol.
Effects of MICT and HIIT protocols on the protein expressions of TGF-β, an anti-angiogenic protein, and VEGF, a pro-angiogenic protein, in the experimental groups. (A) Protein expression of TGF-β, VEGF, and β-Actin. Densitometric analysis of B) TGF-β and C) VEGF proteins in different groups. The data were normalized to the control group and expressed as the mean ± SEM (n = 3/group). ***p < 0.001 vs. control (C) group. #p < 0.05, ##p < 0.01, ###p < 0.001 vs. diabetic (D) animals. HIIT: high-intensity interval training, MICT: moderate-intensity continuous training
Effect of HIIT and MICT on protein expression of TIMP2, pro- and cleaved MMP2 protein levels in diabetic rats
The results of western blotting also revealed that diabetic animals had higher levels of TIMP2 (p < 0.01) protein and lower levels of pro-MMP2 and cleaved MMP2 (p < 0.01 for both) proteins than control rats (Fig. 2). Nevertheless, MICT significantly decreased TIPM2 (p < 0.05) levels while increased pro-MMP2 (p < 0.001) and cleaved MMP2 (p < 0.05) in the diabetic animals. However, HIIT only significantly increased pro-MMP2 (p < 0.01) in the D + HIIT group compared to the D group.
Effects of MICT and HIIT protocols on the protein expressions of TIMP2, pr-MMP2, and cleaved MMP2 in the experimental groups. (A) Protein expressions of TIMP2, pr-MMP2, cleaved MMP2, and β-Actin. Densitometric analysis of B) TIMP2, C) pro-MMP2, and D) cleaved MMP2 protein levels in different groups. The data were normalized to the control group and expressed as the mean ± SEM (n = 3/group). **p < 0.01 vs. control (C) group. #p < 0.05, ##p < 0.01, ###p < 0.001 vs. diabetic (D) animals. HIIT: high-intensity interval training, MICT: moderate-intensity continuous training
Effect of HIIT and MICT on histological changes in the left cardiac ventricle
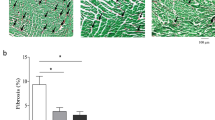
At the microscopic level, diabetic animals showed a significant collagen deposit in the left cardiac tissue (Fig. 3). Moreover, there was a significant increase in cardiac fiber cross-sectional area and a decrease in capillary density in the D group (Fig. 4) compared to the C group. However, MICT protocol obviously reversed these changes and decreased cardiac fibrosis.
Histopathological analysis of the left cardiac ventricular tissue. A) Photomicrographs of the left cardiac ventricle sections stained with Masson trichrome in the experimental groups. The image above shows the appearance of collagen deposit (20x. Scale bar: 100 μm). C) Quantification of interstitial fibrosis in different groups. The data are expressed as the mean ± SEM (n = 5/group). ***p < 0.01 vs. control (C) group. ###p < 0.001 vs. diabetic (D) animals. HIIT: high-intensity interval training, MICT: moderate-intensity continuous training
A) Photomicrographs of the left cardiac ventricle sections stained with Hemotoxylin & Eosin (H&E). The arrows represent the capillary density with a magnification of 20 and 100 μm. B) Quantification of cross-sectional area in different groups. The data are expressed as the mean ± SEM (n = 5/group). ***p < 0.01 vs. control (C) group. ###p < 0.001 vs. diabetic (D) animals. HIIT: high-intensity interval training, MICT: moderate-intensity continuous training
Effect of HIIT and MICT exercise on body weight changes and blood glucose levels in diabetic animals
We also investigated the body weight changes at baseline, 4th and 8th week in the experimental groups. The result showed no significant difference in the initial body weight among different groups. The control animals progressively gained weight from the beginning to the end of the experiment, while D + HIIT and D + MICT groups experienced a slight weight gain during the 4th week, and then showed weight loss during the 8th week. However, diabetic animals showed lower weight gain than the control group and at the 8th weight displayed weight loss (Table 1).
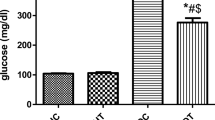
Moreover, the biochemical analysis showed no significant difference in baseline blood glucose among groups. However, following diabetes induction, diabetic animal displayed a higher blood glucose levels than the control group. Although HIIT slightly decreased blood glucose, there was no significant difference between D and D + HIIT groups. However, MICT could significantly decline blood glucose in diabetic rats (Table 2).
Discussions
The results of the present study showed that diabetes induction decreased pro-angiogenic factors (VEGF and cleaved MMP2 levels) and increased anti-angiogenic factors TGF-β and TIMP2 levels), and consequently induced fibrosis and decreased capillary density in the left ventricle. However, exercise trainings, particularly MICT protocol, reversed all these changes.
Recent studies have focused on therapeutic angiogenesis as a therapeutic strategy for the management of diabetes-associated cardiovascular diseases [5]. A growing body of evidence has shown that exercise training is a non-pharmacological strategy for promoting cardiovascular health and decreases myocardial fibrosis in diabetic model [54].
VEGF critically contributes to the blood vessel formation and angiogenesis. Lack of VEGF isoforms is associated with impairment of myocardial angiogenesis and severe left ventricular dysfunction [9]. Several preclinical and clinical studies have reported decreased myocardial VEGF levels in diabetic animal models or diabetic patients, compromising microvascular homeostasis in the myocardium and attenuation of capillary density [10, 26, 28, 35]. Therefore, restoration of VEGF expression may improve microvascular homeostasis and cardiac function. In this regard, Yoon et al. have found that direct intramyocardial injection of plasmid DNA encoding VEGF increased capillary density, decreased fibrosis and apoptosis of cardiomyocytes, and consequently improved cardiac function in diabetic rats by VEGF replenishment [50]. Our results also showed reduced left cardiac ventricle VEGF protein expression in diabetic animals.
Nevertheless, both HIIT and MICT exercise could improve myocardium VEGF protein levels followed by an improvement of capillary density and attenuation of interstitial fibrosis. Erekat et al. have demonstrated that treadmill exercise improved the expression of VEGF in the cardiac muscle of type 1 diabetic rats [12]. Karbalaeifar et al. also reported that 6 weeks of HIIT increases cardiac VEGF mRNA expression and improves myocardial function in rats after myocardial infarction [27].
One of the characteristic features of DCM is interstitial perivascular fibrosis, which exacerbates myocardial ischemia by making a barrier to myocardial perfusion by increasing the oxygen diffusion distance [38]. Diabetes-associated cardiac fibrosis is mainly attributed to the high blood glucose. In fact, hyperglycemia by the alternation of extracellular matrix leads to inflammation and fibrosis [2, 17]. Additionally, evidence shows that TGF-β has a causative role in triggering cardiac fibrosis and hence DCM [51]. In this study, diabetic group displayed increased expression of TGF-β, greater collagen deposition, and cardiac fibrosis. Though MICT significantly reduced blood glucose and cardiac expression of TGF-β associated with decreased cardiac fibrosis. Recently, Li et al. also showed that tetrahydrocurcumin alleviated hyperglycemia-induced oxidative stress and cardiac fibrosis in diabetic mice [32]. It seems that MICT through attenuation of TGF-β expression or improving blood glucose levels could decrease cardiac fibrosis. It is worth noting that MICT was superior to HIIT for enhancing angiogenesis and attenuation of blood glucose and fibrosis in the diabetic rats.
Additionally, we found that diabetic animals had higher TIMP2 protein expression and lower activated MMP2 in the myocardium than control animals. Li et al. have also demonstrated decreased myocardial MMP-2 activity and expression and increased TIMP-2 expression in STZ-induced diabetes in rats [31]. Likewise, Lu et al. have reported elevated levels of TIMPs in aortic intima and left ventricle myocardium and reduced serum levels of MMP2 and MMP9 in STZ-induced diabetes model in minipigs [33]. Evidence shows that MMP-TIMP dysregulation in diabetes induces left ventricle hypertrophy and cardiac dysfunction, as well as increases collagen content and cardiovascular fibrosis [21, 25, 33]. Besides, there is an interaction between VEGF-MMP-TIMP2 pathways. Evidence shows that VEGF could up-regulate MMPs [44], while overexpression of TIMP2 could down-regulate VEGF expression [20].
Nevertheless, MICT exercise protocol could reduce TIMP2 expression while increase cleaved MMP2 levels in diabetic rats. These findings highlight the importance of VEGF-MMPs-TIMPs pathways in exercise-induced myocardial angiogenesis.
Furthermore, our results showed the effectiveness of MICT in decreasing blood glucose. During intense exercise (> 85% of VO2max) results in post-exercise hyperglycemia due to augmentation of the hepatic glucose output by epinephrine. On the other hand, prolonged exercise at moderate intensity (40–60% of VO2max) may expose the patient to the risk of post-exercise hypoglycemia [23].
In conclusion, the present study demonstrated that exercise improved cardiac angiogenesis and fibrosis through increasing the expression of pro-angiogenic factors and attenuation of anti-angiogenic factors, as well as ameliorated glucose levels in the diabetic rat model.
Abbreviations
- ANOVA:
-
analysis of variance
- DCM:
-
diabetic cardiomyopathy
- ECL:
-
enhanced chemiluminescence
- HFD:
-
high-fat diet
- HIIT:
-
high-intensity interval training
- HRP:
-
horseradish peroxidase-conjugated
- MICT:
-
moderate intensity continuous training
- MMP2:
-
matrix metalloproteinase-2
- RIPA:
-
radioimmunoprecipitation assay
- STZ:
-
streptozotocin
- TGF-β:
-
transforming growth factor-beta
- TIMP2:
-
tissue inhibitor of matrix metalloproteinase-2
- T2DM:
-
type2 diabetes mellitusVEGF: vascular endothelial growth factor
References
Alizadeh M, Asad MR, Faramarzi M, Afroundeh R (2017) Effect of eight-week high intensity interval training on omentin-1 gene expression and insulin-resistance in diabetic male rats. Ann Appl Sport Sci 5:29–36
Ban CR, Twigg SM (2008) Fibrosis in diabetes complications: pathogenic mechanisms and circulating and urinary markers. Vasc Health Risk Manag 4:575–596
Bensaid M, Malecaze F, Bayard F, Tauber JP (1989) Opposing effects of basic fibroblast growth factor and transforming growth factor-β on the proliferation of cultured bovine retinal capillary endothelial (BREC) cells. Exp Eye Res 48:791–799
Bernardo MM, Fridman R (2003) TIMP-2 (tissue inhibitor of metalloproteinase-2) regulates MMP-2 (matrix metalloproteinase-2) activity in the extracellular environment after pro-MMP-2 activation by MT1 (membrane type 1)-MMP. Biochem J 374:739–745
Boodhwani M, Sellke FW (2009) Therapeutic angiogenesis in diabetes and hypercholesterolemia: influence of oxidative stress. Antioxid Redox Signal 11:1945–1959
Boodhwani M, Sodha NR, Mieno S, Xu S-H, Feng J, Ramlawi B, Clements RT, Sellke FW (2007) Functional, cellular, and molecular characterization of the angiogenic response to chronic myocardial ischemia in diabetes. Circulation 116:I-31–I-37
Burelle Y, Wambolt RB, Grist M, Parsons HL, Chow JC, Antler C, Bonen A, Keller A, Dunaway GA, Popov KM (2004) Regular exercise is associated with a protective metabolic phenotype in the rat heart. Am J Physiol Heart Circ Physiol 287:H1055–H1063
Carmeliet P (2000) Mechanisms of angiogenesis and arteriogenesis. Nat Med 6:389
Carmeliet P, Ng Y-S, Nuyens D, Theilmeier G, Brusselmans K, Cornelissen I, Ehler E, Kakkar VV, Stalmans I, Mattot V (1999) Impaired myocardial angiogenesis and ischemic cardiomyopathy in mice lacking the vascular endothelial growth factor isoforms VEGF 164 and VEGF 188. Nat Med 5:495
Chou E, Suzuma I, Way KJ, Opland D, Clermont AC, Naruse K, Suzuma K, Bowling NL, Vlahos CJ, Aiello LP (2002) Decreased cardiac expression of vascular endothelial growth factor and its receptors in insulin-resistant and diabetic states: a possible explanation for impaired collateral formation in cardiac tissue. Circulation 105:373–379
Derosa G, D’angelo A, Tinelli C, Devangelio E, Consoli A, Miccoli R, Penno G, Del Prato S, Paniga S, Cicero A (2007) Evaluation of metalloproteinase 2 and 9 levels and their inhibitors in diabetic and healthy subjects. Diabetes Metab 33:129–134
Erekat NS, Al-Jarrah MD, Al Khatib AJ (2014) Treadmill exercise training improves vascular endothelial growth factor expression in the cardiac muscle of type I diabetic rats. Cardiovasc Res 5:23–29
Forsythe JA, Jiang B-H, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL (1996) Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 16:4604–4613
Fràter-Schröder M, Müller G, Birchmeier W, Böhlen P (1986) Transforming growth factor-beta inhibits endothelial cell proliferation. Biochem Biophys Res Commun 137:295–302
Fukai T, Siegfried MR, Ushio-Fukai M, Cheng Y, Kojda G, Harrison DG (2000) Regulation of the vascular extracellular superoxide dismutase by nitric oxide and exercise training. J Clin Invest 105:1631–1639
Ghosh S, Das AK, Sarkar P, Sil PC (2019) Oxidative stress in schistosomiasis, echinococcosis, and trypanosomiasis: a therapeutic approach. In: Discovery and Development of Therapeutics from Natural Products Against Neglected Tropical Diseases. Elsevier, pp 219–239
Gorski DJ, Petz A, Reichert C, Twarock S, Grandoch M, Fischer JW (2019) Cardiac fibroblast activation and hyaluronan synthesis in response to hyperglycemia and diet-induced insulin resistance. Sci Rep 9:1827
Guerrero PA, McCarty JH (2017) TGF-β activation and signaling in angiogenesis. Physiologic and Pathologic Angiogenesis-Signaling Mechanisms and Targeted Therapy
Gustafsson T, Bodin K, Sylven C, Gordon A, Tyni-Lenne R, Jansson E (2001) Increased expression of VEGF following exercise training in patients with heart failure. Eur J Clin Investig 31:362–366
Hajitou A, Sounni N-E, Devy L, Grignet-Debrus C, Lewalle J-M, Li H, Deroanne CF, Lu H, Colige A, Nusgens BV (2001) Down-regulation of vascular endothelial growth factor by tissue inhibitor of metalloproteinase-2: effect on in vivo mammary tumor growth and angiogenesis. Cancer Res 61:3450–3457
Hayashi T, Sohmiya K, Ukimura A, Endoh S, Mori T, Shimomura H, Okabe M, Terasaki F, Kitaura Y (2003) Angiotensin II receptor blockade prevents microangiopathy and preserves diastolic function in the diabetic rat heart. Heart 89:1236–1242
Hinkel R, Howe A, Renner S, Ng J, Lee S, Klett K, Kaczmarek V, Moretti A, Laugwitz K-L, Skroblin P (2017) Diabetes mellitus–induced microvascular destabilization in the myocardium. J Am Coll Cardiol 69:131–143
Ilkhanizadeh B, Shirpoor A, Nemati S, Rasmi Y (2016) Protective effects of ginger (Zingiber officinale) extract against diabetes-induced heart abnormality in rats. Diabetes Metab J 40:46–53
Jaoude J, Koh Y (2016) Matrix metalloproteinases in exercise and obesity. Vasc Health Risk Manag 12:287
Jesmin S, Sakuma I, Hattori Y, Kitabatake A (2003) Role of angiotensin II in altered expression of molecules responsible for coronary matrix remodeling in insulin-resistant diabetic rats. Arterioscler Thromb Vasc Biol 23:2021–2026
Jesmin S, Miyauchi T, Goto K, Yamaguchi I (2006) Down-regulated VEGF expression in the diabetic heart is normalized by an endothelin ETA receptor antagonist. Eur J Pharmacol 542:184–185
Karbalaeifar S, Gaeini AA, Kordi MR, Nuri R, Ghorbani P (2016) The effect of 6-week high intensity interval training on the VEGF/COL-18 ratio and some echocardiographic indices in rats with myocardial infarction. J Kerman Univ Med Sci 20:94–98
Khazaei M, Fallahzadeh AR, Sharifi MR, Afsharmoghaddam N, Javanmard SH, Salehi E (2011) Effects of diabetes on myocardial capillary density and serum angiogenesis biomarkers in male rats. Clinics 66:1419–1424
Kojda G, Hambrecht R (2005) Molecular mechanisms of vascular adaptations to exercise. Physical activity as an effective antioxidant therapy? Cardiovasc Res 67:187–197
Lavie CJ, Johannsen N, Swift D, Sénéchal M, Earnest C, Church T, Hutber A, Sallis R, Blair SN (2014) Exercise is medicine–the importance of physical activity, exercise training, cardiorespiratory fitness and obesity in the prevention and treatment of type 2 diabetes. Eur J Endocrinol 10:18
Li Q, S-z S, Wang Y, Y-j T, M-h L (2007) The roles of MMP-2/TIMP-2 in extracellular matrix remodelling in the hearts of STZ-induced diabetic rats. Acta Cardiol 62:485–491
Li K, Zhai M, Jiang L, Song F, Zhang B, Li J, Li H, Li B, Xia L, Xu L, Cao Y, He M, Zhu H, Zhang L, Liang H, Jin Z, Duan W, Wang S (2019) Tetrahydrocurcumin ameliorates diabetic cardiomyopathy by attenuating high glucose-induced oxidative stress and fibrosis via activating the SIRT1 pathway. Oxidative Med Cell Longev 2019:6746907–6746907
Lu L, Zhang Q, Pu LJ, Peng WH, Yan XX, Wang LJ, Chen QJ, Zhu ZB, Michel J-B, Shen WF (2008) Dysregulation of matrix metalloproteinases and their tissue inhibitors is related to abnormality of left ventricular geometry and function in streptozotocin-induced diabetic minipigs. Int J Clin Exp Pathol 89:125–137
Lu K, Wang L, Wang C, Yang Y, Hu D, Ding R (2015) Effects of high-intensity interval versus continuous moderate-intensity aerobic exercise on apoptosis, oxidative stress and metabolism of the infarcted myocardium in a rat model. Mol Med Rep 12:2374–2382
Marfella R, Esposito K, Nappo F, Siniscalchi M, Sasso FC, Portoghese M, Di Marino MP, Baldi A, Cuzzocrea S, Di Filippo C (2004) Expression of angiogenic factors during acute coronary syndromes in human type 2 diabetes. Diabetes 53:2383–2391
Moreira JB, Bechara LR, Bozi LH, Jannig PR, Monteiro AW, Dourado PM, Wisløff U, Brum PC (2013) High-versus moderate-intensity aerobic exercise training effects on skeletal muscle of infarcted rats. J Appl Physiol 114:1029–1041
Pardali E, ten Dijke P (2009) Transforming growth factor-beta signaling and tumor angiogenesis. Front Biosci 14:4848–4861
Sabbah HN, Sharov VG, Lesch M, Goldstein S (1995) Progression of heart failure: a role for interstitial fibrosis. In: Cellular Interactions in Cardiac Pathophysiology. Springer, pp 29–34
Sedighi M, Sewell RD, Nazari A, Abbaszadeh S, Cheraghi M, Amini A, Heydari Z, Rafieian-Kopaei M (2019) A review on the Most important medicinal plants effective in cardiac ischemia-reperfusion injury. Curr Pharm Des
Son JS, Kim HJ, Son Y, Lee H, Chae SA, Seong JK, Song W (2017) Effects of exercise-induced apelin levels on skeletal muscle and their capillarization in type 2 diabetic rats. Muscle Nerve 56:1155–1163
Srinivasan K, Viswanad B, Asrat L, Kaul C, Ramarao P (2005) Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res 52:313–320
Stetler-Stevenson WG (1999) Matrix metalloproteinases in angiogenesis: a moving target for therapeutic intervention. J Clin Invest 103:1237–1241
Van Craenenbroeck EM, Hoymans VY, Beckers PJ, Possemiers NM, Wuyts K, Paelinck BP, Vrints CJ, Conraads VM (2010) Exercise training improves function of circulating angiogenic cells in patients with chronic heart failure. Basic Res Cardiol 105:665–676
Wang H, Keiser JA (1998) Vascular endothelial growth factor upregulates the expression of matrix metalloproteinases in vascular smooth muscle cells: role of flt-1. Circ Res 83:832–840
Weidner K, Behnes M, Schupp T, Rusnak J, Reiser L, Bollow A, Taton G, Reichelt T, Ellguth D, Engelke N (2018) Type 2 diabetes is independently associated with all-cause mortality secondary to ventricular tachyarrhythmias. Cardiovasc Diabetol 17:125
Westermeier F, Riquelme JA, Pavez M, Garrido V, Díaz A, Verdejo HE, Castro PF, García L, Lavandero S (2016) New molecular insights of insulin in diabetic cardiomyopathy. Front Physiol 7:125
Yang EY, Moses HL (1990) Transforming growth factor beta 1-induced changes in cell migration, proliferation, and angiogenesis in the chicken chorioallantoic membrane. J Cell Biol 111:731–741
Yang Z, Xia W-H, Su C, Wu F, Zhang Y-Y, Xu S-Y, Liu X, Zhang X-Y, Ou Z-J, Lai G-H (2013) Regular exercise-induced increased number and activity of circulating endothelial progenitor cells attenuates age-related decline in arterial elasticity in healthy men. Int J Cardiol 165:247–254
Ying Y, Jiang C, Zhang M, Jin J, Ge S, Wang X (2019) Phloretin protects against cardiac damage and remodeling via restoring SIRT1 and anti-inflammatory effects in the streptozotocin-induced diabetic mouse model. Aging 11:2822
Y-s Y, Uchida S, Masuo O, Cejna M, Park J-S, H-c G, Kirchmair R, Bahlman F, Walter D, Curry C (2005) Progressive attenuation of myocardial vascular endothelial growth factor expression is a seminal event in diabetic cardiomyopathy: restoration of microvascular homeostasis and recovery of cardiac function in diabetic cardiomyopathy after replenishment of local vascular endothelial growth factor. Circulation 111:2073–2085
Yue Y, Meng K, Pu Y, Zhang X (2017) Transforming growth factor beta (TGF-β) mediates cardiac fibrosis and induces diabetic cardiomyopathy. Diabetes Res Clin Pract 133:124–130
Żebrowska A, Hall B, Kochańska-Dziurowicz A, Janikowska G (2018) The effect of high intensity physical exercise and hypoxia on glycemia, angiogenic biomarkers and cardiorespiratory function in patients with type 1 diabetes. Adv Clin Exp Med 27:207–216
Zhang B, Wang D, Ji T-F, Shi L, Yu J-L (2017) Overexpression of lncRNA ANRIL up-regulates VEGF expression and promotes angiogenesis of diabetes mellitus combined with cerebral infarction by activating NF-κB signaling pathway in a rat model. Oncotarget 8:17347
Zheng J, Cheng J, Zheng S, Zhang L, Guo X, Zhang J, Xiao X (2018) Physical exercise and its protective effects on diabetic cardiomyopathy: what is the evidence? Front Endocrinol 9
Acknowledgments
The authors would like to express their gratitude to Dr. Fereshteh Farajdokht from Tabriz University of Medical Sciences, for critical revising the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures of the current study were carried out according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH publication 86–23 revised 1985) and approved by the Ethical Committee of Tabriz University (EC-00212).
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights and informed consent
The current study involves animal subjects (Rats).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yazdani, F., Shahidi, F. & Karimi, P. The effect of 8 weeks of high-intensity interval training and moderate-intensity continuous training on cardiac angiogenesis factor in diabetic male rats. J Physiol Biochem 76, 291–299 (2020). https://doi.org/10.1007/s13105-020-00733-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-020-00733-5