Abstract
Epithelial neutrophil-activating peptide-78 (CXCL5), a member of the subgroup of CXC-type chemokine family, is an inflammatory factor involved in the progression of lung cancer, but the underlying mechanism remains unclear. In this study, we investigated the effects of CXCL5 on proliferation and migration in non-small cell lung cancer (NSCLC) using tissue microarrays from NSCLC patients and H460 cells transfected with a CXCL5-interfered lentivirus vector or stimulated with recombinant CXCL5. We observed that the expression of CXCL5 was significantly higher in lung cancer cell lines, and high CXCL5 was associated with high chemokine (C-X-C motif) receptor 2 expression and was significantly associated with poor differentiation. The high expression of CXCL5 was associated with poor NSCLC prognosis and was an independent predictive factor. Furthermore, downregulation of CXCL5 in H460 cells significantly reduced proliferation and migration. Recombinant CXCL5 promoted H460 cell proliferation and movement by activating MAPK/ERK1/2 and PI3K/AKT signaling. Our study elucidates the important role of CXCL5 in the progression and prognosis of NSCLC. These findings suggested that CXCL5 might be a potential biomarker and novel therapeutic target for lung cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the most commonly diagnosed cancer and the leading cause of cancer-related death, accounting for approximately 13% of total cancer cases and 18% of cancer deaths worldwide each year [10]. Improvement of surgical techniques and perioperative treatments has increased lung cancer survival. However, many patients have local and distant metastasis when they are diagnosed, which contributes to an overall 5-year survival of only 6 to 18% [31]. Better understanding of the molecular pathogenesis of lung cancer would help to improve this situation.
The importance of inflammation in lung cancer has been widely demonstrated. Chemotactic cytokines promote migration of leucocytes or lymphocytes from peripheral blood to tissue and serve as mediators of inflammation. Recent studies have identified chemokines produced by cancer cells essential for the development of inflammatory microenvironments that promote tumor progression [12, 26]. Inflammatory mediators such as tumor necrosis factor (TNF)-α, transforming growth factor (TGF)-β, and interleukin (IL)-1 promote tumor cell proliferation and metastasis [6, 20, 29]. Evidence suggests that chemokines and their receptors are involved in tumor progression. Dysregulated expression of specific chemokines has been implicated to be involved in the progression of inflammatory environment and the initiation of non-small cell lung cancer (NSCLC) [19, 25, 27].
Epithelial neutrophil-activating peptide-78 (CXCL5) is a chemokine that plays an important role in leukocyte recruitment, tumor cell proliferation, and metastasis. It is a member of the CXC chemokine family, which contains an ELR motif similar to that of IL-8, which promotes angiogenesis by interacting with chemokine (C-X-C motif) receptor 2 (CXCR2), its specific G-protein-coupled receptor. CXCL5 promotes bladder cancer cell migration and invasion following binding to CXCR2, activation of PI3K/AKT signaling, and upregulation of matrix metalloprotein 2 and matrix metalloprotein 9 [5]. Osteoblast-derived CXCL5 promotes breast tumor progression by activating the ERK/MSK1/Elk-1/Snail signaling pathway [9]. In studies of lung cancer, DACH1 inhibited lung adenocarcinoma invasion and tumor growth by repressing CXCL5 signaling [8]. The expression of CXCL5 and its activity in NSCLC progression remain elusive. This study assayed the expression of CXCL5 in NSCLC specimens and evaluated the relationship between the CXCL5 expression, clinical characteristics, and overall survival. The effects of both CXCL5 knockdown and exogenous recombinant CXCL5 on proliferation and migration of human H460 NSCLC and the underlying signaling pathways were also investigated.
Materials and methods
Patients and NSCLC tissue
Tissue specimens were obtained from 208 patients with curative resection for NSCLC, including lobectomy and mediastinal lymph node dissection, at Zhongshan Hospital, Shanghai, China, in 2005. Pathological evaluation of formalin-fixed, paraffin-embedded tissue and complete clinical follow-up data were available for the included patients. The tumor/node/metastasis (TNM) stage was determined by the criteria of Union for International Cancer Control Lung Cancer Classification, sixth edition. The pathological classification was based on World Health Organization criteria. The median patient follow-up was 43 months (range, 1–66 months) and was completed in July 2010. Overall survival (OS) was defined as the interval between surgery and death or the last follow-up evaluation, and the data were censored at the last follow-up for living patients. The Zhongshan Hospital Research Ethics Committee approved the study, and all patients gave informed consent prior to enrollment.
Cell lines and reagents
HBE, immortalized normal human bronchial epithelial cells (American Type Culture Collection) and SPC-A-1, H1299, H1650, A549, H460 and H358, and human lung cancer cells (Cell bank of the typical culture preservation Committee, Chinese Academy of Sciences, Shanghai, China) were cultured in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum in standard conditions (37 °C, 20% O2, 5% CO2). Human CXCL5 quantikine enzyme-linked immunosorbent assay (ELISA) kits (DX000), recombinant CXCL5 (254-XB), and anti-human CXCL5 antibody (Clone# 33160) were purchased from R&D Systems (Shanghai, China). Anti-human CXCR2 antibody was purchased from Abcam (ab14935, Hong Kong, China). PI3K/mTOR inhibitor (LY294002) and ERK1/2 inhibitor (PD98059) were purchased from Biovision (Milpitas, CA, USA).
Assay of gene expression
Total RNA was isolated by TRIzol reagent (Invitrogen, Carlsbad, USA) and the concentration was determined by optical density at 260 nm. Then, RNA was reverse transcribed to cDNA. Quantitative real-time PCR (RT-qPCR) was performed with the Advanced Biosystems 7500 Fast qPCR system (Eppendorf, Hamburg, Germany) with two-stage program parameters, as follows: 1 min at 95 °C followed by 40 cycles of 5 s at 95 °C and 30s at 60 °C. Sequences of the primer sets used for this analysis are as follows: CXCL5, 5′-GAGAGCTGCGTTGCGTTTGTTTAC-3′ (forward [F]) and 5′-CCGTTCTTCAGGGAGGCTACCA-3′ (reverse [R]); and for human glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 5′-CCACCCATGGCAAATTCCATGGCA-3′(F) and 5′-TCTACACGGCAGGTCAGGTCCACC-3′(R). Specificity of the amplified products was confirmed by examination of dissociation reaction plots. Each sample was assayed in triplicate, and each experimental group had six wells.
CXCL5 protein assay
CXCL5 protein levels in cell culture supernatants were determined by ELISA following the protocol provided by the assay kit manufacturer (DX000, R&D Systems, Shanghai, China). Briefly, protein samples and standards were added to 96-well polystyrene microplates coated with CXCL5 primary antibody and incubated for 2 h. The plates were washed and incubated with CXCL5 conjugate antibody and incubated for 2 h. After washing twice, substrate solution was added for color development, and the reaction was terminated with stop solution. Absorbance was measured at 450 nm and CXCL5 protein concentration was determined by comparison to the standard curve. The final cell number was counted and the amount of protein secreted by 105 cells was reported as the expression level.
Cell proliferation assay
Cells were seeded in 96-well plates at a density of 2000 per well and incubated for 6 h. Cell counting kit-8 (CCK-8) solution (Dojindo, Japan) was added to each well at a final concentration of 10%, and the plates were incubated for 2 h before measurement of baseline absorbance at 450 nm. Cell proliferation was monitored every 24 h for 96 h.
Colony formation assay
Cells were seeded into culture dishes and incubated for 2 weeks, washed three times in phosphate-buffered saline (PBS), fixed 5 min with 4% paraformaldehyde, washed in PBS, and stained with crystal violet for 20 min at room temperature. After removing the stain solution, the cells were washed two times with tap water and air-dried. Culture dishes with the stained cells were observed and photographed.
Migration assay
Migration assays were performed in transwell permeable inserts with 8-μm pores (Corning Inc.; Corning, NY, USA). Serum-starved cells were trypsinized and 2 × 104 cells in 200 μl serum-free medium were placed into the upper chamber, and the bottom chamber was filled with 600 μl of 10% FBS–RPMI 1640. After 24 h, cells that migrated to the lower chamber were fixed, stained with Giemsa stain, air-dried, and photographed.
Western blot assay
Protein samples (40 μg) were mixed with one-fourth volume of a sodium dodecyl sulfate (SDS) sample buffer, boiled for 5 min, and then separated by 10% SDS-polyacrylamide gel electrophoresis (PAGE). After electrophoresis, proteins were transferred to nylon membranes and incubated with primary antibodies in 5% bovine serum albumin (BSA), at 4 °C overnight after blocking in 5% BSA for 2 h. The primary antibodies and dilutions used were as follows: anti-phospho-AKT (Ser473), anti-AKT, anti-phospho-ERK1/2 (Thr202/Tyr204), anti-ERK1/2, anti-phospho-p38 (Thr180/Tyr182), anti-p38, anti-phospho-JNK (Thr183/Tyr185), anti-JNK, (1:1000, Cell Signaling Technology, Boston, MA); CXCR2(1:500; ab14935, Abcam, Hong Kong, China), and GAPDH (1:5000; Millipore, Bedford, MA). The membranes were incubated in horseradish peroxidase-conjugated anti-rabbit secondary antibody (1:5000, A0208, Beyotime, Shanghai, China) for 2 h at room temperature after washing three times in TBST. After washing, the band densities were read and results calculated by Phoretix 1D software.
Tissue microarray and immunohistochemistry
Tissue microarrays were constructed as previously described [7]. Immunohistochemistry was performed by the avidin-biotin-peroxidase complex method. Briefly, after rehydration and microwave antigen retrieval, antibodies against human anti-human CXCL5 monoclonal antibody (1:20, Clone# 33160, R&D Systems, Shanghai, China) [33] and human CXCR2 antibody (1:50, ab14935, Abcam, Hong Kong, China) [3] were used to sections, incubated at 4 °C overnight, and followed with secondary antibody incubation (GK500705, Gene Tech, China) at 37 °C for 30 min. Staining was carried out with DAB and counter-staining with hematoxylin. CXCL5 expression was evaluated by two pathologists independently. Staining intensity was scored semi-quantitatively from negative (0) to strong (4). The percentage of positively stained cells was scored as 0 (0%), 1 (1 to 33%), 2 (34 to 66%), and 3 (67 to 100%). The staining scores were reported as the sum of the percentage and intensity scores. Staining was reported as indicating low (score of 0–2) or high (score of 3–6) expression.
RNA interference (siRNA) and transfection
Three siRNAs were used to block the expression of CXCL5 in H460 cells. A control siRNA was employed as negative controls. Sequences of the siRNAs used for this analysis are as follows: siRNA-NC, 5′-UUCUCCGAACGUGUCACGUTT-3′(F) and 5′-ACGUGACACGUUCGGAGAATT-3′(R); siRNA-1, 5′-GACCACGCAAGGAGUUCAUTT-3′(F) and 5′-AUGAACUCCUUGCGUGGUCTT-3′(R); siRNA-2, 5′-UGGAAACAAGGAAAACUGATT-3′(F) and 5′-UCAGUUUUCCUUGUUUCCATT-3′(R); and siRNA-3, 5′-UCUGCAAGUGUUCGCCAUATT-3′(F) and 5′-UAUGGCGAACACUUGCAGAT--3′(R).
A lentivirus vector with siRNA-3 was designed and provided by Ribobio Co. (Guangzhou, Guangdong, China), and lentivirus packaging and transduction were performed following the supplier’s recommended protocol. Briefly, H460 cells were placed in 12-well plates and optimal lentivirus (10 μl) was used for transfection. And each experiment included controls containing the transfection reagent with the control vector. After 24 h, CXCL5 were detected by qRT-PCR and western blot analysis. Following exposure to the lentivirus vector, the stable transduced cells were selected by culture with puromycin 5 μg/ml for 2 weeks for our further study.
Statistical analysis
Data were reported as means ± standard error of the mean (SEM) of replicate experiments (n ≥ 3). The statistical significance of between-group differences was determined by Student’s t test, after analysis of variance. The association of clinicopathological variables and CXCL5 expression was determined by the χ2 test. Cumulative survival was estimated by the Kaplan–Meier method and analyzed by the log-rank test. Univariate and multivariate analyses were performed using the Cox proportional hazards regression model. p values < 0.05 were considered significant.
Results
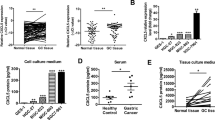
CXCL5 expression is upregulated in NSCLC tissues
Immunohistochemical staining of NSCLC tissue microarrays included tissues from 208 NSCLC patients with different stages and histologic types of disease. Positive CXCL5 staining was observed in the cytoplasm of normal bronchial and alveolar epithelial cells, but the expression was low, with negative or weak intensity. Immunohistochemical staining confirmed that the expression of CXCL5 was significantly higher in NSCLC tissues than in non-tumor tissues (Fig. 1). High expression of CXCL5 was observed in 104 of 208 patients with NSCLC (50%). The correlations of CXCL5 expression with clinicopathological features are shown in Table 1. CXCL5 expression was significantly correlated with lymph node metastasis, TNM stage, and CXCR2 expression. During follow-up, 109 patients died of lung cancer. The overall 5-year survival rate for all patients was 47.6%. CXCL5 expression had a significant negative correlation with prognosis (Fig. 2) and was an independent prognostic factor in NSCLC patients (Table 2).
Positive CXCR2 staining was observed in the cytomembrane, and its expression was significantly upregulated in tumor tissues (Fig. 1). High expression of CXCR2 was also associated with poorer differentiation, lymph node metastasis, and advanced pathological stage (Table 1), while the different expression of CXCR2 did not affect the OS of NSCLC (Table 2).
CXCL5 expression is the highest among the cell lines tested
CXCL5 expression was assayed in six NSCLC cell lines and normal HBE airway epithelial cells by qRT-PCR and ELISA. Expression of CXCR2 protein was assayed in those cell lines in western blots. Expression of CXCL5 mRNA was detected by qPCR only in H460 and H1650 cells (Fig. 3a). ELISA confirmed the high expression of CXCL5 protein in tumor cell lines, with the highest expression in H460 cells, which selected for subsequent experiments (Fig. 3b). CXCR2 protein expression was detected by western blotting in the same two cell lines (Fig. 3c).
CXCL5 and CXCR2 overexpressed in NSCLC cells. a Relative mRNA level of CXCL5 and the protein level of CXCR2 in different cell lines. b Protein level of CXCL5 in different cell line culture supernatants. c Protein level of CXCR2 in different cell lines; the right histogram represents quantification analysis; **p < 0.01 vs. HBE
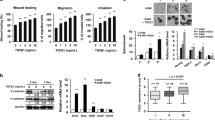
CXCL5 silencing inhibits proliferation, migration, and colony formation of H460 cells
Three siRNAs were used to downregulate CXCL5 expression in H460 cells and the efficiency was assayed by qRT-PCR and ELISA, as shown in Fig. 4a, b. Cultures of H460 transfected by lentivirus with the most effective sequence were established for use in subsequent study procedures.
CXCL5 silencing inhibited proliferation, migration, and colony formation of H460 cells and downregulated the expression of phosphorylated AKT and ERK1/2. a Relative mRNA level of CXCL5 in H460 cells silenced by RNA interference. b Protein level of CXCL5 in H460 culture supernatant silenced by RNA interference. c Effect of CXCL5 silencing on cell colony formation was detected by crystal violet staining. d Effect of CXCL5 silencing on migration was measured by transwell assay. e Effect of CXCL5 silencing on cell proliferation was detected by CCK-8 assay. f Western blot analysis of phosphorylation of AKT, ERK1/2, JNK, and P38 after CXCL5 silencing; the right histogram represents quantification analysis; *p < 0.05, **p < 0.01 vs. control
We tested the effect of CXCL5 on proliferation using CCK-8 assay. Proliferation was significantly suppressed in CXCL5-silenced cells; significantly fewer colonies formed in silenced than in control CXCL5 cell cultures. In the transwell assay, migration of CXCL5 knockdown cells was significantly reduced compared with that of control cells (Fig. 4c–e). The signaling pathways involved in CXCL5-mediated effects on proliferation and invasion of H460 NSCLC cells were investigated by western blotting, which implicated the PI3K/AKT and MAPK/ERK1/2 signaling pathways. CXCL5 knockdown inhibited AKT and ERK1/2 phosphorylation, but had no influence on JNK and P38 phosphorylation (Fig. 4f).
Exogenous CXCL5 promotes proliferation and migration of H460
We stimulated H460 cells with various concentrations of recombinant CXCL5 (0.1, 1, and 10 nM) to investigate the effects on proliferation and migration. Recombinant CXCL5 increased the proliferation and migration of H460. Increases in response to 1- and 10-nM CXCL5 were significant (Fig. 5a, b). Significant increases of phosphorylated AKT and ERK1/2 were observed in H460 cells treated with recombinant CXCL5 (Fig. 5c). These results are consistent with CXCL5 activation of the PI3K/AKT and MAPK/ERK1/2 signaling pathways in H460 cells.
Exogenous CXCL5 promoted proliferation and migration of H460 and the expression of phosphorylated AKT and ERK1/2. a Effect of recombinant CXCL5 on cell proliferation was detected by CCK-8 assay (p < 0.01 compared CXCL5 (1 nM) with the control group; p < 0.01 compared CXCL5 (10 nM) with the control group). b Effect of recombinant CXCL5 on cell migration was detected by transwell assay. c Western blot analysis of phosphorylation of AKT, ERK1/2, JNK, and P38 after recombinant CXCL5 stimulation; the histogram below represents quantification analysis; *p < 0.05, **p < 0.01 vs. control
To evaluate the effects of PI3K/AKT and MAPK/ERK1/2 on the proliferation and migration of H460 cells, we cultured H460 cells with an ERK1/2 inhibitor (PD98059) or a PI3K inhibitor (LY294002). The proliferation and migration induced by CXCL5 were significantly inhibited (Fig. 6).
ERK1/2 or PI3K inhibitor inhibited proliferation and migration induced by CXCL5. a Effect of PI3K inhibitor (LY294002) on cell proliferation induced by CXCL5 (p < 0.01 compared CXCL5+ LY294002 (10uM) with the CXCL5 group; p < 0.01 compared CXCL5+ LY294002 (20uM) with the CXCL5 group; p < 0.01 compared CXCL5+ LY294002 (40uM) with the CXCL5 group). b, c Effect of PI3K inhibitor (LY294002) on cell migration induced by CXCL5. d Effect of ERK1/2 inhibitor (PD98059) on cell proliferation induced by CXCL5 (p < 0.01 compared CXCL5+ PD98059 (10uM) with the CXCL5 group; p < 0.01 compared CXCL5+ PD98059 (20uM) with the CXCL5 group; p < 0.01 compared CXCL5+ PD98059 (40uM) with the CXCL5 group). e, f Effect of ERK1/2 inhibitor (PD98059) on cell migration induced by CXCL5; **p < 0.01 vs. CXCL5 group
Discussion
CXCL5 is a member of a proangiogenic subgroup of the CXC-type chemokine family. Emerging evidence has demonstrated that CXCL5 is involved in carcinogenesis and cancer progression. This investigation of CXCL5 in NSCLC patients demonstrated that high expression of CXCL5 was associated with poor OS. CXCL5 was overexpressed in the NSCLC tissue evaluated in our study, which is in line with previous studies in various cancers [4, 5, 34]. The prognostic value of CXCL5 expression has been previously demonstrated in NSCLC. Gene screening in patients with early stage NSCLC confirmed that CXCL5 was the only one of the 23 candidate genes that had a significant, independent association with both overall and disease-free survival [16]. In the present study, upregulation of CXCL5 was significantly associated with poorer differentiation, lymph node metastasis, advanced pathological stage, and higher CXCR2 expression in NSCLC. Further analysis showed that high expression of CXCL5 was accompanied with worse OS and expression of CXCL5 was identified as an independent prognostic factor in patients with NSCLC. Survival rate of NSCLC patients remains unsatisfactory despite advances in treatments. Therefore, there is a great need for biomarkers to predict NSCLC patients’ prognosis which may help to develop novel therapeutic drugs for the improvement of NSCLC patients’ survival. Our observations from this study prompted us to hypothesize that CXCL5 might be used as a prognostic biomarker in NSCLC.
Lung cancer has diverse functional and biological characteristics, and TNM stage has strong prognostic value. Tumor size and lymph node involvement are predictive of recurrence and metastasis, but they do not reflect the complex nature of NSCLC, in which many biochemical changes underlie progression. The inflammatory microenvironment, with inflammatory cells, cytokines, and chemokines, influences the progress of invasion and metastasis of malignant tumors [28]. Meanwhile, neoplastic tumor cells often overexpress pro-inflammatory mediators to promote cellular proliferation, angiogenesis, resistance to apoptosis, epithelial-to-mesenchymal transition, invasion, and metastasis [24]. CXCL5 was reported to be a powerful proangiogenic chemokine and mediator of inflammation to promote cancer progression. It has been reported that overexpression of CXCL5 promotes hepatic hepatocellular carcinoma (HCC) cell proliferation, invasion, intratumoral neutrophil infiltration, and epithelial-mesenchymal transition [33, 34]. It also promotes proliferation and migration of bladder and colorectal cancer cells [13, 32]. Arenberg et al. first reported that CXCL5 was elevated in fresh NSCLC tissues and correlated with vascular density [1]. Elevated serum levels of CXCL5 were statistically significantly associated with lung cancer risk [23]. Cyclooxygenase-2 contributes to the progression of NSCLC tumorigenesis by enhancing the expression of angiogenic chemokines CXCL8 and CXCL5 [21] while the synergistic effect of HB-EGF (heparin-binding EGF-like growth factor) and CXCL5 exacerbates cancer progression and results in metastasis by potentiating the classical EGFR pathway and the AKT and ERK/RSK1/2 signaling pathways and increasing the phosphorylation of heat shock protein 27 [17]. Consistent with these studies, our study further showed that CXCL5 protein abundance in various types of lung cancer cell lines was higher than that in normal human bronchial epithelial cells. In this study, the effects of CXCL5 on NSCLC development and progression were also investigated by in vitro assays. By downregulating CXCL5 in NSCLC cell lines H460, the decrease of CXCL5 could effectively inhibit the proliferation, colony formation, and migration capacities of H460 cells. Additionally, we further found that recombinant human CXCL5 promoted H460 cell proliferation and migration in a dose-dependent manner. Current studies suggested that both endogenous and exogenous CXCL5 could promote the progression of lung cancer. Together, these results indicated that CXCL5 might be a potential biomarker for NSCLC.
As a CXC chemokine, CXCL5 is an important attractant of granulocytes by binding to its receptor CXCR2. CXCR2 has been studied primarily in stromal cells in NSCLC and is known to increase tumor inflammation and angiogenesis and promote invasion and metastasis in lung adenocarcinoma [22]. CXCR2 blockade eliminated Snail-induced tumor burden which indicated that upstream regulation of Snail in human NSCLC plays a role by promoting tumor progression mediated by CXCR2 ligands [30].CXCR2 was also significantly upregulated in several cancers, where they are key regulators of tumor cell proliferation, metastasis, and angiogenesis. Blocking CXCR2 expression inhibits growth and metastasis of human lung cancer cells by reducing phosphorylation of ERK1/2 and AKT [14]. In our study, we found CXCR2 was upregulated in NSCLC and its high expression was correlated with lymph node metastasis and late pathological stage, but the expression of CXCR2 did not make significant difference to prognosis.
In addition, we investigated the downstream pathway and found that CXCL5 was critical to promote cell proliferation and migration in NSCLC by targeting MAPK/ERK1/2 and PI3K/AKT signaling. AKT and ERK1/2 activation is closely associated with promotion of proliferation, migration, invasion, epithelial-to-mesenchymal transition, and chemo-attraction of neutrophils in malignant tumors. The PI3K/AKT and MAPK/ERK1/2 pathways correlate with lung cancer development, proliferation, metastasis, and chemotherapy resistance [2, 11, 15], as inhibition of PI3K/AKT pathway can reduce migration and invasion of NSCLC cells [18]. In our study, the western blot assays revealed increased phosphorylation of AKT and ERK1/2 after stimulation by CXCL5, while the effects of CXCL5 on cell proliferation and migration were prevented by PI3K/AKT and ERK1/2 inhibitors.
In summary, our study demonstrated that NSCLC overexpressed CXCL5, and high levels of CXCL5 were associated with poor prognosis of NSCLC after resection. Moreover, overexpression of CXCL5 promoted tumor cell proliferation and migration via the activation of PI3K/AKT and MAPK/ERK1/2 signaling pathways. These findings indicate CXCL5 might be a potential prognostic marker to screen patients for unfavorable prognosis.
References
Arenberg DA, Keane MP, DiGiovine B, Kunkel SL, Morris SB, Xue YY, Burdick MD, Glass MC, Iannettoni MD, Strieter RM (1998) Epithelial-neutrophil activating peptide (ENA-78) is an important angiogenic factor in non-small cell lung cancer. J Clin Invest 102:465–472
Coco S, Truini A, Alama A, Dal Bello MG, Vene R, Garuti A, Carminati E, Rijavec E, Genova C, Barletta G, Sini C, Ballestrero A, Boccardo F, Grossi F (2014) Afatinib resistance in non-small cell lung cancer involves the PI3K/AKT and MAPK/ERK signalling pathways and epithelial-to-mesenchymal transition. Target Oncol 10:393-404
Desurmont T, Skrypek N, Duhamel A, Jonckheere N, Millet G, Leteurtre E, Gosset P, Duchene B, Ramdane N, Hebbar M, Van Seuningen I, Pruvot FR, Huet G, Truant S (2015) Overexpression of chemokine receptor CXCR2 and ligand CXCL7 in liver metastases from colon cancer is correlated to shorter disease-free and overall survival. Cancer Sci 106:262–269
Frick VO, Rubie C, Wagner M, Graeber S, Grimm H, Kopp B, Rau BM, Schilling MK (2008) Enhanced ENA-78 and IL-8 expression in patients with malignant pancreatic diseases. Pancreatology 8:488–497
Gao Y, Guan Z, Chen J, Xie H, Yang Z, Fan J, Wang X, Li L (2015) CXCL5/CXCR2 axis promotes bladder cancer cell migration and invasion by activating PI3K/AKT-induced upregulation of MMP2/MMP9. Int J Oncol 47:690–700
Germano G, Allavena P, Mantovani A (2008) Cytokines as a key component of cancer-related inflammation. Cytokine 43:374–379
Gu J, Ding JY, Lu CL, Lin ZW, Chu YW, Zhao GY, Guo J, Ge D (2013) Overexpression of CD88 predicts poor prognosis in non-small-cell lung cancer. Lung Cancer 81:259–265
Han N, Yuan X, Wu H, Xu H, Chu Q, Guo M, Yu S, Chen Y, Wu K (2015) DACH1 inhibits lung adenocarcinoma invasion and tumor growth by repressing CXCL5 signaling. Oncotarget 6:5877–5888
Hsu YL, Hou MF, Kuo PL, Huang YF, Tsai EM (2013) Breast tumor-associated osteoblast-derived CXCL5 increases cancer progression by ERK/MSK1/Elk-1/snail signaling pathway. Oncogene 32:4436–4447
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Kang X, Kong F, Wu X, Ren Y, Wu S, Wu K, Jiang Z, Zhang W (2015) High glucose promotes tumor invasion and increases metastasis-associated protein expression in human lung epithelial cells by upregulating heme oxygenase-1 via reactive oxygen species or the TGF-beta1/PI3K/Akt signaling pathway. Cell Physiol Biochem 35:1008–1022
Kasashima H, Yashiro M, Nakamae H, Kitayama K, Masuda G, Kinoshita H, Fukuoka T, Hasegawa T, Nakane T, Hino M, Hirakawa K, Ohira M (2016) CXCL1-chemokine (C-X-C motif) receptor 2 signaling stimulates the recruitment of bone marrow-derived mesenchymal cells into diffuse-type gastric cancer stroma. Am J Pathol 186:3028–3039
Kawamura M, Toiyama Y, Tanaka K, Saigusa S, Okugawa Y, Hiro J, Uchida K, Mohri Y, Inoue Y, Kusunoki M (2012) CXCL5, a promoter of cell proliferation, migration and invasion, is a novel serum prognostic marker in patients with colorectal cancer. Eur J Cancer 48:2244–2251
Khan MN, Wang B, Wei J, Zhang Y, Li Q, Luan X, Cheng JW, Gordon JR, Li F, Liu H (2015) CXCR1/2 antagonism with CXCL8/interleukin-8 analogue CXCL8(3-72)K11R/G31P restricts lung cancer growth by inhibiting tumor cell proliferation and suppressing angiogenesis. Oncotarget 6:21315–21327
Kim EJ, Juhnn YS (2015) Cyclic AMP signaling reduces sirtuin 6 expression in non-small cell lung cancer cells by promoting ubiquitin-proteasomal degradation via inhibition of the Raf-MEK-ERK (Raf/mitogen-activated extracellular signal-regulated kinase/extracellular signal-regulated kinase) pathway. J Biol Chem 290:9604–9613
Kowalczuk O, Burzykowski T, Niklinska WE, Kozlowski M, Chyczewski L, Niklinski J (2014) CXCL5 as a potential novel prognostic factor in early stage non-small cell lung cancer: results of a study of expression levels of 23 genes. Tumour Biol 35:4619–4628
Kuo PL, Huang MS, Hung JY, Chou SH, Chiang SY, Huang YF, Yang CJ, Tsai MJ, Chang WA, Hsu YL (2014) Synergistic effect of lung tumor-associated dendritic cell-derived HB-EGF and CXCL5 on cancer progression. Int J Cancer 135:96–108
Lee YC, Lin HH, Hsu CH, Wang CJ, Chiang TA, Chen JH (2010) Inhibitory effects of andrographolide on migration and invasion in human non-small cell lung cancer A549 cells via down-regulation of PI3K/Akt signaling pathway. Eur J Pharmacol 632:23–32
Liang JX, Gao W, Liang Y, Zhou XM (2015) Chemokine receptor CXCR4 expression and lung cancer prognosis: a meta-analysis. Int J Clin Exp Med 8:5163–5174
Morrison CD, Parvani JG, Schiemann WP (2013) The relevance of the TGF-beta paradox to EMT-MET programs. Cancer Lett 341:30–40
Pold M, Zhu LX, Sharma S, Burdick MD, Lin Y, Lee PP, Pold A, Luo J, Krysan K, Dohadwala M, Mao JT, Batra RK, Strieter RM, Dubinett SM (2004) Cyclooxygenase-2-dependent expression of angiogenic CXC chemokines ENA-78/CXC ligand (CXCL) 5 and interleukin-8/CXCL8 in human non-small cell lung cancer. Cancer Res 64:1853–1860
Saintigny P, Massarelli E, Lin S, Ahn YH, Chen Y, Goswami S, Erez B, O'Reilly MS, Liu D, Lee JJ, Zhang L, Ping Y, Behrens C, Solis SL, Heymach JV, Kim ES, Herbst RS, Lippman SM, Wistuba II, Hong WK, Kurie JM, Koo JS (2013) CXCR2 expression in tumor cells is a poor prognostic factor and promotes invasion and metastasis in lung adenocarcinoma. Cancer Res 73:571–582
Shiels MS, Pfeiffer RM, Hildesheim A, Engels EA, Kemp TJ, Park JH, Katki HA, Koshiol J, Shelton G, Caporaso NE, Pinto LA, Chaturvedi AK (2013) Circulating inflammation markers and prospective risk for lung cancer. J Natl Cancer Inst 105:1871–1880
Shrihari TG (2017) Dual role of inflammatory mediators in cancer. Ecancermedicalscience 11:721
Spaks A, Svirina D, Spaka I, Jaunalksne I, Breiva D, Tracums I, Krievins D (2016) CXC chemokine ligand 4 (CXCL4) is predictor of tumour angiogenic activity and prognostic biomarker in non-small cell lung cancer (NSCLC) patients undergoing surgical treatment. Biomarkers 21:474–478
Sun JH, Fan N, Zhang Y (2016) Correlation between serum level of chemokine (C-C motif) ligand 18 and poor prognosis in breast cancer. Genet Mol Res 15
Takiguchi S, Korenaga N, Inoue K, Sugi E, Kataoka Y, Matsusue K, Futagami K, Li YJ, Kukita T, Teramoto N, Iguchi H (2014) Involvement of CXCL14 in osteolytic bone metastasis from lung cancer. Int J Oncol 44:1316–1324
Terlizzi M, Casolaro V, Pinto A, Sorrentino R (2014) Inflammasome: cancer’s friend or foe? Pharmacol Ther 143:24–33
Wu Y, Zhou BP (2010) TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and invasion. Br J Cancer 102:639–644
Yanagawa J, Walser TC, Zhu LX, Hong L, Fishbein MC, Mah V, Chia D, Goodglick L, Elashoff DA, Luo J, Magyar CE, Dohadwala M, Lee JM, St JM, Strieter RM, Sharma S, Dubinett SM (2009) Snail promotes CXCR2 ligand-dependent tumor progression in non-small cell lung carcinoma. Clin Cancer Res 15:6820–6829
Youlden DR, Cramb SM, Baade PD (2008) The international epidemiology of lung cancer: geographical distribution and secular trends. J Thorac Oncol 3:819–831
Zheng J, Zhu X, Zhang J (2014) CXCL5 knockdown expression inhibits human bladder cancer T24 cells proliferation and migration. Biochem Biophys Res Commun 446:18–24
Zhou SL, Dai Z, Zhou ZJ, Wang XY, Yang GH, Wang Z, Huang XW, Fan J, Zhou J (2012) Overexpression of CXCL5 mediates neutrophil infiltration and indicates poor prognosis for hepatocellular carcinoma. Hepatology 56:2242–2254
Zhou SL, Zhou ZJ, Hu ZQ, Li X, Huang XW, Wang Z, Fan J, Dai Z, Zhou J (2015) CXCR2/CXCL5 axis contributes to epithelial-mesenchymal transition of HCC cells through activating PI3K/Akt/GSK-3beta/Snail signaling. Cancer Lett 358:124–135
Funding
This study was funded by the National Natural Science Foundation of China (grant number 81372313) and Outstanding Youth Foundation of Zhongshan Hospital (grant number 2017ZSYQ28).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Wang, L., Shi, L., Gu, J. et al. CXCL5 regulation of proliferation and migration in human non-small cell lung cancer cells. J Physiol Biochem 74, 313–324 (2018). https://doi.org/10.1007/s13105-018-0619-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-018-0619-z