Abstract
NOS isoform activation is related to liver failure during sepsis, but the mechanisms driving mitochondrial impairment remain unclear. We induced sepsis by LPS administration to inducible nitric oxide synthase (iNOS−/−) and neuronal nitric oxide synthase (nNOS−/−) mice and their respective wild-type controls to examine the contribution of iNOS to mitochondrial failure in the absence of nNOS. To achieve this goal, the determination of messenger RNA (mRNA) expression and protein content of iNOS in cytosol and mitochondria, the mitochondrial respiratory complex content, and the levels of nitrosative and oxidative stress (by measuring 3-nitrotyrosine residues and carbonyl groups, respectively) were examined in the liver of control and septic mice. We detected strongly elevated iNOS mRNA expression and protein levels in liver cytosol and mitochondria of septic mice, which were related to enhanced oxidative and nitrosative stress, and with fewer changes in respiratory complexes. The absence of the iNOS, but not nNOS, gene absolutely prevented mitochondrial impairment during sepsis. Moreover, the nNOS gene did not modify the expression and the effects of iNOS here shown. Melatonin administration counteracted iNOS activation and mitochondrial damage and enhanced the expression of the respiratory complexes above the control values. These effects were unrelated to the presence or absence of nNOS. iNOS is a main target to prevent liver mitochondrial impairment during sepsis, and melatonin represents an efficient antagonist of these iNOS-dependent effects whereas it may boost mitochondrial respiration to enhance liver survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Liver dysfunction and the subsequent alteration in hemodynamic status and pathogen clearance lead to multiple organ dysfunction syndrome and increased risk of death in septic patients [14]. Thus, the maintenance of liver function is a main goal in antiseptic therapy [39].
It has been postulated that liver function is regulated by nitric oxide (NO·) through a broad range of direct and indirect effects. At physiological levels, NO· is not only critical for healthy hepatocyte blood flow [38, 39] but also plays beneficial roles in inflammation-associated hepatic damage [22, 31, 45, 53]. NO·, however, has also genotoxic and cytotoxic effects, depending on the type of insult, cellular redox status of liver, and source, concentration, and site of NO· production [8]. In this regard, hepatic NO· is formed by three isoenzymes of nitric oxide synthase (NOS): Ca2+-dependent constitutive endothelial (eNOS) and neuronal (nNOS) isoenzymes, which generally produce NO· for regulatory purposes [23], and the Ca2+-independent inducible (iNOS) isoform responsible for generating large and toxic amounts of NO· in response to pathologic and inflammatory conditions [35]. Although these three isoenzymes of NOS are differentially expressed in the liver, nNOS is restricted to nerve endings found in the larger blood vessels with an unknown role [21, 36]. More recently, the presence of iNOS in the mitochondria has been reported in numerous tissues, including the liver [28, 44, 46]. Despite some contradictory issues about the nature of mitochondrial NOS [2, 27, 32], conclusive evidences indicate the existence of both constitutive and inducible NOS isoforms, which derived from cytosolic nNOS and iNOS, respectively [18, 32]. The presence of a constitutive NOS supports a physiological role for the intramitocondrial NO· production, including modulation of oxygen consumption, ATP production, and free radical generation by the reversible inhibition of cytochrome oxidase [30]. In turn, the overproduction of NO· due to mitochondrial iNOS induction is responsible for sepsis-associated mitochondrial dysfunction. Thus, high concentrations of NO· reduce the electron transfer and impair mitochondrial respiration, leading to overproduction of superoxide anion (O2·–) that, in turn, reacts with NO· to yield peroxynitrites (ONOO–) [7, 41]. Both NO· and ONOO– not only are capable to reduce the efficiency of the oxidative phosphorylation, thereby decreasing ATP production [17, 20], but also lead to tyrosine nitration and formation of the other reactive species, profoundly perturbing mitochondrial dysfunction [4, 5, 43].
There is notably evidence that melatonin shows beneficial antioxidant and anti-inflammatory effects in sepsis, including inhibition of iNOS expression and activity, prevention of sepsis-associated mitochondrial oxidative damage, and recovery of electron transport chain (ETC) activity and ATP production [13, 17, 18, 20, 32, 40]. Nevertheless, potential implication of mitochondrial NOS in liver failure during sepsis, as well as the relevance of both mitochondrial isoforms in the antiseptic properties of melatonin, is yet unknown. Here, we induced sepsis by LPS administration in wild-type and nNOS- and iNOS-deficient mice to examine in liver: (a) the role of iNOS in mitochondrial dysfunction including respiratory complex expression and oxidative/nitrosative stress and (b) the existence of any interaction between melatonin and iNOS to promote healthy mitochondria in these conditions.
Materials and methods
Animals and treatments
iNOS knockout B6.129P2-Nos2tm1Lau mice (iNOS−/− mice) and nNOS B6;129S4-Nos1tm1Plh/J-deficient mice and their respective wild-type controls (C57/Bl/6 or iNOS+/+ and B6129SF2/J or nNOS+/+) were obtained from Jackson’s Laboratory through Charles River Labs (Barcelona, Spain). The animals were housed in the animal facility of the University of Granada with a controlled 12-h light-dark cycle at 22 ± 2 °C and on regular chow and tap water. Animals were used at 12–14 weeks of age and 25–30 g body weight. All experiments were conducted in accordance with the Granada’s University Ethics Committee, the Spanish law for animal experimentation (R.D. 53/2013), and the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes (CETS no. 123). All strain mice were divided in the following groups comprising 8–10 animals per group: (a) control group, (b) LPS group, i.p. injected with LPS (Escherichia coli 0111:B4, Sigma-Aldrich, Madrid, Spain) (40 mg/kg b.w., dissolved in 0.3 mL saline), and (c) LPS + aMT group, i.p. injected with LPS (40 mg/kg b.w., dissolved in 0.3 mL saline) and treated with melatonin (30 mg/kg b.w. dissolved in 0.3 mL 0.25% ethanol/saline). The animals received four doses of 30 mg/kg melatonin every one i.p. injection just after LPS administration and the remaining doses at 2, 4, and 6 h after LPS. Preliminary experiments showed that the ethanol-saline volume injected had no effect on the variables studied here [13]. Eight hours after LPS injection, animals were killed by cervical dislocation. Liver was quickly collected, washed, and frozen to −80 °C in liquid nitrogen until the remaining assays were performed.
Isolation of cytosol and mitochondrial fractions
Pure cytosol and mitochondria were prepared by differential centrifugation and Percoll density gradient as previously described, with slight modifications [17]. All procedures were carried out at 4 °C. Briefly, liver was excised, washed with saline, and homogenized (1/10, w/v) in buffer A (0.22 M mannitol, 0.07 M sucrose, 0.5 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N',N'-tetraacetic acid (EGTA), 0.1% BSA, and 2 mM HEPES/KOH, pH 7.4, at 4 °C) at 800 rpm with an SS2 stirrer with a Teflon pestle (Stuart Scientific Co. Ltd., Cambridge, UK). The homogenate was centrifuged at 600×g for 5 min at 4 °C (twice), and the supernatants were centrifuged at 10,300×g for 10 min at 4 °C. An aliquot of the supernatant was used as cytosolic fraction, and the mitochondrial pellets were resuspended in buffer A and poured in ultracentrifuge tubes containing buffer B (0.225 M mannitol, 1 mM EGTA, 25 mM HEPES, and 0.1% BSA, pH 7.4, at 4 °C supplemented with 30% Percoll). The mixture was centrifuged at 95,000×g for 30 min at 4 °C. The fraction with a density of 1052–1075 g/mL, corresponding to a pure mitochondrial fraction, was collected, washed twice with buffer A at 10,300×g for 10 min at 4 °C to remove the Percoll, and frozen to −80 °C. The purity of the mitochondrial fraction obtained with this experimental procedure has been previously validated [32].
Real-time quantitative RT-PCR assay of iNOS mRNA expression
Total RNA from mouse liver was isolated using the Real Total Spin Plus kit (REAL; Durviz SL, Valencia, Spain). RNA purity was estimated on the basis of the OD 260/280 ratio (NanoDrop Technologies, Wilmington, DE, USA). Complementary DNA (cDNA) was synthesized from 2.5 ng of total optimized RNA using the High-Capacity cDNA Reverse Transcription Kit (Life Technologies-Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions. Quantitative real-time PCR (qRT-PCR) was performed on the Stratagene Mx3005P QPCR System (Agilent Technologies, La Jolla, CA, USA) with the FastStart Universal SYBR Green Master mix (Roche Applied Science, Mannheim, Germany). Gene-specific primers for iNOS (sense, 5′-AGACGGATAGGCAGAGATTGG-3′; antisense, 5′-ACTGACACTTCGCACAAAGC-3′) and β-actin (sense, 5′-GCTGTCCCTGTATGCCTCTG-3′; antisense, 5′-CGCTCGTTGCCAATAGTGATG-3′) were designed using the Beacon Designer software (PREMIER Biosoft Int., Palo Alto, CA, USA) and obtained from Thermo Electron GmbH (Ulm, Germany). The PCR program was initiated with 10 min at 95 °C before 40 thermal cycles, each consisting of 15 s at 95 °C and 1 min at 55 °C. Output data were analyzed with MxPro QPCR software (v. 4.0; Agilent Technologies) according to the relative standard curve method, constructed with serial dilutions of cDNA (500, 50, 5, 0.5, and 0.05 ng), and were normalized by β-actin expression. A negative template-free (water) control reaction was also run, and the control group was used as the calibration sample in each strain mouse.
Western blot analysis
Cytosolic and mitochondrial proteins (20 μg) were heated at 95 °C for 5 min, separated by SDS-PAGE on 7.5 or 12.5% acrylamide gels under denaturing conditions, and transferred onto nitrocellulose membranes (PhastSystem; GE Healthcare, Uppsala, Sweden). The membranes were incubated in blocking buffer (5% nonfat dry milk or 5% BSA in PBS plus 0.1% Tween 20, according to primary antibody) and then with the primary antibody diluted in blocking buffer overnight at 4 °C. The primary antibodies used in this study included iNOS (1:100; sc-650, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), nitrotyrosine (1:250; cat. 487924, Calbiochem, San Diego, CA, USA), and MitoProfile Total OXPHOS Rodent WB Antibody Cocktail (dilution 1:250; ab110413, Abcam Inc., Cambridge, MA, USA). Anti-mouse (1:1000; BD Biosciences-Pharmingen, San Diego, CA, USA) and anti-rabbit (1:5000; Thermo Fisher Scientific, Waltham, MA, USA) horseradish peroxidase-conjugated secondary antibodies were used according to the manufacturer’s instructions. OxyBlot Protein Oxidation Detection Kit (Chemicon, Millipore, Billerica, MA, USA) was used for immunoblotting assays of protein carbonyl groups. Dinitrophenylhydrazine (DNPH) derivatization and SDS/PAGE electrophoresis were performed following the manufacturer’s instructions. Derivatized proteins were blotted onto nitrocellulose membranes, incubated in blocking buffer during 3 h at room temperature, and incubated overnight at 4 °C with the specific antibody against the DNP moiety of the proteins (1:150). This step was followed by incubation with goat anti-rabbit IgG secondary antibody (1:300) for 1 h at room temperature. The immunoreactions were detected with the Western Lightning Plus-ECL system (Perkin-Elmer Life Sciences Inc., Boston, MA, USA) according to the manufacturer’s protocol. Plots were digitized and quantified on a Kodak Image Station 2000R (Eastman Kodak Co., Rochester, NY, USA).
Statistical analysis
Data are expressed as means ± SEM, where n indicates the number of independent experiments. Significance was determined using ANOVA followed by Bonferroni’s test for multiple comparisons (Prism 6; GraphPad, San Diego, CA, USA). The Brown–Forsythe test was used to assess the equality of group variances. The level of statistical significance was taken as p < 0.05.
Results
LPS induction of cytosolic and mitochondrial iNOS was unrelated to nNOS form and counteracted by melatonin.
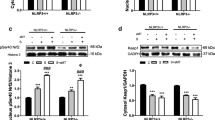
In response to i.p. LPS administration, we observed a significant induction of iNOS gene expression in liver in both wild-type and nNOS-deficient mice (Fig. 1a). These mouse strains showed similar induction of iNOS messenger RNA (mRNA) expression. As expected, mice lacking iNOS did not show iNOS gene expression in either untreated or LPS- and melatonin-treated mice (Fig. 1a). In all cases, melatonin administration blunted the effects of LPS on iNOS expression (Fig. 1a).
a Gene expression of iNOS in liver of all mouse strains tested after LPS and melatonin treatment. Representative western blot and densitometric analysis of iNOS protein levels in cytosol (b) and mitochondria (c), respectively, of wild-type, iNOS−/− and nNOS−/− mice. Data are presented as mean ± SEM of five experiments per group. ***p < 0.001 vs. control; ###p < 0.001 vs. LPS group
The protein levels of cytosolic and mitochondrial iNOS match the gene expression (Fig. 1b, c). Western blot confirmed a significant increase in iNOS protein levels in liver cytosol and mitochondria of both wild-type and nNOS-deficient mice after LPS treatment (Fig. 1b, c). iNOS protein, however, was not detected in both cytosol and mitochondria of untreated or LPS-treated iNOS-deficient mice (Fig. 1b, c). In all cases, melatonin administration counteracted the enhanced iNOS protein content (Fig. 1b, c).
iNOS, but not nNOS, mediates the effects of LPS on protein nitration and carbonylation in liver cytosol and mitochondria, which was neutralized by melatonin.
Nitration of free and protein-associated tyrosine residues to 3-nitrotyrosine is a biomarker of nitro-oxidative stress linked to altered protein structure and function [42]. According to the results shown in Fig. 1, an LPS-dependent increase in iNOS expression and protein levels was accompanied by high levels of 3-nitrotyrosine residues in cytosol and mitochondria of both wild-type mice and nNOS-deficient mice, whereas no changes were observed in both cytosol and mitochondria of iNOS-deficient mice (Figs. 2 and 3). Interestingly, 3-nitrotyrosine levels were more elevated in iNOS+/+ mice than in nNOS+/+ or nNOS−/− mice. Melatonin administration protected cytosolic and mitochondrial proteins from nitrosative damage, normalizing or even reducing nitrotyrosine residues below its basal levels (Figs. 2 and 3). As expected, melatonin had no effect on nitrosative damage in iNOS-deficient mice (Figs. 2 and 3).
In addition to 3-nitrotyrosine residues, introduction of carbonyl groups into protein side chains is another stable marker of LPS-induced nitro-oxidative stress. Levels of carbonyl groups of oxidized proteins reported similar increase after LPS treatment in iNOS+/+, nNOS+/+, and nNOS−/− mice in liver cytosol and mitochondria, while it remains unchanged in iNOS-deficient mice (Fig. 4). In contrast to its lack of effect in iNOS−/− mice, melatonin treatment absolutely counteracted protein oxidation to control values in both wild-type and nNOS-deficient strain mice (Fig. 4).
Western blot analysis of the effects of melatonin treatment on LPS-induced carbonyl group levels in the cytosol and mitochondria from iNOS+/+, iNOS−/−, nNOS+/+, and nNOS−/− mice. Data are expressed as means ± SEM of five experiments per group. p < 0.05 and ***p < 0.001 vs. control; ###p < 0.001 vs. LPS group
LPS has minimal effect on the expression of respiratory complexes, but melatonin enhanced them significantly.
Finally, we investigated whether LPS or melatonin treatment was affected by proteins involved in oxidative phosphorylation. For this purpose, we used the MitoProfile Total OXPHOS Rodent WB Antibody Cocktail for western blot analysis of OXPHOS complexes. The kit contains five mouse antibodies, one each against CI subunit NDUFB8, CII-30 kDa, CIII-Core protein 2, CIV subunit 1, and CV α subunit as an optimized premixed cocktail. The protein content of the CI, CIII, CIV, and CV subunits is normalized by CII, which is exclusively coded by nuclear DNA (Abcam). The results revealed that the content of mitochondria-encoded subunits in CI, CIII (Core2), CIV (Cox1), and CV (Vα), was slightly increased in iNOS+/+ and nNOS−/− mice (Fig. 5a, d), whereas they were unchanged in iNOS−/− and nNOS+/+ mice (Fig. 5c, d) after LPS administration. The protein levels of these subunits, however, increased significantly above the control and LPS groups after melatonin treatment in iNOS+/+, nNOS+/+, and nNOS−/− mice. Interestingly, Core2, Cox1, and complex I levels remain unchanged after melatonin treatment in iNOS−/− mice, although Vα protein level increased notably (Fig. 5).
Discussion
This study clearly shows that the lack of iNOS gene prevents the cytosolic and mitochondrial oxidative/nitrosative damage and respiratory changes in mouse liver after LPS administration. Moreover, our study also sustained that the antioxidant and mitochondrial effects of melatonin on liver were related to the inhibition of the expression and protein levels of iNOS/i-mtNOS, both of which increased by LPS. Overall, these data further support and extend the important therapeutic implications for the potential use of melatonin due to its ability to suppress LPS-induced iNOS expression, oxidative damage, and subsequent liver failure.
Besides multiple physiological functions, the liver plays key roles in metabolic and immune responses during sepsis through a wide range of actions, including pathogen clearance, detoxification, and protein synthesis for metabolic and immune and coagulation functions [38]. A main point of discussion is, however, the role of nitric oxide (NO·) in hepatic function. NO·, as synthesized by constitutive neuronal or endothelial NO synthases (nNOS and eNOS, respectively), maintains local distribution of perfusion and portal pressure while protecting against hepatic inflammatory damage to prevent platelet adhesion or polymorphonuclear neutrophil accumulation [8, 34, 37]. Nonetheless, the role of NO· derived from inducible NO synthase (iNOS) in the liver under pathologic conditions, including endotoxemia, remains controversial. Whereas some reports indicate that administration of iNOS-selective inhibitors had a beneficial effect on liver damage and dysfunction in LPS-treated rats [46], others suggest that iNOS inhibitors or lack of iNOS gene only prevents circulatory failure but does not attenuate the liver damage or necrosis caused by the endotoxin [33, 51]. We have previously reported that LPS administration increases iNOS expression and activity leading to an excess of NO· production and NO·-dependent oxidative/nitrosative damage, which may contribute to the increased risk of multiorgan failure and death during sepsis [12, 18]. Importantly, several groups have shown the presence of both constitutive and inducible NOS isoforms in the liver mitochondria. In this regard, the constitutive isoform or c-mtNOS was identified as a post-translationally modified variant of nNOS [45], with significant implications in the regulation of mitochondrial function and cell proliferation [1, 24, 31]. Soon after the identification of c-mtNOS, the existence of inducible isoform or i-mtNOS was reported [16, 47]. Besides its relationship with the cytosolic iNOS, several reports also support that i-mtNOS is closely related to the nitrosative/oxidative damage and mitochondrial dysfunction during endotoxemia [17, 19, 31, 39].
Having in mind the existence of two NOS isoforms in the mitochondria, we then examined the relative contribution of nNOS/c-mtNOS vs. iNOS/i-mtNOS to liver dysfunction during endotoxemia. As mentioned earlier, NO· can play cytoprotective or cytotoxic effects depending on its levels and NOS isoform-specific production, the presence of oxidative stress, and the specific inter- and intracellular localization [10]. This dual role of NO· is particularly evident at mitochondrial level from tissues with substantial energetic demands such as the liver. Under normal conditions, NO· competes with O2 and reversibly inhibits complex IV of the mitochondrial electron transport chain (ETC), thus regulating the rate of cellular energy supply [3, 5]. Alternatively, NO· also regulates mitochondrial energy production through reversible nitrosylation of mitochondrial protein [9, 41]. After LPS administration, however, high levels of NO· produced by iNOS/i-mtNOS induction reduce electron transfer along the mitochondrial ETC, increasing electron leakage and subsequent superoxide anion (O2 —•) production [16]. In turn, elevated levels of both O2 —• and NO· react to yield ONOO–, which irreversibly inhibits the mitochondrial ETC and ATP synthase, thereby decreasing ATP production [7, 16, 17, 19, 31]. NO· and NO·-related free radicals can also directly inhibit mitochondrial enzymes by tyrosine nitration, oxidation of residues, and/or damage to the iron sulfur center of the respiratory complex, which further increases mitochondrial dysfunction during endotoxemia [5, 6]. The increased iNOS/i-mtNOS protein and gene expression, protein oxidation levels and nitration of protein-associated tyrosine residues found in our experimental conditions support their contribution to mitochondrial dysfunction after LPS administration. Moreover, we also observe a significant oxidative/nitrosative damage at cytosolic level, resulting in massive free radical damage and eventually death cell [11, 49].
Although we have previously reported that oxidative/nitrosative damage derived from i-mtNOS induction triggers ETC dysfunction [16, 18, 19, 31, 39], our results do not show significant changes in the protein levels of the ETC complex. According to previous report [23], these data suggest that mitochondrial dysfunction is not due to a reduced synthesis of OXPHOS proteins during sepsis or LPS administration. As expected, similar patterns of iNOS/i-mtNOS changes, oxidative/nitrosative stress, and mitochondrial dysfunction during endotoxemia were observed in the livers of both wild-type and nNOS-deficient mice. Importantly, the lack of the iNOS gene prevents LPS-associated aforementioned changes, supporting a detrimental role of iNOS/i-mtNOS during lipopolysaccharide-induced endotoxemia in the mouse liver.
Mitochondrial damage during sepsis supports that targeting antioxidants to mitochondria may be of therapeutic benefit in patients with this pathology [15, 24]. Considering its antioxidant and anti-inflammatory actions and the fact that mitochondria uptake melatonin in a concentration- and time-dependent manner [32, 48], it is not surprising that melatonin therapy was beneficial in restoring mitochondrial homeostasis in sepsis. We previously reported that melatonin administration clearly prevents multiple organ failure and improves survival in septic animals through a broad spectrum of actions, including the inhibition of the expression and activity of both cytosolic iNOS and mitochondrial i-mtNOS, directly scavenging oxygen- and nitrogen-derived free radicals, inducing the expression of antioxidant enzymes, and restoring the mitochondrial glutathione (GSH) pool and mitochondrial homeostasis [12, 14, 16–19, 25, 31, 36, 39]. Importantly, we have also shown that beneficial effects of melatonin were directly related to inhibition of iNOS/i-mtNOS but lacking effects on nNOS/c-mtNOS [17, 39]. Our results confirm these protective effects of melatonin in liver of septic mice. Here, melatonin treatment inhibits both expression and protein levels of iNOS/i-mtNOS and protects proteins against both oxidation and nitrosylation at cytosolic and mitochondrial levels due to the antioxidant activity of this indolamine. These beneficial effects of melatonin were also proved in aged animals, which showed an exaggerated response to sepsis that was also prevented by the indoleamine [18, 50]. Moreover, a direct effect of melatonin on mitochondrial function is supported by its ability to increase protein levels of ETC complexes. Of note, the therapeutic effects of melatonin here reported were also related to the inhibition of iNOS/i-mtNOS but not to nNOS.
Overall, the data presented support the existence of an inducible mitochondrial NOS isoform (i-mtNOS), derived from cytosolic iNOS. Moreover, mice lacking nNOS have similar LPS-associated mitochondrial dysfunction than wild-type septic mice, while the absence of the iNOS gene prevented hepatic mitochondria impairment after LPS administration, which supports the implication of the iNOS gene in the pathophysiological events following inflammation. Importantly, iNOS/i-mtNOS emerges as specific pharmacological targets to prevent acute liver dysfunction, a critical complication profoundly related to multiple organ dysfunction syndrome in septic patients. Melatonin behaves as a selective iNOS/i-mtNOS inhibitor that, together with its antioxidant properties and the ability of mitochondria to take up and retain melatonin, supports the efficacy of this indolamine as a therapy in septic patients elsewhere reported [28].
A last point of the discussion is the mechanism involved in the translocation of iNOS from the cytosol into the mitochondrion. To date, there is no current information explaining the transport of iNOS; in the case of nNOS, however, it was reported that one of the nNOS proteins is synthesized in the cytosol, and it is under post-translational modifications including reversible acylation with myristic acid that may serve for subcellular targeting or membrane anchoring [29, 45]. Moreover, it was shown that the amino terminus of nNOS contains a mitochondrial targeting signal that is necessary and sufficient for import into mitochondria [52]. Thus, the post-translational modifications required for the iNOS carrying across the cytosol to mitochondria remains to be clarified.
We conclude that melatonin, which has multiple mechanisms of action on mitochondria, prevented absolutely the induction of iNOS, blunting its activity in cytosol and mitochondria, boosting mitochondrial OXPHOS capacity, and reducing oxidative and nitrosative stress. Whereas the effects of melatonin were unrelated to the presence or absence of nNOS, the former represents an efficient enhancer of liver survival during sepsis.
References
Acuña-Castroviejo D, Escames G, López LC, Hitos AB, León J (2005) Melatonin and nitric oxide: two required antagonists for mitochondrial homeostasis. Endocrine 27:159–168
Brookes PS (2004) Mitochondrial nitric oxide synthase. Mitochondrion 3:187–204
Boveris A, Alvarez S, Navarro A (2002) The role of mitochondrial nitric oxide synthase in inflammation and septic shock. Free Radic Biol Med 33:1186–1193
Brown GC (1999) Nitric oxide and mitochondrial respiration. Biochim Biophys Acta 1411:351–369
Brown GC (2001) Regulation of mitochondrial respiration by nitric oxide inhibition of cytochrome c oxidase. Biochim Biophys Acta 1504:46–57
Brown GC, Borutaite V (2004) Inhibition of mitochondrial respiratory complex I by nitric oxide, peroxynitrite and S-nitrosothiols. Biochim Biophys Acta 1658:44–49
Cadenas E, Poderoso JJ, Antunes F, Boveris A (2000) Analysis of the pathways of nitric oxide utilization in mitochondria. Free Radic Res 33:747–756
Chen T, Zamora R, Zuckerbraun B, Billiar TR (2003) Role of nitric oxide in liver injury. Curr Mol Med 3:519–526
Chouchani ET, Nadtochiy SM HTR, Brookes PS, Fearnley IM, Lilley KS, Smith RA, Murphy MP (2010) Identification of S-nitrosated mitochondrial proteins by S-nitrosothiol difference in gel electrophoresis (SNO-DIGE): implications for the regulation of mitochondrial function by reversible S-nitrosation. Biochem J 430:49–59
Clemens MG (1999) Nitric oxide in liver injury. Hepatology 30:1–5
Cowley HC, Bacon PJ, Goode HF, Webster NR, Jones JG, Menon DK (1996) Plasma antioxidant potential in severe sepsis: a comparison of survivors and nonsurvivors. Crit Care Med 24:1179–1183
Crespo E, Macias M, Pozo D, Escames G, Martín M, Vives F, Guerrero JM, Acuña-Castroviejo D (1999) Melatonin inhibits expression of the inducible NO synthase II in liver and lung and prevents endotoxemia in lipopolysaccharide-induced multiple organ dysfunction syndrome in rats. FASEB J 13:1537–1546
Dhainaut JF, Marin N, Mignon A, Vinsonneau C (2001) Hepatic response to sepsis: interaction between coagulation and inflammatory processes. Crit Care Med 29:S42–S47
Doerrier C, Garcia JA, Volt H, Díaz-Casado ME, Lima-Cabello E, Ortiz F, Luna-Sánchez M, Escames G, López LC, Acuña-Castroviejo D (2015) Identification of mitochondrial deficits and melatonin targets in liver of septic mice by high-resolution respirometry. Life Sci 121:158–165
Escames G, Acuña-Castroviejo D, López LC, Tan DX, Maldonado MD, Sánchez-Hidalgo M, León J, Reiter RJ (2006) Pharmacological utility of melatonin in the treatment of septic shock: experimental and clinical evidence. J Pharm Pharmacol 58:1153–1165
Escames G, León J, Macias M, Khaldy H, Acuña-Castroviejo D (2003) Melatonin counteracts lipopolysaccharide-induced expression and activity of mitochondrial nitric oxide synthase in rats. FASEB J 17:932–934
Escames G, López LC, Ortiz F, López A, García JA, Ros E, Acuña-Castroviejo D (2007) Attenuation of cardiac mitochondrial dysfunction by melatonin in septic mice. FEBS J 274:2135–2147
Escames G, López LC, Ortiz F, Ros E, Acuña-Castroviejo D (2006) Age-dependent lipopolysaccharide-induced iNOS expression and multiorgan failure in rats: effects of melatonin treatment. Exp Gerontol 41:1165–1173
Escames G, López LC, Tapias V, Utrilla P, Reiter RJ, Hitos AB, León J, Rodríguez MI, Acuña-Castroviejo D (2006) Melatonin counteracts inducible mitochondrial nitric oxide synthase-dependent mitochondrial dysfunction in skeletal muscle of septic mice. J Pineal Res 40:71–78
Esteban FJ, Pedrosa JA, Jimenez A, Jiménez A, Fernández AP, Bentura ML, Martínez-Murillo R, Rodrigo J, Peinado MA (1997) Distribution of neuronal nitric oxide synthase in the rat liver. Neurosci Lett 226:99–102
Farinelli SE, Park DS, Greene LA (1996) Nitric oxide delays the death of trophic factor-deprived PC12 cells and sympathetic neurons by a cGMP-mediated mechanism. J Neurosci 16:2325–2334
Forstermann U, Boissel JP, Kleinert H (1998) Expressional control of the ‘constitutive’ isoforms of nitric oxide synthase (NOS I and NOS III). FASEB J 12:773–790
Fredriksson K, Tjader I, Keller P, Petrovic N, Ahlman B, Schéele C, Wernerman J, Timmons JA, Rooyackers O (2008) Dysregulation of mitochondrial dynamics and the muscle transcriptome in ICU patients suffering from sepsis induced multiple organ failure. PLoS One 3:e3686
Galley HF (2011) Oxidative stress and mitochondrial dysfunction in sepsis. Br J Anaesth 107:57–64
Garcia JA, Volt H, Venegas C, Doerrier C, Escames G, López LC, Acuña-Castroviejo D (2015) Disruption of the NF-kappaB/NLRP3 connection by melatonin requires retinoid-related orphan receptor-alpha and blocks the septic response in mice. FASEB J 29:3863–3875
Ghafourifar P, Cadenas E (2005) Mitochondrial nitric oxide synthase. Trends Pharmacol Sci 26:190–195
Ghafourifar P, Richter C (1997) Nitric oxide synthase activity in mitochondria. FEBS Lett 418:291–296
Gitto E, Karbownik M, Reiter RJ, Tan DX, Cuzzocrea S, Chiurazzi P, Cordaro S, Corona G, Trimarchi G, Barberi I (2001) Effects of melatonin treatment in septic newborns. Pediatr Res 50:756–760
Giulivi C (2003) Characterization and function of mitochondrial nitric-oxide synthase. Free Radic Biol Med 34:397–408
Kim YM, Talanian RV, Billiar TR (1997) Nitric oxide inhibits apoptosis by preventing increases in caspase-3-like activity via two distinct mechanisms. J Biol Chem 272:31138–31148
López LC, Escames G, Tapias V, Utrilla P, León J, Acuña-Castroviejo D (2006) Identification of an inducible nitric oxide synthase in diaphragm mitochondria from septic mice: its relation with mitochondrial dysfunction and prevention by melatonin. Int J Biochem Cell Biol 8:267–278
López A, Garcia JA, Escames G, Venegas C, Ortiz F, López LC, Acuña-Castroviejo D (2009) Melatonin protects the mitochondria from oxidative damage reducing oxygen consumption, membrane potential, and superoxide anion production. J Pineal Res 46:188–198
MacMicking JD, Nathan C, Hom G et al (1995) Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell 1(81):641–650
MacMicking J, Xie QW, Nathan C (1997) Nitric oxide and macrophage function. Annu Rev Immunol 5:323–350
McNaughton L, Puttagunta L, Martinez-Cuesta MA, Kneteman N, Mayers I, Moqbel R, Hamid Q, Radomski MW (2002) Distribution of nitric oxide synthase in normal and cirrhotic human liver. Proc Natl Acad Sci U S A 99:17161–11766
Martín M, Macías M, Escames G, León J, Acuña-Castroviejo D (2000) Melatonin but not vitamins C and E maintains glutathione homeostasis in t-butyl hydroperoxide-induced mitochondrial oxidative stress. FASEB J 14:1677–1679
Mittal MK, Gupta TK, Lee FY, Sieber CC (1994) Groszmann RJ. Nitric oxide modulates hepatic vascular tone in normal rat liver. Am J Phys 267: G416-G422.
Nesseler N, Launey Y, Aninat C, Aninat C, Morel F, Mallédant Y, Seguin P (2012) Clinical review: the liver in sepsis. Crit Care 16:235
Ortiz F, García JA, Acuña-Castroviejo D, Doerrier C, López A, Venegas C, Volt H, Luna-Sánchez M, López LC, Escames G (2014) The beneficial effects of melatonin against heart mitochondrial impairment during sepsis: inhibition of iNOS and preservation of nNOS. J Pineal Res 56:71–81
Poderoso JJ, Carreras MC, Lisdero C, Riobó N, Schöpfer F, Boveris A (1996) Nitric oxide inhibits electron transfer and increases superoxide radical production in rat heart mitochondria and submitochondrial particles. Arch Biochem Biophys 328:85–92
Prime TA, Blaikie FH, Evans C et al (2009) A mitochondria-targeted S-nitrosothiol modulates respiration, nitrosates thiols, and protects against ischemia-reperfusion injury. Proc Natl Acad Sci U S A 106:10764–10769
Quijano C, Romero N, Radi R (2005) Tyrosine nitration by superoxide and nitric oxide fluxes in biological systems: modeling the impact of superoxide dismutase and nitric oxide diffusion. Free Radic Biol Med 39:728–741
Schild L, Reinheckel T, Reiser M, Horn TF, Wolf G, Augustin W (2003) Nitric oxide produced in rat liver mitochondria causes oxidative stress and impairment of respiration after transient hypoxia. FASEB J 7:2194–2201
Takemura S, Minamiyama Y, Inoue M, Kubo S, Hirohashi K, Kinoshita H (2000) Nitric oxide synthase inhibitor increases hepatic injury with formation of oxidative DNA damage and microcirculatory disturbance in endotoxemic rats. Hepato-Gastroenterology 47:1364–1370
Tatoyan A, Giulivi C (1998) Purification and characterization of a nitric-oxide synthase from rat liver mitochondria. J Biol Chem 273:11044–11048
Thiemermann C, Ruetten H, Wu CC, Vane JR (1995) The multiple organ dysfunction syndrome caused by endotoxin in the rat: attenuation of liver dysfunction by inhibitors of nitric oxide synthase. Br J Pharmacol 116:2845–2851
Valdez LB, Zaobornyj T, Boveris A (2005) Functional activity of mitochondrial nitric oxide synthase. Methods Enzymol 396:444–455
Venegas C, Garcia JA, Escames G, Ortiz F, López A, Doerrier C, García-Corzo L, López LC, Reiter RJ, Acuña-Castroviejo D (2012) Extrapineal melatonin: analysis of its subcellular distribution and daily fluctuations. J Pineal Res 52:217–227
Victor VM, Rocha M, De la Fuente M (2004) Immune cells: free radicals and antioxidants in sepsis. Int Immunopharmacol 4:327–347
Volt H, Garcia JA, Doerrier C, Diaz-Casado ME, Guerra-Librero A, López LC, Escames G, Tresguerres JA, Acuna-Castroviejo D (2016) Same molecule but different expression: aging and sepsis trigger NLRP3 inflammasome activation, a target of melatonin. J Pineal Res 60:193–205
Wray GM, Millar CG, Hinds CJ, Thiemermann C (1998) Selective inhibition of the activity of inducible nitric oxide synthase prevents the circulatory failure, but not the organ injury/dysfunction, caused by endotoxin. Shock 9:329–335
Zemojtel TL, Kolanczyk M, Kossler N, Stricker S, Lurz R, Mikula I, Duchniewicz M, Schuelke M, Ghafourifar P, Martasek P, Vingron M, Mundlos S (2006) Mammalian mitochondrial nitric oxide synthase: characterization of a novel candidate. FEBS Lett 580:455–462
Zhu W, Fung PC (2000) The roles played by crucial free radicals like lipid free radicals, nitric oxide, and enzymes NOS and NADPH in CCl(4)-induced acute liver injury of mice. Free Radic Biol Med 29:870–880
Acknowledgements
This study was partially supported by grants from the Instituto de Salud Carlos III (Ministerio de Economía y Competitividad and Fondos Feder, Spain no. RD12/0043/0005, PI08-1664; PI13-00981) and from the Consejería de Innovación, Ciencia y Empresa, Junta de Andalucía (P07-CTS-03135, P10-CTS-5784, and CTS-101), Spain.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
ᅟ
All experiments were conducted in accordance with the Granada’s University Ethics Committee, the Spanish law for animal experimentation (R.D. 53/2013), and the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes (CETS no. 123)
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
García, J.A., Ortiz, F., Miana, J. et al. Contribution of inducible and neuronal nitric oxide synthases to mitochondrial damage and melatonin rescue in LPS-treated mice. J Physiol Biochem 73, 235–244 (2017). https://doi.org/10.1007/s13105-017-0548-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-017-0548-2