Abstract
The prostate gland is a part of the male reproductive tract which produces both angiotensin II (Ang II) and relaxin 2 (RLN2). The present study analyzes the effect of both these peptide hormones at concentration 10−8M on viability, proliferation, adhesion, migration, and invasion of normal prostate epithelial cells (PNT1A). Improved survival in two- and three-dimensional cell cultures was noted as well as visual changes in colony size and structure in Geltrex™. Stimulatory influence on cell viability of each peptide applied single was lower than in combination. Enhanced survival of PNT1A cells appears to be associated with increased BCL2/BAX messenger RNA (mRNA) expression ratio. Modulation of cell spreading and cell-extracellular matrix adhesion dynamics were also altered as an influence of tested hormone application. However, long-term Ang II and RLN2 effects may lead to an increase of normal prostate cell migration and invasion abilities. Moreover, gelatin zymography revealed that both gelatinases A and B were augmented by Ang II treatment, whereas RLN2 significantly stimulated only MMP-9 secretion. These results support the hypothesis that deregulation of locally secreted peptide hormones such as Ang II and RLN2 may take part in the development of certain cancers, including prostate cancer. Moreover, the observed ability of relaxin 2 to act as a regulator of mRNA expression levels not only LGR7 but also classic angiotensin receptors suggested that renin-angiotensin system and relaxin family peptide system are functionally linked.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
While angiotensin II (Ang II) is an octapeptide hormone best known for its role in the maintenance of blood pressure and water-electrolyte balance, the most well-studied function of relaxin 2 (RLN2) is associated with pregnancy and parturition. However, many other features of both these peptide hormones have been observed and described in tissues of different origins [11, 22]. For example, the main elements of the renin-angiotensin system (RAS) and relaxin family peptide system (RFPS), i.e., angiotensin II, angiotensin receptors AT1 and AT2, relaxin 2, relaxin receptors RXFP1/LGR7 and RXFP2/LGR8, are expressed in the prostate gland [24, 29]. There is no doubt that both systems play important role in the physiology and pathology of the prostate. It has been established that angiotensin II and relaxin 2 can influence sperm quality by increasing sperm count and motility [13, 25]. On the other hand, these peptide hormones have been associated with a chronic inflammatory, benign prostatic hyperplasia (BPH), and prostate carcinogenesis. It seems that cell processes such as proliferation, apoptosis, invasion, and metastasis of prostate cells are modulated by these local peptide hormones [5–7, 18, 34].
Relatively few studies have been published on the interactions between the RAS and the RFPS. Angiotensin and relaxin have been described to have both chronotropic and inotropic properties; however, their actions are opposed to each other [14, 35]. Moreover, relaxin may act by the renin-angiotensin system to induce the release of vasopressin and oxytocin in the nervous system [17] and a recently conducted study revealed that relaxin needs the AT2 receptor subtype of angiotensin receptors to abrogate renal interstitial fibrosis [2].
To our knowledge, the present study is the first to analyze the impact of angiotensin II and relaxin 2 on PNT1A, a normal prostate epithelial cell line. This non-tumorigenic, normal prostate epithelial cell line appears to be a valuable tool for the study of initial steps leading to cancer transformation of the prostate gland [28].
This process is complex and encompasses disruption of many cellular processes such as cell survival and increased number of uncontrolled divisions. Moreover, penetration of the basement membrane and remodeling of the extracellular matrix (ECM) proteins, as well as focalized cell-matrix adhesion, are critical steps in the process of tumor cell invasion [16, 27, 32]; thus, within this study, we assessed the influence of Ang II and RLN2 in terms of basic cellular processes which alteration initiates cancerogenesis. To enhance the cognitive value of this study and mimic natural cell-matrix adhesion and to assess the viability and proliferation of the PNT1A cells, two-dimensional (2D) and three-dimensional (3D) Geltrex™ cultures were used. 3D cell culture models better mimic the in vivo environment compared with monolayer cultures. One of the most striking differences observed when comparing cells in 2D and 3D is a dissimilarity in morphology, due to the fact that 3D culture systems allow the complex interactions between normal cell-cell and cell-extracellular matrix while cells grown in a monolayer are less able to form communication networks [16, 21]. The cell survival abilities after treatment with peptide hormones were estimated with BCL2/BAX messenger RNA (mRNA) ratio. This ratio is known to correlate positively with cell survival and is frequently used as a prognostic factor in many types of cancers [26, 36]; thus, its usage in the abovementioned assessment of Ang II and RLN2 influence seems to be rationale.
Many studies have reported that both relaxin 2 and angiotensin II induce a breakdown of the tissue basement membrane by affecting the expression of matrix metalloproteinases (MMPs) and their inhibitors (TIMPs). Penetration of the basement membrane and remodeling of the ECM proteins, as well as the occurrence of focalized cell-matrix adhesion, are critical early steps in the process of tumor cell invasion and further metastasis [6, 7, 18, 34]. Hence, this study was also designed to investigate the biological effects of Ang II and RLN2, alone and in combination, on adhesion, migration, and invasion of normal epithelial prostate cells. To fulfill the overview of the influence of both hormones on cell behavior connected with invasiveness, potential changes in metalloproteinases secretion were also estimated.
Materials and methods
2D and 3D cell culture and reagents
Immortalized, normal prostate epithelial PNT1A cells were obtained from the European Collection of Cell Cultures (ECACC; 95012614). Cell line authenticity was confirmed by short-tandem repeat (STR) DNA profiling (LGC Standards Cell Line Authentication Service, Germany; 2014). At least two passages were performed after thawing from liquid nitrogen. For core experiments, cells with passage number between 9 and 18 were taken. The cells were grown in a classical 2D cell culture system or as 3D cultures on matrix basement membrane. Cells cultured on 2D were seeded on plates or flasks (BD Biosciences). For 3D culture, experiments were performed in 48-well culture plates, coated with Geltrex™ LDEV-Free Basement Membrane Matrix. Cells resuspended in 2 % Geltrex™ solution (1 × 105 cells/ml) were added to the wells and allowed to grow for several days. The medium containing 2 % Geltrex™ was replaced at least every 4 days. Both 2D and 3D cultures were maintained in advanced RPMI 1640 supplemented with 5 % fetal bovine serum, 2 mM l-glutamine, 1 mM sodium pyruvate, and antibiotics. After 3–5 days, the cells were harvested with 0.25 % trypsin/EDTA.
In our previous report, we observed the effect of submission of angiotensin peptides on prostate cancer cell viability in a range of concentrations from 0.005 to 5000 nM [8]. Moreover, the dose-dependent response of cancer cells both to angiotensin [38] and relaxin [12] has been also done by other groups but at higher concentrations; thus, we decided to use in this study angiotensin II (Bachem; H-1705) and relaxin 2 (Bachem; H-6784) at a final concentration of 10−8 M for all experiments. The chosen concentrations might be slightly higher than the physiologically reported ones, but, in any case, are still meaningful. The RPMI medium and all culture supplements/reagents were purchased from Gibco® (Thermo Fisher Scientific, Inc.) unless otherwise specified.
Cell viability/proliferation assay
WST-1 assay and Alamar Blue® assay
The RLN2 and Ang II were added to a cell culture medium, either in combination or individually, in particular periods of time after cell seeding, i.e., 24 h for 2D PNT1A cell culture or 7 days for 3D culture. The WST-1 or Alamar Blue® was added 48 h later, and the wells were incubated for 1–3 h. During incubation, the color of WST-1 or Alamar Blue changed from red to yellow or from blue to red, respectively. The final color intensity, which reflects the metabolic activity of the cells, was measured using a BioTek microplate reader. Absorbance was monitored at 450 nm for WST-1 or 570 nm for Alamar Blue. The metabolic activity/cell viability (%) was calculated to control cells cultured in complete growth medium without RLN2 and Ang II.
BrdU assay
BrdU cell proliferation ELISA (Roche Diagnostics; 11647229001) was performed as described elsewhere [9]. Briefly, cells were plated on a 96-well flat bottom plate and allowed to attach overnight at 37 °C in 5 % CO2. The cells were then treated with the indicated concentration of peptide hormones for 24 or 48 h. Bromodeoxyuridine (BrdU) was added to the culture medium in the last 4 h of incubation, and absorbance was measured at 450 nm on a BioTek microplate reader. Cell proliferation (% of control) was calculated in relation to untreated controls.
Automated method of counting cells
The cells were seeded in a 12-well cell culture plate and incubated with or without peptide hormones at a concentration of 10−8 M. After 24–48 h, the cells were collected by trypsinization. The viable cells were then counted using 0.1 % trypan blue dye staining with an automated cell counter (Countess®, Thermo Fisher Scientific, Inc.).
Cell migration/invasion assay
Wound healing assay
The detailed procedures for the wound healing test have been described previously [9]. A pipette tip was used to make a straight scratch on a near-confluent cell monolayer. Peptide hormones (10−8 M) were added, and wound closure was documented by a series of photographs. The area of the wounded surface and its closure was calculated by ImageJ software at different times from 0 to 60 h. The results were expressed as a percentage of initial wound size at time zero.
Transwell migration/invasion assay
The transwell migration/invasion assays were performed as described previously [9, 10]. Firstly, the cells were seeded on a six-well plate and incubated with the addition of peptide hormones at a concentration of 10−8 M for 24 h. The control cells were grown in standard growth medium. After incubation, time cells were collected by trypsinization, resuspended in serum-free medium (1 × 106 cells/ml) with addition (experimental wells) or without (control wells) peptide hormones (10−8 M), and then added to upper chambers. The membranes of upper inserts were perforated, having 8- or 3-μm pores, and to better access the invasion potential, a part of used chambers was coated with Matrigel (a mixture of ECM proteins). The lower chamber contained medium with 10 % fetal bovine serum (FBS). The chambers were incubated at 37 °C with 5 % CO2 for 24–48 h. After this period, non-migrating/non-invasion cells from the upper surface of the transwell/separating membrane were mechanically removed. Cells which migrated to the lower surface of the membrane were stained with staining solution (10 mg crystal violet, 5 ml ethanol 95 %, and 45 ml distilled water) and then dissolved in 10 % acetic acid. The cell migration/invasion potential was quantified by measuring the absorbance at 570 nm using a BioTek microplate reader. Results are shown as percentage inhibition or stimulation as compared to the 100 % untreated control.
Gelatin zymography assay
The matrix metalloproteinase activity (MMP-2 and MMP-9) of the normal prostate epithelial cells, before and after peptide hormone treatment (24 and 48 h), was determined by gelatin zymography as described previously [15, 30]. Briefly, conditioned media were subjected to gel electrophoresis containing 4 % gelatin. Protein extracts (5–10 μg) were subjected to electrophoresis, followed by washing in 2.5 % Triton X-100. The gels were then incubated overnight at 37 °C in the reaction buffer, before being stained (Coomassie blue) and destained (30 % methanol and 10 % acetic acid). Areas of enzymatic activity appeared as clear bands over a dark background. The density of the gel bands was analyzed using ImageJ software.
Adhesion assay
The influence of RLN2 and Ang II, either alone or in combination, on the ability of prostate cancer cells to adhere to ECM components was also evaluated. The adhesion assay was performed in 24-well culture plates coated with ECM proteins: collagen I (BD Biosciences; 354408), collagen IV (BD Biosciences; 354429), laminin (BDBiosciences; 354412), and fibronectin (BD Biosciences; 354411). Firstly, the cells were seeded on a six-well plate with addition of peptide hormones at a concentration of 10−8 M. The control cells were grown in standard growth medium. After 24 or 48 h, the cells were collected by trypsinization and were added to ECM-coated wells in RPMI/FBS-free medium at a concentration of 105 cells/well. After 90 min incubation at 37 °C, the cells were washed three times with PBS to remove non-adherent cells. Samples were fixed and stained with a 0.1 % solution of crystal violet in 25 % ethanol, stored at room temperature, and rinsed in water. Finally, cells were solubilized by adding 10 % acetic acid and quantified using a microplate reader at 570 nm (BioTek). The data was represented as percentage relative to the untreated control.
Real-time RT-PCR
With quantitative reverse transcription PCR (RT-qPCR), we assessed changes in expression level of BAX, BCL2, AT1, AT2, LGR7, and LGR8 after RLN2 and Ang II treatment. PNT1A cells (passage number between 5 and 10) were exposed to RLN2 and/or Ang II (1 × 10−8 M) for 24 or 48 h. The RNA was then extracted using a universal kit for total RNA isolation (A&A Biotechnology). Reverse transcriptase was used to synthesize cDNA for further qPCR analysis [10]. Amplification reactions were performed with a LightCycler 480 Real-Time PCR System. Quantitative data from gene expression experiments were normalized to expression of two housekeeping genes: H3F3A and RPLPO as endogenous control. The Universal Human Reference RNA (Stratagene), composed of total RNA from ten human cell lines, was used as a calibrator. Expression ratios were calculated according to Pfaffl method with REST-MCS software. REST 2009 Software (version 2.0.13) was used to determine significant differences between control and sample groups. The software provides proper error propagation and robust statistical analysis by using a random reallocation algorithm with 2000 iterations. The following reaction conditions and sequences of used primers are presented in Table 1 [31].
Statistical analysis
The results are presented as mean ± SD or SEM of at least three independent experiments. The measurements were subjected to analysis of variance (two-way ANOVA) and Tukey’s multiple comparison test. Relationships were regarded as statistically significant if p < 0.05.
Results
The impact of Ang II and RLN2, alone and in combination, on cell viability and proliferation of normal prostate epithelial cells
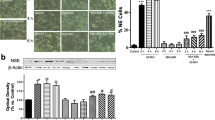
WST-1 and Alamar Blue® assays showed that investigated peptide hormones increased the viability of normal prostate epithelial cells in both 2D and 3D cultures; however, the results were statistically significant for particular experimental conditions (Fig. 1b). In all culture models, Ang II and RLN2 were found to have a higher impact on cell viability when administered together, rather than separately. However, only in 2D model, this PNT1A pro-viability influence of hormones administered separately was statistically significant, except for WST-1 Ang II result.
The changes in the cell viability and proliferation of PNT1A cells after exposure to peptide hormones at concentration 10−8 M (Ang II, RLN2, Ang II + RLN2) in a classical 2D cell culture system or in 3D cultures in a basement membrane matrix. a PNT1A cells grown on Geltrex™ for 1 day and next were treated with peptides for another 5 days. Representative images are shown to illustrate the effect of peptide hormones on the morphology and size of 3D structures compared with control group. b The level of metabolic activity in PNT1A cells, after 48-h treatment with peptides, evaluated in WST-1 assay (n = 8) and Alamar Blue® assay (n = 6), respectively. c The rates of intensity cell division, after 24–48-h exposure of PNT1A cells to peptide hormones, evaluated in BrdU assay. (X ± SD; n ≥ 6; Tukey’s test *p < 0.05)
This dependency of better additive effect of examined hormone combination was evident in the case of cell viability in 3D culture. In terms of proliferation changes accessed with BrdU assay, a slight increase of BrdU incorporation into DNA in 2D cell culture after the 48-h incubation with tested peptides was observed. The results were not significant for Ang II (Fig. 1c).
The results obtained with the BrdU assay were verified with the trypan blue assay using an automated cell counter (data not shown). Also in this case, extension of the incubation time from 24 to 48 h enables to observe the increase of cell division potential. Moreover, the multi-cellular structure formation of PNT1A cells in 3D cultures was positively influenced by both peptide hormones. After a 5-day treatment, larger colony sizes were observed in all the tested cultures than controls (Fig. 1a).
The impact of Ang II and RLN2, alone and in combination, on BCL2 and BAX mRNA expression in normal prostate epithelial cells
The BCL-2 and BAX mRNA expression levels in PNT1A cells after 24- or 48-h exposure to Ang II and RLN2, according to real-time RT-PCR analysis, are summarized in Fig. 2a. BAX gene expression level decreased while BCL2 increased after 48-h treatment. The smallest changes in mRNA expression were associated with both genes after Ang II induction, and the greatest after joint Ang II and RLN2 administration. Furthermore, alteration of BCL2 and BAX mRNA expression resulted in an increased ratio of anti-apoptotic index, however, only insignificant for Ang II. As shown in Fig. 2b, Ang II and RLN2 seem to have an additive effect on BCL2/BAX mRNA ratio in normal prostate epithelial cells. This result also explains the viability changes indicating better cell survival after hormone treatment.
The impact of Ang II and RLN2, alone and in combination, on adhesion of normal prostate epithelial cells
After 90 min of incubation, the PNT1A cells suspended in serum-free medium adhered efficiently to fibronectin, but less to both types of collagen and showed a very weak adhesion to laminin. As shown in Fig. 3, reduction in PNT1A adhesion to ECM components was noted after 24-h incubation time; however, the results were not significant for all hormone combination conditions. Prolonged exposure to examined peptide hormones significantly increased PNT1A cell adhesion to ECM proteins; however, this effect was not observed for their combine application. Similarly, there was no significant increase in adhesion to fibronectin and laminin after 48 h of treatment with RLN2.
The changes in the ability of PNT1A cell adhesion on extracellular matrix (ECM) proteins: laminin, fibronectin, collagen I, and collagen IV after exposure to peptide hormones at concentration 10−8 M (Ang II, RLN2, Ang II + RLN2). It shows statistical analysis adhesion assay at different incubation times, 24 and 48 h, and representative images illustrated the binding affinity of PNT1A cells to ECM proteins after 48 h of incubation with peptides. (X ± SD; n = 8; Tukey’s test *p < 0.05)
The impact of Ang II and RLN2, alone and in combination, on cell migration and invasion of normal prostate epithelial cells
The cell migration/invasion assays showed that the tested peptide hormones can modulate both the motility (Fig. 4a, b) and invasiveness (Fig. 4c) of normal prostate epithelial cells, but significant changes were observed only after long-time incubation (72 h). RLN2 facilitates colonization of PNT1A cells into new areas (Fig. 4a) and increases cell migration, but only across 8-μm pore size filters, while migration through a PET membrane with a smaller pore size was promoted mainly by Ang II and, to lesser extent, by a combination of both Ang II and RLN2 (Fig. 4b). The ability of PNT1A cells to cross basement membranes (Fig. 4c), i.e., thin but cross-linked with extracellular matrices (ECMs), was clearly higher in cells after 72 h of exposure to the hormones than the control group.
The changes in the migration and invasion potential of PNT1A cells observed after exposure to peptide hormones at concentration 10−8 M (Ang II, RLN2, Ang II + RLN2). a shows the results of the wound healing assay (0–60 h) (X ± SEM; n = 24) whereas the b, c present the results in transwell migration assay (48 h; n = 6) and invasion chamber assays (24 and 72 h; n = 5), respectively (X ± SD; n ≥ 5; Tukey’s test *p < 0.05)
The impact of Ang II and RLN2, alone and in combination, on MMP-2 and MMP-9 secretion in normal prostate epithelial cells
Gelatin zymography revealed that PNT1A cells secreted into the culture medium proteins with gelatinase activity (Fig. 5). Clear bands were observed at 92 and 72 kDa against a dark background, which correspond to pro-MMP-9 and pro-MMP-2, respectively. In addition, two faintly visible complexes of MMPs were detected, with molecular masses of 240 and 130 kDa (data not shown). No important differences were observed in the level of MMPs after 24-h exposure of PNT1A cells to Ang II and RLN2 (individually or together). The 48-h treatment with Ang II augmented both gelatinases A and B whereas RLN2 significantly stimulated only MMP-9 secretion. In this case, the joint action of the two peptides did not show additive or synergistic effect.
The changes in the level of MMP-2 and MMP-9 secretion in PNT1A cells evaluated after 48-h exposure to peptide hormones at concentration 10−8 M (Ang II, RLN2, Ang II + RLN2) determined in the gelatin zymography assay. a The representative image of a gelatin zymogram. b The statistical analyses of MMP-2 and MMP-9 activity. Band staining intensities were measured by densitometry, using ImageJ software (X ± SD; n = 5; Tukey’s test *p < 0.05)
The impact of Ang II and RLN2, alone and in combination, on angiotensin and relaxin receptor mRNA expression in normal prostate epithelial cells
Real-time RT-PCR showed that both classic angiotensin receptors AT1 (1.439 ± 0.49552) and AT2 (0.653 ± 0.11273) are expressed in PNT1A cells. This normal prostate epithelial cell line also expresses relaxin receptors such as LGR7 (10.41 ± 1.71) and LGR8 (21.32 ± 2.59). Furthermore, we observed that relaxin 2 can change mRNA expression level not only LGR7 but also angiotensin type 1 and type 2 receptors. Meanwhile, Angiotensin II can only alter the mRNA level of classic angiotensin receptors. Expression of LGR8 receptor was not influenced by either RLN2 or Ang II (Table 2).
Discussion
The mechanisms of prostate tumorigenesis initiation and progression associated with changes in the local renin-angiotensin system and relaxin family peptide system still remain unclear [5, 6, 18, 34]. We know that Ang II and RLN2 can modulate cell proliferation and apoptosis, invasion, and spread of human prostate cancer cell lines such as lymph node carcinoma of the prostate (LNCaP), DU-145, and PC-3. As the LNCaP cell line was established from lymph node carcinoma, and PC-3 and DU-145 from bone and brain metastases, respectively [39], these cell lines do not represent good models for the research on primary tumors and causes of prostate cancer formation.
This study is the first to consider the impact of angiotensin II and relaxin 2 on the viability, proliferation, adhesion, migration, and invasion of normal prostate epithelial cells. Also for the first time, we present mRNA expression for key receptors for RAS (AT1 and AT2) and RFPS (LGR7 and LGR8) in PNT1A cells. The PNT1A cell line represents a valuable tool for the study of initial steps leading to transformation of the prostate gland. To create the line, normal adult prostatic epithelial cells were immortalized by retroviral transduction with the Simian virus 40 (SV40). As the PNT1A cells show c-MYC gene amplification and functional inactivation of p53, they exhibit molecular and biochemical properties similar to a normal prostate epithelium but are more sensitive to progression towards malignant transformation [1, 4, 33].
As already mentioned, interactions between RAS and RFPS have been reported in kidney fibrosis [2], the cardiovascular [14, 35], and the central nervous system [17], but so far, never in the carcinogenesis of reproductive tissues. Physical interaction between AT2 receptors and LGR7 was also demonstrated previously [2]. Our study for the first time noted the possibility of mutual regulation of gene expression in both systems via relaxin 2. In PNT1A cells, treatment with relaxin 2 resulted in change of mRNA expression level of LGR7 and both classic angiotensin receptors (Table 2). These results provide a strong basis to evaluate the combined effect of angiotensin II and relaxin 2 on viability, proliferation, adhesion, migration, and invasion of normal prostate epithelial cells (Ryc. 1–5).
Angiotensin II and relaxin 2 improved maintenance of cellular viability of normal prostate cell lines. Our results also indicate that the peptide combination exhibits an additive effect on PNT1A cell survival. In the case of both used methods (WST-1 assay and Alamar Blue assay) and for both types of cultures (2D and 3D), this trend was observed (Fig. 1). However, the trend of additive effect of hormone application on cell viability on 3D cell culture was less pronounced and statistically nonsignificant. This result may be a result of an obvious dissimilarity between the culture systems, as many differences were observed between the 3D and 2D cell cultures with regard to their sensitivity to chemicals and other agents. It is worth noting that cancer cells grown on Matrigel were significantly more sensitive to most prominent cancer drugs than cells grown in 2D cultures [23].
3D cell culture models better mimic the in vivo environment than monolayer cultures, because they enable cell-cell and cell-ECM interactions. Härmä V et al. [21] report that Matrigel strongly supports the growth and differentiation of both normal and prostate cancer spheroids. Generally speaking, the aggregates formed in Matrigel can be divided into four morphological groups: branching/round phenotype, mass phenotype, grape-like phenotype, and a stellate phenotype. Normal primary prostate epithelial (PrECs) and in vitro immortalized cell lines such as RWPE-1 and PWR-1E simultaneously form round spheroids and acinar branching structures [21, 37]. Our findings confirm that the cellular morphology and architecture of PNT1A cells grown in Geltrex™ and in standard cell culture are different. Furthermore, our results show that Ang II and RLN2 influence the morphology and multi-cellular structure formation of PNT1A cells in 3D culture: the colonies are larger and they have a greater tendency to form more branched aggregates than in the control group after a 5-day treatment (Fig. 1a).
The BCL2 family, the essential integrators of survival and death, plays an important role in the accumulation of prostate cancer cells. The BCL2/BAX (or BAX/BCL2) ratio is known to be a prognostic factor for the development and aggressiveness of several diseases, including reproductive cancers [23, 36]. Our findings suggest that the changes in the ratio of BCL2/BAX mRNA expression, corresponding to anti-apoptotic and pro-apoptotic genes, may contribute to increased viability of normal prostate epithelial cells after exposure to the peptide hormones. The strongest induction of BCL2 and downregulation of BAX were observed for the combination of Ang II and RLN2.
Both relaxin 2 and angiotensin II are secreted from the prostate gland into the seminal fluid. Several previous studies have reported that both have a positive influence on many sperm functions, including sperm motility and cervical mucus penetration [24, 29]. However, many other types of cell from a wide variety of human tissues are also capable of movement. Cell motility is associated with several physiological processes, such as embryonic morphogenesis and adult wound healing, as well as some pathological conditions including tumor invasion and metastasis; however, this active movement is realized by very different mechanisms. In 2D models, migration is mainly dependent on adhesion [16, 19].
The present study examined the ability of normal prostate cells to adhere to the major structural elements of the ECM. PNT1A cells were found to adhere efficiently to fibronectin, both collagen types to a lesser extent and very poorly to laminin. Furthermore, Ang II and RLN2 were seen to modulate the adhesive potential of normal epithelial prostate cells. The initial reduction in adhesion to ECM components was reversed after prolonged treatment with peptides. Cytoskeleton rearrangements and dynamic changes in adhesion properties are required for cell detachment and migration [16]. Our findings demonstrate that both Ang II and RLN2 can increase cellular motility and invasiveness in immortalized PNT1A cells. In this regard, the results for Ang II and RLN2 used in combination were between those of each hormone used individually. However, it must be strongly emphasized that stimulatory effect on PNT1A cells was observed only after two, or even three, days of continuous treatment at 10−8 M. Hence, only long-term exposure to Ang II and RLN2 may significantly increase normal prostate cell migration and invasion.
It is interesting to note that our results for wound healing appear to coincide with the results of transwell migration assay through inserts containing 8-μm pore size filters, while the results received from invasion assay are similar to those of migration assay with inserts containing 3-μm pore size filters. A smaller pore size, such as a solidified Matrigel layer, results in a greater challenge for cell movement. Mechanical forces are among the many environmental factors that play an important role in the regulation of cellular behavior and function, including adhesion, migration, or proliferation. Recent studies report that the mechanotransduction is not restricted to elements located at the cell surface but can occur in the nucleus. The forces may affect the cytoskeleton and local focal adhesions, intracellular messengers, or induce long-term phenotypic changes in cells, associated with the modulation of gene expression [3, 19].
During migration/invasion, cells must pass through structural barriers such as basement membranes. Many previous studies have reported that both angiotensin II and relaxin 2 play an important role in the remodeling of the extracellular matrix in several reproductive tract tissues [5–7, 18, 34]. Normal and pathological tissue remodeling is regulated by the activity of matrix metalloproteinases. Increased expression of MMPs has been observed in various cancers, and many reports suggest their prognostic value in patients with prostate cancer [20]. In the present study, gelatin zymography revealed that the PNT1A cells secreted MMP-2 and MMP-9 into the culture medium. Furthermore, Ang II treatment augmented both gelatinases A and B whereas RLN2 significantly stimulated only MMP-9 secretion. It is no surprise that these results coincide closely with the results in transwell invasion assay.
The present study for the first time takes into account the impact of Ang II and RLN2, alone and in combination, on normal prostate epithelial cell line and examined their influence on basic cell biological processes, which alteration may initiate tumor development. Basing on our results, it seems that the examined hormones increase cell viability, with an additive effect observed for both peptides in combination, with changes in the BCL2/BAX expression ratio being related to better survival of PNT1A cells. Moreover, the hormone application resulted in occurrence of dynamic changes in the efficiency of PNT1A cell adhesion to ECM components. Furthermore, the potential for migration and invasion was significantly enhanced, but only after long-term exposure to Ang II or RLN2, with no additive effect observed for when they were applied in combination. Our findings indicate that exposure to Ang II and RLN2 could result in increased levels of matrix metalloproteinases such as MMP-2 and MMP-9 gelatinases, resulting in enhanced metastatic properties of the PNT1A cells. The obtained results are compatible with emerging evidence that deregulation of locally secreted peptide hormones such as RLN2 or Ang II can increase the risk of certain cancers, including prostate cancer. Moreover, the results revealed that the ability of relaxin 2 to act as a regulator of mRNA expression levels not only LGR7 but also of classic angiotensin receptors, suggesting that renin-angiotensin system and relaxin family peptide system are probably functionally linked.
References
Avancès C, Georget V, Térouanne B, Orio F, Cussenot O, Mottet N, Costa P, Sultan C (2001) Human prostatic cell line PNT1A a useful tool for studying androgen receptor transcriptional activity and its differential subnuclear localization in the presence of androgens and antiandrogens. Mol Cell Endocrinol 184:13–24
Chow BS, Kocan M, Bosnyak S, Sarwar M, Wigg B, Jones ES, Widdop RE, Summers RJ, Bathgate RA, Hewitson TD, Samuel CS (2014) Relaxin requires the angiotensin II type 2 receptor to abrogate renal interstitial fibrosis. Kidney Int 86:75–85
Dahl KN, Ribeiro AJ, Lammerding J (2008) Nuclear shape mechanics and mechanotransduction. Circ Res 102:1307–1318
Degeorges A, Hoffschir F, Cussenot O, Gauville C, Le Duc A, Dutrillaux B, Calvo F (1995) Recurrent cytogenetic alterations of prostate carcinoma and amplification of c-myc or epidermal growth factor receptor in subclones of immortalized PNT1 human prostate epithelial cell line. Int J Cancer 62:724–731
Domińska K (2009) The importance of the local rennin-angiotensin system in the pathology of prostate. Folia Medica Lodziensia 36:73–86
Domińska K (2013) Relaxin 2—a pregnancy hormone involved in the process of carcinogenesis. Ginekol Pol 84:126–130
Domińska K, Lachowicz-Ochedalska A (2008) The involvement of the renin-angiotensin system (RAS) in cancerogenesis. Postepy Biochem 54:294–300
Domińska K, Piastowska AW, Rebas E, Lachowicz-Ochedalska A (2009) The influence of peptides from the angiotensin family on tyrosine kinase activity and cell viability in a human hormone-dependent prostate cancer line. Endokrynol Pol 60:363–9
Domińska K, Piastowska-Ciesielska AW, Lachowicz-Ochędalska A, Ochędalski T (2012) Similarities and differences between effects of angiotensin III and angiotensin II on human prostate cancer cell migration and proliferation. Peptides 37:200–206
Dominska K, Piastowska-Ciesielska AW, Pluciennik E, Lachowicz-Ochedalska A, Ochedalski T (2013) A comparison of the effects of Angiotensin IV on androgen-dependent and androgen-independent prostate cancer cell lines. J Renin Angiotensin Aldosterone Syst 14:74–81
Dschietzig T, Bartsch C, Baumann G, Stangl K (2006) Relaxin—a pleiotropichormone and its emerging role for experimental and clinical therapeutics. Pharmacol Ther 112:38–56
Feng S, Agoulnik IU, Bogatcheva NV, Kamat AA, Kwabi-Addo B, Li R, Ayala G, Ittmann MM, Agoulnik AI (2007) Relaxin promotes prostate cancer progression. Clin CancerRes 13:1695–1702
Ferlin A, Menegazzo M, Gianesello L, Selice R, Foresta C (2012) Effect of relaxin on human sperm functions. J Androl 33:474–482
Ferreira VM, Gomes TS, Reis LA, Ferreira AT, Razvickas CV, Schor N, Boim MA (2009) Receptor-induced dilatation in the systemic and intrarenal adaptation to pregnancy in rats. PLoS One 4:e4845
Frederiks WM, Mook OR (2004) Metabolic mapping of proteinase activity with emphasis on in situ zymography of gelatinases: review and protocols. J Histochem Cytochem 52:711–722
Friedl P, Wolf K (2003) Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer 3:362–374
Geddes BJ, Summerlee AJ (1995) The emerging concept of relaxin as a centrally acting peptide hormone with hemodynamic actions. J Neuroendocrinol 7:411–417
George AJ, Thomas WG, Hannan RD (2010) The renin-angiotensin system and cancer: old dog new tricks. Nat Rev Cancer 10:745–759
Guilluy C, Osborne LD, Van Landeghem L, Sharek L, Superfine R, Garcia-Mata R, Burridge K (2014) Isolated nuclei adapt to force and reveal a mechanotransduction pathway in the nucleus. Nat Cell Biol 16:376–381
Hadler-Olsen E, Winberg JO, Uhlin-Hansen L (2013) Matrix metalloproteinases in cancer: their value as diagnostic and prognostic markers and therapeutic targets. Tumor Biol 34:2041–2051
Härmä V, Virtanen J, Mäkelä R, Happonen A, Mpindi JP, Knuuttila M, Kohonen P, Lötjönen J, Kallioniemi O, Nees M (2010) A comprehensive panel of three-dimensional models for studies of prostate cancer growth invasion and drug responses. PLoS One 5:e10431
Haulica I, Bild W, Serban DN (2005) Angiotensin peptides and their pleiotropicactions. J Renin Angiotensin Aldosterone Syst 6:121–131
Hongisto V, Jernström S, Fey V, Mpindi JP, Sahlberg KK, Kallioniemi O, Perälä M (2013) High-throughput 3D screening reveals differences in drug sensitivities between culture models of JIMT1 breast cancer cells. PLoS One 8:e77232
Ivell R, Kotula-Balak M, Glynn D, Heng K, Anand-Ivell R (2011) Relaxin family peptides in the male reproductive system—a critical appraisal. Mol Hum Reprod 17:71–84
Leung PS, Sernia C (2003) The renin-angiotensin system and male reproduction: new functions for old hormones. J MolEndocrinol 30:263–270
Lin Y, Fukuchi J, Hiipakka RA, Kokontis JM, Xiang J (2007) Up-regulation of Bcl-2 is required for the progression of prostate cancer cells from an androgen-dependent to an androgen-independent growth stage. Cell Res 17:531–536
Mazaris E, Tsiotras A (2013) Molecular pathways in prostate cancer. Nephrourol Mon 5:792–800
Mitchell S, Abel P, Ware M, Stamp G, Lalani E (2000) Phenotypic and genotypic characterization of commonly used human prostatic cell lines. BJU Int 85:932–944
O’Mahony OA, Barker S, Puddefoot JR, Vinson GP (2005) Synthesis and secretion of angiotensin II by the prostate gland in vitro. Endocrinology 146:392–398
Piastowska-Ciesielska AW, Dominska K, Nowakowska M, Gajewska M, Gajos-Michniewicz A, Ochedalski T (2013) Angiotensin modulates human mammary epithelial cell motility. J Renin Angiotensin Aldosterone Syst 15:419–429
Pluciennik E, Krol M, Nowakowska M, Kusinska R, Potemski P, Kordek R, Bednarek AK (2010) Breast cancer relapse prediction based on multi-gene RT-PCR algorithm. Med Sci Monit 16:CR132–136
Schrecengost R, Knudsen KE (2013) Molecular pathogenesis and progression of prostate cancer. SeminOncol 40:244–258
Schwab TS, Stewart T, Lehr J, Pienta KJ, Rhim JS, Macoska JA (2000) Phenotypic characterization of immortalized normal and primary tumor derived human prostate epithelial cell cultures. Prostate 44:164–171
Silvertown JD, Summerlee AJ, Klonisch T (2003) Relaxin-like peptides in cancer. Int J Cancer 107:513–519
Teichman SL, Unemori E, Dschietzig T, Conrad K, Voors AA, Teerlink JR, Felker GM, Metra M, Cotter G (2009) Relaxin a pleiotropic vasodilator for the treatment of heart failure. Heart Fail Rev 4:321–329
Tolonen TT, Tommola S, Jokinen S, Parviainen T, Martikainen PM (2007) Bax and Bcl-2 are focally overexpressed in the normal epithelium of cancerous prostates. Scand J UrolNephrol 41:85–90
Tyson DR, Inokuchi J, Tsunoda T, Lau A, Ornstein DK (2007) Culture requirements of prostatic epithelial cell lines for acinar morphogenesis and lumen formation in vitro: role of extracellular calcium. Prostate 67:1601–1613
Uemura H, Ishiguro H, Nagashima Y, Sasaki T, Nakaigawa N, Hasumi H, Kato S, Kubota Y (2005) Antiproliferative activity of angiotensin II receptor blocker through cross-talk between stromal and epithelial prostate cancer cells. Mol Cancer Ther 4:1699–1709
Wu X, Gong S, Roy-Burman P, Lee P, Culig Z (2013) Current mouse and cell models in prostate cancer research. Endocr Relat Cancer 20:R155–170
Acknowledgments
This work was financially supported by the National Science Center, research grant: NN403 2081 39.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Domińska, K., Ochędalski, T., Kowalska, K. et al. A common effect of angiotensin II and relaxin 2 on the PNT1A normal prostate epithelial cell line. J Physiol Biochem 72, 381–392 (2016). https://doi.org/10.1007/s13105-016-0489-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-016-0489-1